文章信息
- 段书众, 马思佳, 于文会, 王欢欢, 张华, 王景福
- DUAN Shuzhong, MA Sijia, YU Wenhui, WANG Huanhuan, ZHANG Hua, WANG Jingfu
- 内质网应激参与高磷诱导的血管平滑肌细胞钙化的作用机制
- The mechanism of endoplasmic reticulum stress on vascular smooth muscle cell calcification induced by high phosphorus
- 中国医科大学学报, 2022, 51(7): 599-603
- Journal of China Medical University, 2022, 51(7): 599-603
-
文章历史
- 收稿日期:2021-01-25
- 网络出版时间:2022-06-21 13:44
研究显示,慢性肾脏病患者血管钙化的抑制和促进因素之间稳态失衡,导致血管平滑肌细胞(vascular smooth muscle cell,VSMC) 向成骨细胞转分化[1],血管内膜、中膜及心脏瓣膜钙化[2]。血管钙化是心脑血管疾病的重要危险因素,与慢性肾衰竭患者心血管并发症和全因死亡率相关[3]。研究[4]显示高磷血症是慢性肾脏病患者血管钙化的决定性因素;高磷血症导致血管钙化的机制包括VSMC向成骨/软骨细胞转分化、凋亡小体形成、细胞外囊泡释放、细胞外基质蓄积、炎性细胞因子释放、氧化应激反应等。内质网应激参与VSMC凋亡,导致动脉粥样硬化、高血压、血管瘤和血管钙化等血管疾病[5]。目前,仍无预防血管钙化的有效手段,因此需要探索可能的药物治疗靶点[6]。内质网应激是否参与高磷诱导的VSMC钙化鲜有报道。本研究探讨内质网应激是否参与高磷诱导的血管钙化过程及其可能的作用机制。
1 材料与方法 1.1 细胞培养及分组人胸主动脉VSMC (美国ScienCell Research Laboratories)于含10%胎牛血清、100mg/mL链霉素、100U/mL青霉素的DMEM培养基中,37℃、5%CO2、湿度70%~80%恒温培养箱中培养。每2d更换1次培养液,第5~8代细胞用于实验。设置对照组(正常培养)、磷正常浓度(1.3mmol/L,NP) 组、磷高浓度(2.6mmol/L,HP) 组、HP+ SP600125组[应用c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK) 抑制剂SP600125 (美国Sigma-Aldrich公司,10μmol/L) 预处理30min后,高浓度磷(2.6mmol/L) 培养基培养10 d]、HP+siCHOP组[(C/EBP homologous protein,CHOP)siRNA使CHOP基因沉默,高浓度磷(2.6mmol/L) 培养基培养10d]。
1.2 方法 1.2.1 茜素红染色观察各组VSMC钙沉积VSMC接种到6孔板中,生长至60%时,各组实施干预措施,PBS冲洗3次,4%多聚甲醛4℃固定10min,PBS冲洗3次,放入1%茜素红S溶液内染色30min,然后0.2%醋酸溶液快速冲洗1次,滤纸吸干后乙醇脱水,二甲苯固定、封片,显微镜下观察并拍照。
1.2.2 实时定量PCR检测各组内质网应激及钙化相关基因表达弃去培养瓶内上清液,PBS冲洗,Trizol法提取总RNA。取RNA 1 μg,用反转录试剂盒逆转录获得cDNA。以cDNA作为PCR扩增模板,按照说明书配比反应体系,以GAPDH为内参基因。引物序列:骨形态发生蛋白2 (bone morphogenetic protein 2,BMP-2),正向引物5’-GGGACCCGCTGTCTTCTAGT-3’,反向引物5’-TCAACTCAAATTCGCTGAGGAC-3’;Runt相关转录因子2 (Runt-related transcription factor 2,Runx-2),正向引物5’-ACTGTGGTTACCGTCATGGC-3’,反向引物5’-ACTTGGTTTTTCATAACAGCGGA-3’;CHOP,正向引物5’-GGAAACAGAGTGGTCATTCC C-3’,反向引物5’- CTGCTTGAGCCGTTCATTCTC;RNA依赖的蛋白激酶样内质网激酶(RNA-dependent protein kinase-like endoplasmic reticulum kinase,PERK),正向引物5’-ACGATGAGACAGAGTTGCGA C-3’,反向引物5’-ATCCAAGGCAGCAATTCTCCC-3’;肌醇需求因子1 (inositol-requiring enzyme1,IRE1),正向引物5’-CATCCCCATGCCGAAGTTCA-3’,反向引物5’-CTGCTTCTCTCCGGTCAGGA-3’;GAPDH,正向引物5’-AGAAGGCTGGGGCTCATTTG-3’,反向引物5’-AGGGGCCATCCACAGTCTTC-3’。反应条件:预变性95℃、5min;循环反应95℃、15s,60℃、30s,共40个循环;溶解曲线95℃、15s,60℃、1min,95℃、15s,60℃、15s。基因相对表达量采用2-ΔΔCt法进行计算。
1.2.3 Western blotting检测内质网应激蛋白CHOP、磷酸化PERK (phosphorylated PERK,p-PERK)、IRE1、磷酸化c-Jun氨基末端激酶(phosphorylated c-Jun N-terminal kinase,p-JNK) 及钙化蛋白BMP-2、Runx-2表达于75 cm2培养瓶中培养各组VSMC,PBS洗涤3次,经裂解液在4 ℃下裂解30 min,离心、取上清液,BCA试剂盒测定蛋白含量。SDS-PAGE电泳分离蛋白组分,转至PVDF膜上5%脱脂奶粉封闭60 min,一抗[分别为抗CHOP(1∶1000)、抗BMP-2(1∶1000)、抗IRE1(1∶1000)、抗p-PERK (1∶1000)、抗p-JNK(1∶500)、抗Runx-2(1∶1000) 和抗-β-actin(1∶5000)]孵育液内4 ℃过夜,TBST洗涤3次,与相应辣根过氧化物酶标记的二抗结合,室温孵育1 h。化学发光法显色发光,全自动凝胶成像系统扫描、拍照,Image J软件分析数据。
1.3 统计学分析采用SPSS 22.0软件处理数据。计量资料以x±s表示,2组比较采用独立样本t检验,多组比较采用单因素方差分析,两两比较采用LSD-t检验。P < 0.05为差异有统计学意义。
2 结果 2.1 各组VSMC钙沉积比较结果显示,对照组与NP组未见钙化表现;HP组VSMC染成深红色,提示存在细胞钙化;HP+SP600125组钙化较HP组明显减轻,提示JNK诱导细胞凋亡参与了高磷诱导的VSMC钙化;HP+siCHOP组VSMC钙化较HP组减轻,提示CHOP诱导的VSMC凋亡也参与了高磷诱导的VSMC钙化。见图 1。
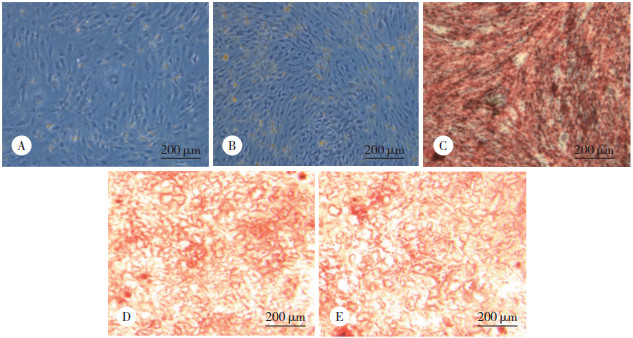
|
| A, control group; B, NP group; C, HP group; D, HP+SP600125 group; E, HP+siCHOP group. 图 1 各组VSMC钙沉积比较 Fig.1 Comparison of VSMC calcium deposition in each group |
2.2 各组VSMC钙化相关基因BMP-2、Runx-2和内质网应激相关基因IRE1、PERK、JNK、CHOP表达比较
结果显示,与对照组比较,NP组VSMC相关基因BMP-2、Runx-2表达无明显增加,提示正常浓度磷无诱导VSMC钙化的作用;而HP组BMP-2、Runx-2表达较对照组及NP组明显增高(P < 0.05),提示高浓度磷可诱导VSMC钙化。与对照组及NP组比较,HP组内质网应激相关基因IRE1、PERK、JNK及CHOP表达均显著增加(均P < 0.05)。见图 2。

|
| * P < 0.05 vs control group; ** P < 0.01 vs control and NP groups. 图 2 各组VSMC钙化相关基因BMP-2、Runx-2,内质网应激相关基因IRE1、PERK、JNK、CHOP表达比较 Fig.2 The expression of BMP-2, Runx-2, IRE1, PERK, JNK, and CHOP in each group |
2.3 高磷导致VSMC内质网应激反应增强
结果显示,与对照组及NP组比较,HP组内质网应激蛋白IRE1、p-PERK、CHOP、p-JNK表达均显著增高(均P < 0.05),提示高磷培养细胞可增强内质网应激反应,并且同时激活了pPERK-CHOP和IRE1-p-JNK 2条途径。见图 3。
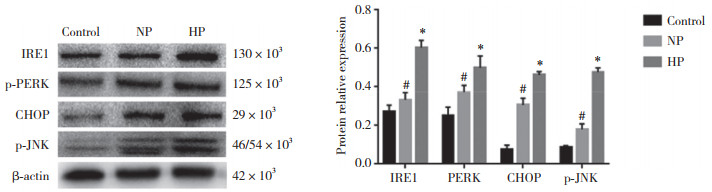
|
| # P < 0.05 vs control group; * P < 0.01 vs control and NP group. 图 3 各组内质网应激相关蛋白IRE1、p-PERK、JNK、CHOP表达 Fig.3 Protein expression of IRE1, p-PERK, JNK, and CHOP in each group |
2.4 抑制内质网应激信号传递可减轻高磷血症导致的钙化
Western blotting结果显示,与对照组比较,CHOPsiRNA使CHOP基因沉默后凋亡蛋白CHOP表达下调,说明转染成功敲低了CHOP表达,见图 4。与HP组比较,chopsiRNA使CHOP基因沉默后,CHOP表达减少,说明抑制了内质网应激p-PERK-CHOP途径。应用JNK抑制剂sp600125抑制内质网应激的IRE1-p- JNK途径,与HP组比较,sichop+HP组与sp600125+HP组钙化蛋白BMP-2及Runx-2表达均显著减少(均P < 0.05),提示内质网应激参与了高磷诱导的VSMC钙化。见图 5。

|
| *P < 0.05 vs control group. 图 4 VSMC转染siCHOP后CHOP蛋白表达 Fig.4 Expression of CHOP protein in VSMC transfected with siCHOP |
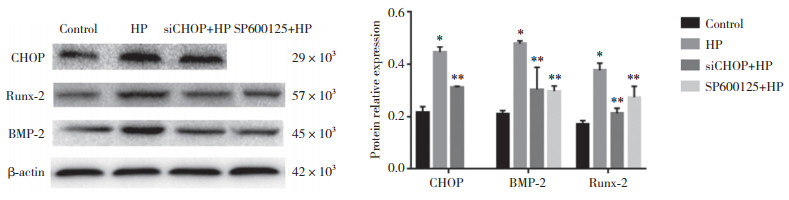
|
| *P < 0.05 vs control group; **P < 0.05 vs HP group. 图 5 各组钙化蛋白BMP-2及Runx-2表达比较 Fig.5 Comparison of expression of calcifying proteins BMP-2 and Runx-2 in each group |
3 讨论
本研究结果显示,高磷可诱导VSMC BMP-2及Runx-2表达增加,提示VSMC发生了成骨细胞表型转分化。血管钙化是VSMC向成骨细胞、软骨细胞、成脂细胞及巨噬细胞表型转分化驱动的主动过程[6]。随着肾脏功能逐渐减退,肾脏排泄磷功能障碍,慢性肾脏病患者普遍存在高磷血症。研究[7]显示,高磷启动和促进血管钙化进展的机制包括:(1) 促进VSMC向软骨细胞表型转变,增加细胞外基质钙化;(2) 诱导VSMC凋亡;(3) 抑制单核细胞/巨噬细胞向破骨样细胞分化;(4) 增加成纤维细胞生长因子23 (fibroblast growth factor 23,FGF23) 水平;(5) 减少Klotho蛋白表达。已有研究[8]显示内质网应激机制通过多种机制诱导血管钙化。
本研究应用高磷培养基刺激VSMC表达p-JNK等内质网应激特异性蛋白,探讨内质网应激是否参与高磷诱导的VSMC钙化。结果显示,高磷培养细胞可增强内质网应激反应;同时发现p-JNK、chop等凋亡相关蛋白表达增多,提示内质网应激诱导了VSMC凋亡。已有体外实验[9]证实VSMC凋亡先于血管钙化,凋亡小体含有高浓度钙,它沉积到细胞外基质,作为钙沉积的内核而引起钙化。内质网是细胞内负责合成蛋白和脂类的细胞器,同时又是主要的钙储存库,维持细胞钙稳态;当内质网腔内未折叠蛋白或者错误折叠蛋白过多时,导致内质网应激稳态破坏、发生内质网应激,从而导致多种疾病发生[10]。内质网应激主要通过诱导VSMC凋亡的方式促进血管钙化[11],其诱导凋亡的信号通路包括:(1) PERK活化后,磷酸化真核细胞起始因子(eukaryotic initiation factor 2,eIF2) 的α亚单位,进而促进转录激活因子4 (activating transcription factor 4,ATF4) 转位入核,激活凋亡蛋白CHOP,诱导细胞凋亡[12]。(2) IRE1发生二聚化和自身磷酸化,生成的IRE1α可以募集肿瘤坏死因子受体相关因子2 (TNF receptor associated factor 2,TRAF2),依次激活凋亡信号调节激酶1 (apoptosis signal-regulating kinase 1,ASK1)、JNK,JNK通过抑制B淋巴细胞瘤-2基因而诱导细胞凋亡[13]。(3) 内质网应激时,caspase-12活化激活caspase家族蛋白而诱导凋亡。内质网应激除了通过诱导凋亡的方式参与血管钙化外,还激活转录活化因子X盒结合蛋白1 (X-box binding protein 1,XBP1) 结合Runx-2启动子,促进血管平滑肌细胞转分化[14]。
本研究在沉默CHOP基因后再高磷刺激VSMC,结果显示BMP-2及Runx-2表达均减少,提示内质网应激的PERK-eIF2-chop途径参与高磷诱导的VSMC钙化。应用JNK抑制剂SP600125预处理高磷细胞后发现预处理后的VSMC钙沉积减轻,提示内质网应激的IRE1-TRAF2-JNK途径亦参与了高磷诱导的VSMC钙化。已有研究[15]显示体外培养子宫颈癌细胞时,细胞外液高磷环境可激活区细胞内质网应激,细胞凋亡蛋白裂解的caspase-3表达增加,但加入SP600125抑制JNK后表达减少。研究[16]显示在糖尿病大鼠模型中,铁死亡通过内质网应激参与心肌缺血/再灌注损伤。可见内质网应激诱导凋亡的机制可能发生在多种细胞内,因此可能成为与凋亡相关的血管钙化等疾病的潜在治疗靶点。
综上所述,内质网应激机制参与了高磷诱导的VSMC钙化,其作用机制可能是激活了pPERK-CHOP和IRE1-p-JNK两条途径。本研究不足之处:(1) 未进行SP600125和chopsiRNA对VSMC钙定量和碱性磷酸酶活性影响的检测;(2) 为体外实验,仅采用细胞培养,今后应设计动物实验来进一步论证。
| [1] |
KAKANI E, ELYAMNY M, AYACH T, et al. Pathogenesis and management of vascular calcification in CKD and dialysis patients[J]. Semin Dial, 2019, 32(6): 553-561. DOI:10.1111/sdi.12840 |
| [2] |
SCHLIEPER G, SCHURGERS L, BRANDENBURG V, et al. Vascular calcification in chronic kidney disease: an update[J]. Nephrol Dial Transplant, 2016, 31(1): 31-39. DOI:10.1093/ndt/gfv111 |
| [3] |
JOHNSON RC, LEOPOLD JA, LOSCALZO J. Vascular calcification: pathobiological mechanisms and clinical implications[J]. Circ Res, 2006, 99(10): 1044-1059. DOI:10.1161/01.RES.0000249379.55535.21 |
| [4] |
VOELKL J, LANG F, ECKARDT KU, et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia[J]. Cell Mol Life Sci, 2019, 76(11): 2077-2091. DOI:10.1007/s00018-019-03054-z |
| [5] |
SHANAHAN CM, FURMANIK M. Endoplasmic Reticulum stress in arterial smooth muscle cells: a novel regulator of vascular disease[J]. Curr Cardiol Rev, 2017, 13(2): 94-105. DOI:10.2174/1573403X12666161014094738 |
| [6] |
SINGH A, TANDON S, TANDON C. An update on vascular calcification and potential therapeutics[J]. Mol Biol Rep, 2021, 48(1): 887-896. DOI:10.1007/s11033-020-06086-y |
| [7] |
DURHAM AL, SPEER MY, SCATENA M, et al. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness[J]. Cardiovasc Res, 2018, 114(4): 590-600. DOI:10.1093/cvr/cvy010 |
| [8] |
COZZOLINO M, CICERI P, GALASSI A, et al. The key role of phosphate on vascular calcification[J]. Toxins, 2019, 11(4): 213. DOI:10.3390/toxins11040213 |
| [9] |
LEE SJ, LEE IK, JEON JH. Vascular calcification-new insights into its mechanism[J]. Int J Mol Sci, 2020, 21(8): 2685. DOI:10.3390/ijms21082685 |
| [10] |
KAPUSTIN AN, DAVIES JD, REYNOLDS JL, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization[J]. Circ Res, 2011, 109(1): e1-e12. DOI:10.1161/CIRCRESAHA.110.238808 |
| [11] |
GUZEL E, ARLIER S, GUZELOGLU-KAYISLI O, et al. Endoplasmic Reticulum stress and homeostasis in reproductive physiology and pathology[J]. Int J Mol Sci, 2017, 18(4): 792. DOI:10.3390/ijms18040792 |
| [12] |
CHANG JR, SUN N, LIU Y, et al. Erythropoietin attenuates vascular calcification by inhibiting endoplasmic reticulum stress in rats with chronic kidney disease[J]. Peptides, 2020, 123: 170181. DOI:10.1016/j.peptides.2019.170181 |
| [13] |
MIYAZAKI-ANZAI S, MASUDA M, DEMOS-DAVIES KM, et al. Endoplasmic reticulum stress effector CCAAT/enhancer-binding protein homologous protein(CHOP) regulates chronic kidney disease-induced vascular calcification[J]. J Am Heart Assoc, 2014, 3(3): e000949. DOI:10.1161/JAHA.114.000949 |
| [14] |
IURLARO R, MUÑOZ-PINEDO C. Cell death induced by endoplasmic reticulum stress[J]. FEBS J, 2016, 283(14): 2640-2652. DOI:10.1111/febs.13598 |
| [15] |
HE P, MANN-COLLURA O, FLING J, et al. High phosphate actively induces cytotoxicity by rewiring pro-survival and pro-apoptotic signaling networks in HEK293 and HeLa cells[J]. FASEB J, 2021, 35(1): e20997. DOI:10.1096/fj.202000799RR |
| [16] |
LI WY, LI W, LENG Y, et al. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress[J]. DNA Cell Biol, 2020, 39(2): 210-225. DOI:10.1089/dna.2019.5097 |
 2022, Vol. 51
2022, Vol. 51




