文章信息
- 杨森林, 张国秋, 李超, 李克文
- YANG Senlin, ZHANG Guoqiu, LI Chao, LI Kewen
- miR-133a调控RUNX2/BMP2信号通路参与激素性股骨头坏死的机制
- Mechanism of microRNA-133a regulating the RUNX2/BMP2 signaling pathway in steroid-induced osteonecrosis of the femoral head
- 中国医科大学学报, 2023, 52(11): 984-991
- Journal of China Medical University, 2023, 52(11): 984-991
-
文章历史
- 收稿日期:2023-03-02
- 网络出版时间:2023-11-02 11:33:45
激素性股骨头坏死(steroid-induced osteonecrosis of the femoral head,SONFH)是破坏退行性疾病,是近年来随着糖皮质激素广泛应用而引发的股骨坏死疾病[1]。皮质类固醇是引起SONFH发生的最常见原因[2]。SONFH病理上表现为股骨头局部血液循环障碍导致股骨头供血减少、骨髓间充质干细胞(bone manrrow mesenchymal stem cells,BMSCs)成骨分化受到抑制、破骨细胞异常活化,导致骨吸收和新骨重建失衡,最终股骨头坏死、塌陷[3]。目前,SONFH发病机制尚不明确,缺乏统一的治疗方法。因此,寻找相关治疗靶点至关重要。微RNA(microRNA,miRNA)异常表达影响骨骼代谢[4]。其中,miR-133a在骨骼增殖、分化等功能中发挥重要作用[5],沉默miR-133a表达能够缓解糖皮质激素诱导的骨质疏松,并影响间充质干细胞功能,在骨代谢中发挥重要作用[6]。生长相关转录因子2(runt-related transcription factor 2,RUNX2)在骨发育及后期均调解骨形成,RUNX2失活抑制间充质干细胞向成骨分化[7];骨形态蛋白2(bone morphogenetic protein 2,BMP2)能够诱导骨和软骨形成,其靶细胞是未分化的间充质干细胞[8]。进一步研究[9]发现miR-133a能够靶向调控RUNX2,抑制miR-133a能够增强RUNX2的活性和增加骨骼修复能力,推测miR-133a在SONFH中可能发挥类似功效影响骨代谢。本研究分析SONFH患者BMSCs中miR-133a的表达情况,探讨miR-133a调控RUNX2/ BMP2信号通路参与SONFH的机制,旨在为SONFH靶向治疗提供参考依据。
1 材料与方法 1.1 样本来源及处理收集2021年6月至2022年6月青海大学附属医院接受髋关节置换的SONFH患者(SONFH组)骨髓(2 mL)及股骨颈骨折不愈合患者(对照组)骨髓(2 mL)。本研究获得青海大学附属医院伦理委员会批准(20210403)。将骨髓与含有抗凝剂的10%胎牛血清α-MEM培养液轻轻混合,取BMSCs(1.073 g/mL)分离液在离心管中放置至室温,转移至15 mL离心管中,液面上缓慢滴加BMSCs分离液,2 000 r/min离心15 min;离心后箭头吸管轻轻吸取云雾状细胞层,经磷酸缓冲液清洗、2 000 r/min离心5 min,弃上清后加10%胎牛血清α-MEM培养液1 000 r/min离心5 min,弃上清后加10%胎牛血清α-MEM培养液,调整细胞浓度为5×105/mL接种至6 cm板中,置于37 ℃、CO2培养箱培养。
1.2 试剂与仪器引物由上海生工生物工程有限公司合成,RUNX2-3’UTR-WT、RUNX2-3’UTR-MUT、miR-133a模拟物(miR-133a mimic)、miR-133a模拟物对照(mimic con)、miR-133a抑制剂(miR-133a inhibitor)、miR-133a抑制剂对照(inhibitor con)、si-RUNX2均由广州锐博生物科技有限公司提供。一抗RUNX2、BMP2、骨钙素(osteocalcin,OCN)、Ⅰ型胶原蛋白(collagen Ⅰ,COL-Ⅰ),二抗山羊抗兔,CCK-8试剂盒(英国Abcam公司,货号分别为ab236639、ab214821、ab108397、ab34710、ab6721、ab228554);茜素红染色液(上海爱必信生物科技有限公司,abs42012987);油红O染色液(上海经科化学科技有限公司,WB1016);cDNA第一条链合成试剂盒(碧云天科技有限公司,D7178S);双荧光素酶报告基因检测试剂盒(上海翌圣生物科技股份有限公司,11401ES)。主要仪器包括流式细胞仪(美国BD公司,FACSCalibur)、实时定量PCR(real-time quantitative PCR,RT-qPCR)仪(赛默飞世尔公司,7500)、蛋白凝胶成像仪(美国Bio-Rad公司,BIO-RADXR)、酶标仪(深圳汇松科技发展有限公司,MB-530)。
1.3 方法 1.3.1 BMSCs鉴定: 1.3.1.1 细胞表型鉴定细胞培养至第3代,经胰蛋白酶消化、1 500 r/min离心15 min,收集细胞沉淀,经磷酸缓冲液清洗后制成1×105/mL细胞,取100 μL荧光标记的抗体[CD71、CD44、CD34、人类白细胞抗原DR等位基因(human leukocyte antigen DR allele,HLA-DR)]10 μL室温孵育30 min,磷酸缓冲液洗去未标记抗体,上清中添加300 μL磷酸缓冲液,流式细胞术检测细胞表型。
1.3.1.2 定向诱导BMSCs成骨分化细胞按照1×104/mL接种至细胞爬片上,置于6孔板中,37 ℃、CO2培养箱中培养24 h,吸取原培养液,加入1×10-7 mol/L地塞米松、10 mmol/L β-甘油磷酸钠、50 mg/L L-抗坏血酸、5 μg/L rhTGF-β1、10%胎牛血清α-MEM培养液;空白对照添加10%胎牛血清α-MEM培养液培养,倒置显微镜每天观察细胞生长及增殖情况。第21天取出载玻片,茜素红染色鉴定成骨分化能力。
1.3.1.3 定向诱导BMSCs成脂分化细胞按照3×103/mL接种至细胞爬片上,置于6孔板中,待细胞80%~ 90%时,加入1 μmol/L地塞米松、0.5 mmol/L IBMX、10 mg/L牛胰岛素、1 mmol/L吲哚美辛、10%胎牛血清α-MEM培养液;空白对照添加10%胎牛血清α-MEM培养液培养,7 d后取出玻片,油红O染色并拍照。
1.3.2 RT-qPCR检测BMSCs中miR-133a、RUNX2 mRNA表达对照组、SONFH组经鉴定的1×105/mL BMSCs,Trizol法提取总RNA,cDNA第一条链合成试剂盒合成cDNA,RT-qPCR仪检测miR-133a、RUNX2 mRNA表达。miR-133a,正向5’-TTTGGTCCCCTTCAAC-3’,反向5’-TAGCTATCCTTTGCT-3’;U6,正向5’-CGCTTCGGCAGCACATATAC-3’,反向5’-CAGGGGCCATGCTAATCTT-3’;RUNX2,正向5’-TTGGAATCGATGGTAATTATCTTTAG-3’,反向5’-AATCCTATTGCGGTAATCTTACCTTAAT-3’;GAPDH,正向5’-ACGGCAAGTTCAACGGCACAG-3’,反向5’-GAAGACGCCAGTAGACTCCACGAC-3’。20 μL反应体系:400 ng/μL cDNA 1 μL、2×SYBR qPCR Mix 10 μL、正向/反向引物(10 μmol/L)各0.5 μL,ddH2O 8 μL。反应条件:95 ℃、30 s;95 ℃、30 s,miR-133a:61 ℃、30 s;RUNX2:60 ℃、30 s,40个循环。2-ΔΔCt法计算miR-133a、RUNX2 mRNA相对表达水平。
1.3.3 Western blotting检测BMSCs中RUNX2蛋白表达对照组、SONFH组经鉴定的BMSCs细胞添加蛋白裂解酶冰上裂解细胞20 min,12 000 r/min 4 ℃离心20 min,上清即为总蛋白。每个样品(20 μg)分离蛋白,PVDF膜280 mA 40 min转膜、5%脱脂奶粉室温封闭2 h,对应一抗RUNX2、GAPDH,4 ℃孵育过夜;加入对应二抗室温孵育1 h,蛋白显色液显膜,蛋白凝胶成像仪拍照并定量分析。
1.3.4 双荧光素酶验证miR-133a与RUNX2的靶向位点生物信息学软件ENCORI/starBase(https://rnasysu.com/encori/)预测miR-133a与RUNX2存在结合位点。设计合成RUNX2的3’UTR-WT和MUT,分别与miR-133a mimic或mimic con共转染至SONFH患者骨髓提取的BMSCs中,双荧光素酶报告基因检测试剂盒检测各组荧光素酶活性。
1.3.5 实验分组实验分为对照组、SONFH组、inhibitor con组、miR-133a inhibitor组、miR-133a inhibitor+si-RUNX2组。除对照组外,其他各组均用SONFH分离的BMSCs实验,按照Lipofectamine 2000试剂说明书分别转染inhibitor con、miR-133a inhibitor、miR-133a inhibitor+si-RUNX2,转染6 h后更换为10%胎牛血清α-MEM培养液。
1.3.6 RT-qPCR检测各组细胞中miR-133a、RUNX2 mRNA各组细胞继续培养48 h,参照1.3.2方法检测。
1.3.7 Western blotting检测各组BMSCs中RUNX2蛋白各组细胞继续培养48 h,参照1.3.3方法检测。
1.3.8 CCK-8检测细胞增殖情况各组细胞按照1×105/mL置于96孔板中,每孔100 μL,10%胎牛血清的α-MEM培养液置于37 ℃、5%CO2培养箱中继续培养,分别在培养24、48、72 h时各组对应添加CCK-8试剂(10 μL)继续培养2 h,酶标仪检测450 nm处吸光度(optical density,OD)值。
1.3.9 茜素红染色鉴定细胞矿化能力各组细胞按照1×105/mL置于6孔板中,继续培养21 d,茜素红染液染色,倒置显微镜下观察矿化结节情况。显微镜视野下每个样品计数5个孔,采用Image-J软件定量分析,每个视野下矿化结节数量为矿化结节相对数量。
1.3.10 Western blotting检测BMSCs中BMP2、BGP、COL-Ⅰ蛋白各组细胞继续培养48 h,参照1.3.3方法检测。
1.4 统计学分析利用GraphPad Prism7.0软件进行统计分析,计量资料采用x ±s表示,两组比较采用t检验;多组比较采用单因素方差分析,组内两两比较采用snk-q检验。P < 0.05为差异有统计学意义。
2 结果 2.1 BMSCs鉴定结果结果显示,对照组和SONFH组细胞表面CD71、CD44表达,CD34、HLA-DR不表达。见图 1。成骨分化21 d茜素红染色对照组、SONFH组均出现矿化结节,结节呈红色散落分布;空白对照细胞为阴性。见图 2。成脂分化7 d油红O染色,显微镜下对照组、SONFH组均可见脂滴被染,呈橙红色;细胞内脂滴沉着,且数量较多,部分细胞内脂滴融合变大成泡状,细胞核位于中央或被挤向外周。空白对照显微镜下未见橙红色出现,未形成脂滴。见图 3。以上结果提示BMSCs提取成功。
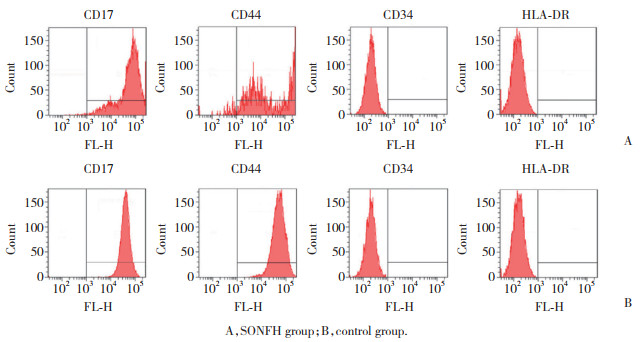
|
| A, SONFH group; B, control group. 图 1 BMSCs鉴定结果 Fig.1 BMSCs identification results |
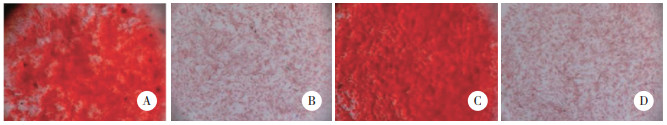
|
| A, control group by induction of osteogenic differentiation; B, blank control in control group; C, SONFH group by induction of osteogenic differentiation; D, blank control in SONFH group. 图 2 茜素红染色结果 ×40 Fig.2 Alizarin red staining results ×40 |
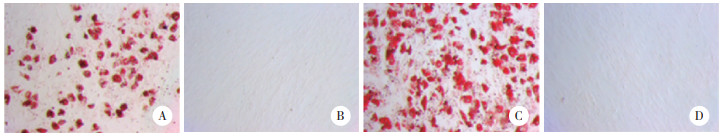
|
| A, control group by induction of lipogenic differentiation; B, blank control in control group; C, SONFH group by induction of lipogenic differentiation; D, blank control in SONFH group. 图 3 油红O染色结果×100 Fig.3 Oil red O staining results×100 |
2.2 对照组和SONFH组BMSCs中miR-133a、RUNX2 mRNA及蛋白表达
结果显示,与对照组比较,SONFH组BMSCs中miR-133a表达升高(P < 0.05),RUNX2 mRNA和蛋白表达降低(P < 0.05)。见图 4、表 1。

|
| 图 4 对照组和SONFH组BMSCs中RUNX2蛋白表达 Fig.4 Expression of RUNX2 protein in BMSCs in the control group and SONFH group |
| Group | miR-133a | RUNX2 mRNA | RUNX2 protein |
| Control | 1.03±0.13 | 1.00±0.08 | 0.86±0.08 |
| SONFH | 5.23±0.39 | 0.26±0.03 | 0.31±0.03 |
| t | 25.025 | 21.215 | 15.768 |
| P | < 0.001 | < 0.001 | < 0.001 |
2.3 miR-133a与RUNX2的靶向关系验证
结果显示,miR-133a与RUNX2存在特异性结合位点,见图 5。双荧光素酶验证结果显示,与mimic con+RUNX2 3’UTR-WT组(1.01±0.08)相比,miR-133a mimic+RUNX2 3’UTR-WT组(0.52±0.06)细胞荧光素酶活性下降(P < 0.05)。而mimic con+RUNX2 3’UTR-MUT组(0.99±0.07)与miR-133a mimic+RUNX2 3’UTR-MUT组(1.00±0.11)细胞荧光素酶活性比较差异无统计学意义(P > 0.05)。

|
| 图 5 预测miR-133a与RUNX2的特异性结合位点 Fig.5 Predicted specific binding sites of miR-133a and RUNX2 |
2.4 各组BMSCs中miR-133a、RUNX2 mRNA和蛋白表达
结果显示,与对照组相比,SONFH组、inhibitor con组、miR-133a inhibitor组、miR-133a inhibitor+ si-RUNX2组BMSCs中miR-133a表达升高(P < 0.05),RUNX2 mRNA和蛋白表达降低(P < 0.05)。与SONFH组和inhibitor con组比较,miR-133a inhibitor组、miR-133a inhibitor+si-RUNX2组BMSCs中miR-133a表达降低(P < 0.05),RUNX2 mRNA和蛋白表达升高(P < 0.05)。与miR-133a inhibitor组比较,miR-133a inhibitor+si-RUNX2组BMSCs中miR-133a表达升高(P < 0.05),RUNX2 mRNA和蛋白表达降低(P < 0.05)。见图 6、表 2。
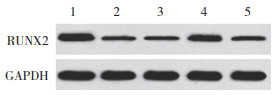
|
| 1, control group; 2, SONFH group; 3, inhibitor con group; 4, miR-133a inhibitor group; 5, miR-133a inhibitor+si-RUNX2 group. 图 6 各组BMSCs中RUNX2蛋白表达比较 Fig.6 Comparison of RUNX2 protein expression in BMSCs in each group |
| Group | miR-133a | RUNX2 mRNA | RUNX2 protein |
| Control | 1.01±0.12 | 1.00±0.10 | 0.89±0.08 |
| SONFH | 4.86±0.521) | 0.28±0.031) | 0.34±0.041) |
| Inhibitor con | 4.78±0.491) | 0.29±0.041) | 0.32±0.031) |
| miR-133a inhibitor | 2.48±0.311),2),3) | 0.77±0.081),2),3) | 0.65±0.061),2),3) |
| miR-133a inhibitor+si-RUNX2 | 3.46±0.391,)2,)3),4) | 0.52±0.061,)2,)3),4) | 0.41±0.041,)2,)3),4) |
| F | 12.488 | 129.96 | 126.745 |
| P | 0.001 | 0.001 | 0.001 |
| 1)P < 0.05 compared with the control group;2)P < 0.05 compared with SONFH group;3)P < 0.05 compared with inhibitor con group;4)P < 0.05 compared with miR-133a inhibitor group. | |||
2.5 miR-133a对各组BMSCs增殖的影响
24、48、72 h检测细胞增殖的结果显示,与对照组相比,SONFH组、inhibitor con组、miR-133a inhibitor组、miR-133a inhibitor+si-RUNX2组OD值降低(P < 0.05)。与SONFH组和inhibitor con组相比,miR-133a inhibitor组、miR-133a inhibitor+si-RUNX2组OD值升高(P < 0.05)。与miR-133a inhibitor组相比,miR-133a inhibitor+si-RUNX2组OD值降低(P < 0.05)。见表 3。
| Group | OD at 450 nm | ||
| 24 h | 48 h | 72 h | |
| Control | 0.56±0.04 | 0.97±0.10 | 1.56±0.16 |
| SONFH | 0.27±0.031) | 0.51±0.041) | 0.64±0.051) |
| Inhibitor con | 0.26±0.031) | 0.50±0.051) | 0.65±0.061) |
| miR-133a inhibitor | 0.48±0.051),2),3) | 0.79±0.081),2),3) | 1.24±0.131),2),3) |
| miR-133a inhibitor+si-RUNX2 | 0.34±0.041),2),3),4) | 0.67±0.071),2),3),4) | 0.87±0.071),2),3),4) |
| F | 70.480 | 46.441 | 89.703 |
| P | < 0.001 | < 0.001 | < 0.001 |
| 1)P < 0.05 compared with the control group;2)P < 0.05 compared with SONFH group;3)P < 0.05 compared with inhibitor con group;4)P < 0.05 compared with miR-133a inhibitor group. | |||
2.6 miR-133a对BMSCs矿化的影响
结果显示,与对照组(68.48±7.18)相比,SONFH组(23.42±3.26)、inhibitor con组(23.51±3.44)、miR-133a inhibitor组(53.67±6.26)、miR-133a inhibitor+ si-RUNX2组(34.85±4.09)BMSCs矿化结节相对数量减少(P < 0.05)。与SONFH组和inhibitor con组相比,miR-133a inhibitor组、miR-133a inhibitor+si-RUNX2组BMSCs矿化结节相对数量增加(P < 0.05)。与miR-133a inhibitor组相比,miR-133a inhibitor+si-RUNX2组BMSCs矿化结节相对数量减少(P < 0.05)。见图 7。
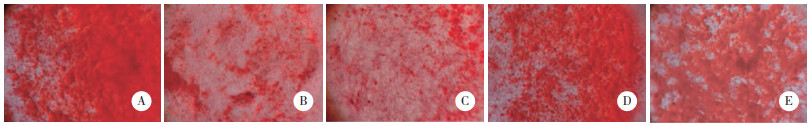
|
| A, control group; B, SONFH group; C, inhibitor con group; D, miR-133a inhibitor group; E, miR-133a inhibitor+si-RUNX2 group. 图 7 各组BMSCs矿化情况比较×40 Fig.7 Comparison of BMSCs mineralization in each group ×40 |
2.7 miR-133a对各组BMP2、BGP、COL-Ⅰ蛋白表达的影响
与对照组相比,SONFH组、inhibitor con组、miR-133a inhibitor组、miR-133a inhibitor+si-RUNX2组BMSCs中BMP2、BGP、COL-Ⅰ蛋白表达降低(P < 0.05);与SONFH组、inhibitor con组相比,miR-133a inhibitor组BMSCs中BMP2、BGP、COL-Ⅰ蛋白表达,miR-133a inhibitor+si-RUNX2组BMSCs中BMP2、COL-Ⅰ蛋白表达升高(P < 0.05);与miR-133a inhibitor组相比,miR-133a inhibitor+si-RUNX2组BMSCs中BMP2、BGP、COL-Ⅰ蛋白表达降低(P < 0.05)。见图 8、表 4。
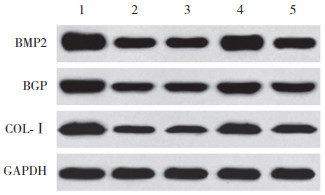
|
| 1, control group; 2, SONFH group; 3, inhibitor con group; 4, miR-133a inhibitor group; 5, miR-133a inhibitor+si-RUNX2 group. 图 8 各组BMSCs中BMP2、BGP、COL-Ⅰ蛋白表达比较 Fig.8 Comparison of BMP2, BGP and COL-Ⅰ protein expression in BMSCs among all groups |
| Group | BMP2 | BGP | COL-Ⅰ |
| Control | 2.62±0.41 | 1.16±0.14 | 1.26±0.09 |
| SONFH | 1.26±0.131) | 0.66±0.071) | 0.53±0.041) |
| Inhibitor con | 1.23±0.141) | 0.65±0.061) | 0.54±0.051) |
| miR-133a inhibitor | 2.11±0.231),2),3) | 0.96±0.091),2),3) | 1.02±0.111),2),3) |
| miR-133a inhibitor+si-RUNX2 | 1.47±0.151),2),3),4) | 0.73±0.081),4) | 0.81±0.071),2),3),4) |
| F | 39.500 | 34.697 | 101.579 |
| P | 0.001 | 0.001 | 0.001 |
| 1)P < 0.05 compared with control group;2)P < 0.05 compared with SONFH group;3)P < 0.05 compared with inhibitor con group;4)P < 0.05 compared with miR-133a inhibitor group. | |||
3 讨论
近年来由于激素的不规范使用,SONFH发生率明显增加;激素已成为股骨头坏死的第一致病因子[10]。研究[11]发现股骨头坏死患者成骨分化能力明显降低、坏死组织中出现大量凋亡细胞,成骨细胞数量减少和功能障碍,导致成骨能力不足,引起骨修复障碍,加重疾病。BMSCs具有多向分化潜能,在特定条件下能够成骨分化,在伤口愈合、股骨头坏死的治疗中具有重要作用[12]。因此,发现与BMSCs定向分化有关的因子,进而寻找SONFH治疗靶点尤为重要。本研究发现SONFH组BMSCs矿化结节相对数量比对照组减少,提示SONFH患者BMSCs中成骨分化能力降低,进而影响成骨细胞数量,导致骨修复障碍。
miR-133a抑制剂增强转化生长因子-β1诱导的肌成纤维细胞分化,产生高水平胶原蛋白的肌成纤维细胞,从而导致肺弹性和功能丧失[13]。miR-133a模拟物可诱导线粒体生成和成肌细胞分化,而miR-133a抑制剂会减弱细胞分化[14];miR-133a抑制剂可显著促进糖皮质激素(地塞米松)处理的间充质干细胞的细胞增殖、活力和成骨细胞分化,并抑制向脂肪细胞分化[6]。本研究结果显示,SONFH患者BMSCs中miR-133a高表达,BMSCs增殖受到抑制、矿化结节数量减少;降低miR-133a水平后促进细胞增殖、矿化结节数量增加,提示miR-133a可能抑制SONFH患者BMSCs的成骨分化能力和增殖能力。
miRNA靶向调控基因表达影响疾病进展。RUNX2是miR-133a靶基因之一,miR-133a能够靶向抑制RUNX2/BMP2信号通路抑制骨形成,从而负向调节骨折愈合[15]。RUNX2作为成熟成骨分化重要指标和促进分子,在成骨细胞和间充质干细胞中高表达,是骨形成必要转录因子。RUNX2能够通过结合RUNX共有序列(PuACCPuCA)调控骨谱系细胞[16],可调控多种因子发挥作用,且这些调控元件在成骨细胞基因启动子中均可找到[17]。BMP2作为RUNX2下游调控因子之一,可作用间充质干细胞,促进间充质干细胞的成骨分化[18]。BGP、COL-Ⅰ作为成骨细胞晚期表型和功能蛋白,在成骨合成、矿化反应、骨形成中发挥作用[19]。本研究发现miR-133a与RUNX2存在靶向结合位点,并经双荧光素酶验证。降低miR-133a表达能够上调RUNX2表达,进而调控下游BMP2水平,升高BGP、COL-Ⅰ表达促进成骨分化。在降低miR-133a基础上干扰RUNX2可逆转上述过程。提示抑制miR-133a的表达能够靶向上调RUNX2的表达,从而促进BMSCs成骨分化和增殖,进而可能缓解疾病进展。
综上所述,SONFH患者BMSCs中miR-133a高表达,抑制miR-133a表达可通过激活RUNX2/BMP2信号通路促进BMSCs成骨分化、增殖。本研究为临床上SOFNH的治疗提供一定理论依据,但RUNX2仅是miR-133a靶基因之一,miR-133a亦可能通过别的靶基因发挥作用,今后需进一步研究论证。
| [1] |
JIANG CL, ZHOU ZB, LIN YW, et al. Astragaloside IV ameliorates steroid-induced osteonecrosis of the femoral head by repolarizing the phenotype of pro-inflammatory macrophages[J]. Int Immunopharmacol, 2021, 93: 107345. DOI:10.1016/j.intimp.2020.107345 |
| [2] |
CASEY KM, GORE F, VILCHES-MOURE JG, et al. Management of morbidity and mortality in a New Zealand white rabbit model of Ste-roid Induced osteonecrosis of the femoral head[J]. Comp Med, 2021, 71(1): 86-98. DOI:10.30802/aalas-cm-20-000071 |
| [3] |
KONG LC, ZUO RT, WANG MW, et al. Silencing microRNA-137-3p, which targets RUNX2 and CXCL12 prevents steroid-induced osteonecrosis of the femoral head by facilitating osteogenesis and angiogenesis[J]. Int J Biol Sci, 2020, 16(4): 655-670. DOI:10.7150/ijbs.38713 |
| [4] |
SIKORA M, MARYCZ K, MIESZEK A. Small and long non-coding RNAs as functional regulators of bone homeostasis, acting alone or cooperatively[J]. Mol Ther Nucleic Acids, 2020, 21: 792-803. DOI:10.1016/j.omtn.2020.07.017 |
| [5] |
MENCÍA CASTAÑO I, CURTIN CM, DUFFY GP, et al. Next generation bone tissue engineering: non-viral miR-133a inhibition using collagen-nanohydroxyapatite scaffolds rapidly enhances osteogenesis[J]. Sci Rep, 2016, 14(6): 27941-27949. DOI:10.1038/srep27941 |
| [6] |
WANG G, WANG FB, ZHANG LC, et al. MiR-133a silencing rescues glucocorticoid-induced bone loss by regulating the MAPK/ERK signaling pathway[J]. Stem Cell Res Ther, 2021, 12(1): 1-14. DOI:10.1186/s13287-021-02278-w |
| [7] |
FAN DW, FAN DY, YUAN WQ. CMTM3 suppresses bone formation and osteogenic differentiation of mesenchymal stem cells through inhibiting Erk1/2 and RUNX2 pathways[J]. Genes Dis, 2021, 8(6): 882-890. DOI:10.1016/j.gendis.2020.12.003 |
| [8] |
HSU SL, CHOU WY, HSU CC, et al. Shockwave therapy modulates the expression of BMP2 for prevention of bone and cartilage loss in the lower limbs of postmenopausal osteoporosis rat model[J]. Biomedicines, 2020, 8(12): 614. DOI:10.3390/biomedicines8120614 |
| [9] |
CASTAÑO IM, RAFTERY R, CHEN G, et al. Rapid bone repair with the recruitment of CD206+M2-like macrophages using non-viral scaffold-mediated miR-133a inhibition of host cells[J]. Acta Biomater, 2020, 109(5): 267-279. |
| [10] |
WU ZX, WEN YX, FAN GL, et al. HEMGN and SLC2A1 might be potential diagnostic biomarkers of steroid-induced osteonecrosis of femoral head: study based on WGCNA and DEGs screening[J]. BMC Musculoskelet Disord, 2021, 22(1): 85. DOI:10.1186/s12891-021-03958-7 |
| [11] |
温家福, 韦标方. 激素性股骨头坏死骨髓间充质干细胞成骨分化的研究进展[J]. 解放军医学杂志, 2020, 45(11): 1207-1214. DOI:10.11855/j.issn.0577-7402.2020.11.16 |
| [12] |
YU HP, LIU P, ZHU DY, et al. Chrysophanic acid shifts the differentiation tendency of BMSCs to prevent alcohol-induced osteonecrosis of the femoral head[J]. Cell Prolif, 2020, 53(8): e12871. DOI:10.1111/cpr.12871 |
| [13] |
WEI P, XIE Y, ABEL PW, et al. Transforming growth factor (TGF) β1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis[J]. Cell Death Dis, 2019, 10(9): 670. DOI:10.1038/s41419-019-1873-x |
| [14] |
ZHANG JL, HUA CJ, ZHANG Y, et al. KAP1-associated transcriptional inhibitory complex regulates C2C12 myoblasts differentiation and mitochondrial biogenesis via miR-133a repression[J]. Cell Death Dis, 2020, 11(9): 732. DOI:10.1038/s41419-020-02937-5 |
| [15] |
PENG H, LU SL, BAI Y, et al. MiR-133a inhibits fracture healing via targeting RUNX2/BMP2[J]. Eur Rev Med Pharmacol Sci, 2018, 22(9): 2519-2526. DOI:10.26355/eurrev_201805_14914 |
| [16] |
KIM JM, YANG YS, PARK KH, et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation[J]. Nat Commun, 2020, 11(1): 2289. DOI:10.1038/s41467-020-16038-6 |
| [17] |
吴钰坤, 韩杰, 温帅波. 骨折愈合过程中Runx2基因的作用机制[J]. 中国组织工程研究, 2021, 25(14): 2274-2279. DOI:10.12122/j.issn.1674-4500.2019.03.26 |
| [18] |
WU XH, DOU B, SUN NY, et al. Astragalus saponin IV promotes osteogenic differentiation of bone marrow mesenchymal stem cells via miR-21/NGF/BMP2/Runx2 pathway[J]. Acta Histochem, 2020, 122(4): 151549. DOI:10.1016/j.acthis.2020.151549 |
| [19] |
SUN LJ. Icariin stimulates hFOB 1.19 osteoblast proliferation and differentiation via OPG/RANKL mediated by the estrogen receptor[J]. Curr Pharm Biotechnol, 2021, 22(1): 168-175. DOI:10.2174/18734316mtazyoduex |
 2023, Vol. 52
2023, Vol. 52




