文章信息
- 宋倩, 康惠文, 蒋守芳, 周秀云, 王晓波, 章义利
- SONG Qian, KANG Huiwen, JIANG Shoufang, ZHOU Xiuyun, WANG Xiaobo, ZHANG Yili
- 大麻素受体2在脓毒症大鼠急性肺损伤中的作用及其机制
- Effect and mechanism of cannabinoid receptor 2 on acute lung injury in septic rats
- 中国医科大学学报, 2022, 51(9): 826-831
- Journal of China Medical University, 2022, 51(9): 826-831
-
文章历史
- 收稿日期:2021-11-12
- 网络出版时间:2022-08-15 8:57
2. 浙江大学医学院附属金华医院血液净化中心, 浙江 金华 321000;
3. 浙江大学医学院附属金华医院内科, 浙江 金华 321000;
4. 浙江大学医学院附属金华医院健康管理中心, 浙江 金华 321000
2. Blood Purification Center, Affiliated Jinhua Hospital, School of Medicine, Zhejiang University, Jinhua 321000, China;
3. Department of Internal Medicine, Affiliated Jinhua Hospital, School of Medicine, Zhejiang University, Jinhua 321000, China;
4. Health Management Center, Affiliated Jinhua Hospital, School of Medicine, Zhejiang University, Jinhua 321000, China
脓毒症是宿主对感染产生的失控性反应, 同时出现危及生命的器官功能障碍[1]。急性肺损伤(acute lung injury, ALI)是脓毒症严重的并发症之一[2]。ALI发生发展过程中, 核因子κB (nuclear factor-κB, NF-κB)及丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)信号通路被激活, 可使炎症介质大量释放, 进而加重肺损伤[3-4]。细胞外信号调节激酶1/2 (extracellular signal regulated kinase1/2, ERK1/2)是最具代表性的MAPK之一, 在脓毒症ALI的发生发展中起重要作用[5]。目前, 对脓毒症所致ALI尚无特效治疗方法, 抗炎治疗是一种重要和有效的治疗手段[6]。大麻素受体2 (cannabinoid receptor 2, CB2R)属于G蛋白耦联受体, 主要在免疫细胞中表达[7]。激活的CB2R具有抗炎和免疫抑制作用[8], 可作为抗炎药物用于类风湿性关节炎等多种疾病的治疗[9], 因此推测CB2R有可能成为治疗脓毒症的一个新靶点。本研究拟通过脂多糖(lipopolysaccharide, LPS)诱导建立大鼠脓毒症ALI模型, 并探讨CB2R在脓毒症ALI中的作用及其机制。
1 材料与方法 1.1 主要试剂及仪器LPS (中国Solarbio公司); 大鼠肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)、白细胞介素-1β (intertleukin-1β, IL-1β)试剂盒(中国欣博盛生物科技有限公司); JHW133、AM630 (美国APExBIO公司); 兔抗IL-1β (美国bio-world公司); 兔源ERK1/2、p-ERK1/2 (美国Cell Signaling公司); 兔源TNF-α、NF-κB p65、NF-κB p65、β-actin (美国Affinity Biosciences公司); 反转录试剂盒、实时定量PCR扩增试剂盒(加拿大abm公司)。Fluor Chem HD2型凝胶成像系统(美国Alpha公司)。
1.2 实验动物分组及模型制备健康SPF级SD大鼠48只, 6~8周龄, 体质量(180± 20)g, 购自北京华阜康生物科技股份公司[动物合格证号: 华阜康SCXK (京)2019-0008]。实验动物处理符合实验动物福利伦理原则(批准文号: LX2019093)。适应性喂养1周后, 将大鼠随机分为对照组(C组)、模型组(LPS组)、CB2R激动剂组(JWH133组)和CB2R拮抗剂组(AM630组), 每组12只。LPS组大鼠腹腔注射LPS (10 mg/kg)建立脓毒症模型, JWH133组及AM630组在注射LPS前30 min腹腔注射JWH133 (3.0 mg/kg)及AM630 (3.0 mg/kg)。LPS注射后, LPS组和AM630组各有1只大鼠死亡。所有动物在给予LPS 6 h后腹腔注射5%水合氯醛麻醉, 放血处死, 收集支气管肺泡灌洗液(bronchoalveolar lavage fluid, BALF)及肺组织标本。
1.3 检测指标及方法 1.3.1 肺组织病理学检测(1) 取右下肺组织, 以4%多聚甲醛固定36 h后, 常规修块、脱水、透明、包埋、切片, HE染色后, 在光镜下观察肺组织病理变化。(2)取肺组织进行固定、包埋、超薄切片, 透射电镜下观察肺泡上皮细胞、线粒体、细胞核、板层小体等肺组织超微结构的改变。
1.3.2 肺组织含水率检测取右中肺, 用滤纸拭去表面血渍, 立即称其湿质量(W); 置于80 ℃恒温干燥箱, 烘烤24 h后称其干质量(D)。肺组织含水率= (W-D)/ W。
1.3.3 BALF中炎性细胞因子含量测定放血处死大鼠后, 打开胸腔, 结扎右侧肺部, 剪开气管, 将生理盐水从气管缓慢注入大鼠左侧肺泡内, 然后缓慢抽出, 重复3次, 收集BALF。按ELISA试剂盒说明书步骤检测BALF中TNF-α、IL-1β含量。
1.3.4 肺组织ERK1/2及NF-κB p65 mRNA表达量检测TRIzol法提取肺组织RNA, 应用逆转录试剂盒合成cDNA, 按照实时定量PCR试剂盒说明书步骤, 检测mRNA表达水平, β-actin作内参照。通过2-ΔΔCt法计算mRNA表达量。引物序列见表 1。
| Gene | Primer sequences (5’-3’) |
| ERK1/2 | CTACACGCAGCTGCAGTACATC |
| GTGCGCTGACAGTAGGTTTGA | |
| NF-κB p65 | ATGCGCTTCCGCTACAA |
| GTGACCAGGGAGATGCG | |
| β-actin | CCGCGAGTACAACCTTCTTG |
| CCCATACCCACCATCACACC |
1.3.5 肺组织TNF-α、IL-1β、ERK1/2、p-ERK1/2、NF-κB p65、p-NF-κB p65蛋白表达检测
采用Western blotting法检测。称量适量肺组织, 匀浆裂解后于4 ℃下14 000 r/min离心15 min, 提取上清液, 测定样品蛋白浓度。取20 μg蛋白样品, 经10%聚丙烯酰胺凝胶电泳分离后, 转至PVDF膜上, 将膜置于2%BSA中37 ℃摇床封闭2 h。在含一抗2%BSA的TBST溶液中孵育2 h或4 ℃过夜。加入HRP标记的山羊抗兔IgG二抗37 ℃摇床孵育1 h, 显影。采用图像分析软件计算蛋白相对表达量。
1.4 统计学分析采用SPSS 20.0统计软件进行分析。数据以x±s表示, 组间比较采用单因素方差分析, 组间两两比较采用LSD检验。P < 0.05为差异有统计学意义。
2 结果 2.1 肺组织病理学改变光镜下C组肺泡结构完整、清晰、肺泡壁厚度均匀, 肺间质未见水肿现象; LPS组肺泡结构破坏严重, 肺泡隔明显增厚, 肺间质及肺泡出现严重水肿, 大量炎症细胞浸润; 与LPS组相比, JWH133组肺泡结构较完整, 肺泡中炎症细胞浸润和水肿不同程度减轻, AM630组肺泡结构破坏严重, 肺泡中炎症细胞浸润和水肿加剧。见图 1。
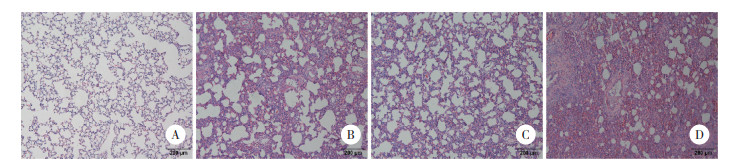
|
| A, C group; B, LPS group; C, JWH133 group; D, AM630 group. 图 1 大鼠肺组织病理变化HE ×100 Fig.1 Pathological changes of lung tissue in rats HE ×100 |
电镜下C组可见形态正常的Ⅰ型肺泡上皮细胞, 核仁清晰, 胞质内可见线粒体, 核糖体等细胞器, 细胞器形态完整, 数量正常; LPS组胞质内可见部分内质网扩张, 细胞器减少; JWH133组受损程度较LPS组明显改善, 胞质内可见大量细胞器, 且形态结构基本正常; AM630组受损程度较LPS组重, 可见核周隙扩张, 内质网扩张明显, 个别线粒体有水肿。见图 2。
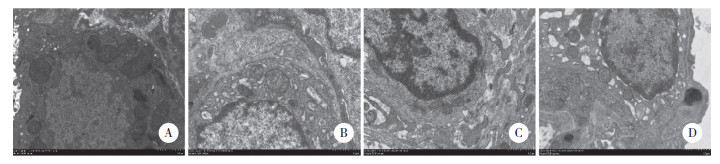
|
| A, C group; B, LPS group; C, JWH133 group; D, AM630 group. 图 2 大鼠肺组织超微结构变化×8 000 Fig.2 ultrastructural changes of lung tissue in rats ×8 000 |
2.2 肺组织含水率比较
与C组比较, LPS组和AM630组肺组织含水率均显著升高(P < 0.05)。与LPS组相比, JWH133组肺组织含水率显著下降(P < 0.05), AM630组肺组织含水率无统计学差异, 但有升高的趋势。见表 2。
| Group | Water content | F | P |
| Control | 0.785±0.013 | 7.384 | 0.001 |
| LPS | 0.820±0.0191) | ||
| JWH133 | 0.775±0.0122) | ||
| AM630 | 0.822±0.0431) | ||
| 1)P < 0.05 vs control group;2)P < 0.05 vs LPS group. | |||
2.3 BALF中炎性细胞因子TNF-α、IL-1β水平的变化
与C组相比, LPS组、JWH133组和AM630组BALF中TNF-α水平显著升高(P < 0.05); LPS组、AM630组BALF中IL-1β水平显著升高(P < 0.05)。与LPS组相比, JWH133组TNF-α、IL-1β水平显著下降(P < 0.05), AM630组IL-1β水平显著升高(P < 0.05)。见表 3。
| Group | TNF-α | IL-1β | |
| Control | 84.51±8.28 | 25.29±3.74 | |
| LPS | 207.45±31.911) | 60.53±10.501) | |
| JWH133 | 138.60±5.871), 2) | 31.38±6.612) | |
| AM630 | 210.59±9.441) | 70.44±3.221), 2) | |
| 1)P < 0.05 vs control group; 2)P < 0.05 vs LPS group. | |||
2.4 肺组织中ERK1/2、NF-κB p65mRNA表达水平比较
与C组比较, LPS组、JWH133组及AM630组ERK1/2、NF-κB p65 mRNA表达水平显著升高(P < 0.05); 与LPS组比较, JWH133组ERK1/2 mRNA表达水平显著降低(P < 0.05)。见图 3。
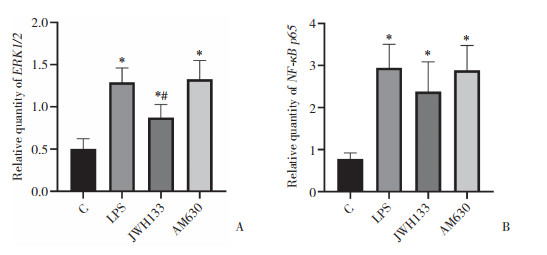
|
| A, ERK1/2;B, NF-κB p65. *P < 0.05 vs C group; #P < 0.05 vs LPS group. 图 3 各组大鼠肺组织ERK1/2和NF-κB p65 mRNA表达水平比较 Fig.3 Comparison of ERK1/2 and NF-κB p65 mRNA expression levels in rat lung tissues |
2.5 肺组织中TNF-α、IL-1β、ERK1/2、p-ERK1/2、NF-κB p65、NF-κB p65蛋白表达水平比较
与C组比较, LPS组和AM630组TNF-α、IL-1β、p-ERK1/2、NF-κB p65蛋白表达水平显著升高(P < 0.05); 与LPS组比较, JWH133组TNF-α、IL-1β、p-ERK1/2、NF-κB p65蛋白表达水平显著降低(P < 0.05), AM630组p-ERK1/2、p-NF-κBp65蛋白表达水平显著升高(P < 0.05)。见图 4。
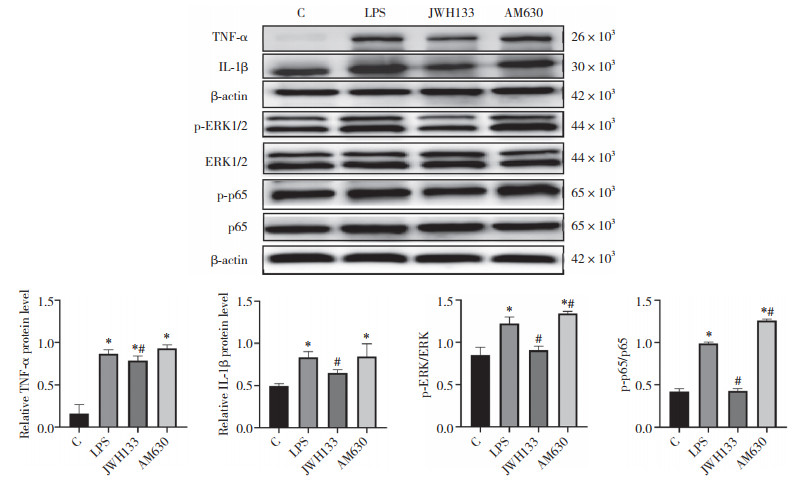
|
| *P < 0.05 vs C group; #P < 0.05 vs LPS group. 图 4 大鼠肺组织ERK/NF-κB通路相关蛋白表达 Fig.4 Expression of ERK/NF-κB pathway related proteins in rat lung |
3 讨论
脓毒症的最新定义为因宿主对感染的反应失调而导致的危及生命的器官功能障碍[10]。目前, 虽然在控制脓毒症感染反应方面的研究取得了一定进展, 但仍无有效的治疗药物。内源性大麻素系统可以调节先天性免疫和获得性免疫, 改善炎症反应、疼痛和氧化应激等[11-12]。CB1R主要在中枢神经系统表达, 而CB2R主要在外周组织表达[13]。CB2R是体内重要的炎症调节因子, 通过抑制白细胞募集, 减少趋化因子、黏附分子、活性氧和促炎细胞因子等的合成和释放, 产生抗炎作用[14-16]。在增生性玻璃体视网膜病早期, CB2R激动剂干预可减少眼部炎症和疾病的严重程度[17], 用CB2R激动剂还可预防类风湿关节炎局部和全身炎症性骨破坏[18]; 在大鼠自发性高血压模型中, JWH133通过减轻延髓头端腹外侧区炎症降低大鼠血压[19]; 但CB2R在脓毒症中的研究较少。
本研究选择CB2R激动剂JWH133和CB2R拮抗剂AM630对大鼠ALI模型进行干预, 结果发现, JWH133可显著减轻LPS所致大鼠肺损伤及肺水肿程度, 减少炎性细胞因子TNF-α、IL-1β的表达, 而AM630则使上述反应均加重。提示CB2R参与了脓毒症肺损伤的过程, 可显著减轻脓毒症时肺部的炎症反应, 减轻LPS导致的肺损伤。
MAPK作为真核细胞胞质内的信号转导终末通路, 与脓毒症ALI的发病机制密切相关[20]。ERK是最具代表性的MAPK之一。ERK是一种丝氨酸/苏氨酸蛋白激酶, 是传递有丝分裂原信号的信号转导蛋白。ERK一般位于细胞质中, 激活后, ERK通过磷酸化包括转录因子在内的多种底物控制下游反应[21]。NF-κB激活在炎性细胞因子基因转录中起关键作用, 能触发炎症的级联反应, 可诱导包括TNF-α、IL-1β、IL-6等多种炎症介质的表达[22]。
本研究通过腹腔注射LPS诱导脓毒症肺损伤模型, 发现ERK1和ERK2的2种同工型THr-Glu-Try基序双重磷酸化, 同时NF-κB p65磷酸化, mRNA表达水平也上调。重要的是, JWH133预处理可显著抑制ERK及NF-κB p65的磷酸化及下调基因表达水平, 炎性细胞因子TNF-α、IL-1β的表达也下降。抑制CB2R后, ERK及NF-κB p65的磷酸化水平升高, 炎性细胞因子水平也随之上升。
综上所述, 激活CB2R可抑制脓毒症ALI大鼠的肺组织炎症反应, 改善肺组织损伤情况, 其机制可能是通过调控ERK/NF-κB信号通路实现。本研究为临床治疗脓毒症ALI提供了新的思路和方向, 不足之处在于仅探讨了ERK1/2-NF-κB信号通路在此过程中的作用, 未探讨CB2R是否还通过其他途径产生作用, 尚有待进一步研究探讨CB2R的肺保护机制。肺泡上皮屏障和肺微血管内皮屏障的完整性破坏及通透性改变是ALI发生的根本原因。在神经系统的保护作用的研究中发现, CB2R在改善脑屏障功能、减轻脑水肿过程中发挥重要作用。因此, 未来的研究将从CB2R对肺泡上皮细胞间屏障结构的作用等方面探讨其肺保护机制。
| [1] |
HUANG M, CAI SL, SU JQ. The pathogenesis of sepsis and potential therapeutic targets[J]. Int J Mol Sci, 2019, 20(21): 5376. DOI:10.3390/ijms20215376 |
| [2] |
WANG YM, JI R, CHEN WW, et al. Paclitaxel alleviated Sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-κB pathway[J]. Drug Des Devel Ther, 2019, 13: 3391-3404. DOI:10.2147/DDDT.S222296 |
| [3] |
HUANG Y, HUANG LX, ZHU GF, et al. Downregulated microRNA-27b attenuates lipopolysaccharide-induced acute lung injury via activation of NF-E2-related factor 2 and inhibition of nuclear factor κB signaling pathway[J]. J Cell Physiol, 2019, 234(5): 6023-6032. DOI:10.1002/jcp.27187 |
| [4] |
GUO TT, SU ZZ, WANG Q, et al. Vanillin protects lipopolysaccharide-induced acute lung injury by inhibiting ERK1/2, p38 and NF-κB pathway[J]. Future Med Chem, 2019, 11(16): 2081-2094. DOI:10.4155/fmc-2018-0432 |
| [5] |
XU QL, WANG J. IGFBP7 aggravates sepsis-induced acute lung injury by activating the ERK1/2 pathway[J]. Folia Histochem Cytobiol, 2020, 58(4): 247-254. DOI:10.5603/FHC.a2020.0028 |
| [6] |
欧海燕, 段娅娟, 陈兰. 外周血单核细胞NLRP3炎性小体对脓毒症急性肺损伤患者病情严重程度的诊断价值[J]. 实用医学杂志, 2020, 36(3): 380-384. DOI:10.3969/j.issn.1006-5725.2020.03.021 |
| [7] |
PARLAR A, ARSLAN SO. CB2 agonist (AM1241)improving effect on ovalbumin-induced asthma in rats[J]. Iran J Pharm Res, 2020, 19(1): 3-17. DOI:10.22037/ijpr.2019.1101002 |
| [8] |
GONÇALVES ED, DUTRA RC. Cannabinoid receptors as therapeutic targets for autoimmune diseases: where do we stand?[J]. Drug Discov Today, 2019, 24(9): 1845-1853. DOI:10.1016/j.drudis.2019.05.023 |
| [9] |
LOWIN T, SCHNEIDER M, PONGRATZ G. Joints for joints: cannabinoids in the treatment of rheumatoid arthritis[J]. Curr Opin Rheumatol, 2019, 31(3): 271-278. DOI:10.1097/BOR.0000000000000590 |
| [10] |
BRACHT H, HAFNER S, WEIB M. Sepsis update: definition and epidemiology[J]. Anasthesiol Intensivmed Notfallmed Schmerzther, 2019, 54(1): 10-20. DOI:10.1055/a-0625-5492 |
| [11] |
HE QW, XIAO F, YUAN QH, et al. Cannabinoid receptor 2:a potential novel therapeutic target for sepsis?[J]. Acta Clin Belg, 2019, 74(2): 70-74. DOI:10.1080/17843286.2018.1461754 |
| [12] |
TAY MZ, POH CM, RÉNIA L, et al. The trinity of COVID-19:immunity, inflammation and intervention[J]. Nat Rev Immunol, 2020, 20(6): 363-374. DOI:10.1038/s41577-020-0311-8 |
| [13] |
HOWLETT AC, ABOOD ME. CB 1 and CB 2 receptor pharmacology[J]. Adv Pharmacol, 2017, 80: 169-206. DOI:10.1016/bs.apha.2017.03.007 |
| [14] |
PORTER RF, SZCZESNIAK AM, TOGURI JT, et al. Selective cannabinoid 2 receptor agonists as potential therapeutic drugs for the treatment of endotoxin-induced uveitis[J]. Molecules, 2019, 24(18): 3338. DOI:10.3390/molecules24183338 |
| [15] |
STASIULEWICZ A, ZNAJDEK K, GRUDZIEŃ M, et al. A guide to targeting the endocannabinoid system in drug design[J]. Int J Mol Sci, 2020, 21(8): 2778. DOI:10.3390/ijms21082778 |
| [16] |
HUSSAIN MT, GREAVES DR, IQBAL AJ. The impact of cannabinoid receptor 2 deficiency on neutrophil recruitment and inflammation[J]. DNA Cell Biol, 2019, 38(10): 1025-1029. DOI:10.1089/dna.2019.5024 |
| [17] |
SZCZESNIAK AM, PORTER RF, TOGURI JT, et al. Cannabinoid 2 receptor is a novel anti-inflammatory target in experimental proliferative vitreoretinopathy[J]. Neuropharmacology, 2017, 113(Pt B): 627-638. DOI:10.1016/j.neuropharm.2016.08.030 |
| [18] |
ZHU M, YU BQ, BAI JX, et al. Cannabinoid receptor 2 agonist prevents local and systemic inflammatory bone destruction in rheumatoid arthritis[J]. J Bone Miner Res, 2019, 34(4): 739-751. DOI:10.1002/jbmr.3637 |
| [19] |
SHI HK, GUO HC, LIU HY, et al. Cannabinoid type 2 receptor agonist JWH133 decreases blood pressure of spontaneously hypertensive rats through relieving inflammation in the rostral ventrolateral medulla of the brain[J]. J Hypertens, 2020, 38(5): 886-895. DOI:10.1097/HJH.0000000000002342 |
| [20] |
CHEN QH, LIU JJ, WANG WQ, et al. Sini decoction ameliorates sepsis-induced acute lung injury via regulating ACE2-Ang (1-7)- Mas axis and inhibiting the MAPK signaling pathway[J]. Biomed Pharmacother, 2019, 115: 108971. DOI:10.1016/j.biopha.2019.108971 |
| [21] |
PATEL AL, SHVARTSMAN SY. Outstanding questions in developmental ERK signaling[J]. Development, 2018, 145(14): dev143818. DOI:10.1242/dev.143818 |
| [22] |
MITCHELL JP, CARMODY RJ. NF-κB and the transcriptional control of inflammation[J]. Int Rev Cell Mol Biol, 2018, 335: 41-84. DOI:10.1016/bs.ircmb.2017.07.007 |
 2022, Vol. 51
2022, Vol. 51




