文章信息
- 韩孟冉, 张晨钰, 杜岑, 都健, 敖娜
- HAN Mengran, ZHANG Chenyu, DU Cen, DU Jian, AO Na
- 血小板/白细胞比值与2型糖尿病合并非酒精性脂肪性肝病患者肝纤维化进展的相关性
- Correlation between platelet to white blood cell ratio and the progression of liver fibrosis in patients with type 2 diabetes and non-alcoholic fatty liver disease
- 中国医科大学学报, 2022, 51(6): 492-496
- Journal of China Medical University, 2022, 51(6): 492-496
-
文章历史
- 收稿日期:2021-12-09
- 网络出版时间:2022-06-02 9:19
2型糖尿病(type 2 diabetes,T2DM) 与非酒精性脂肪性肝病(non-alcoholic fatty liver disease,NAFLD) 关系密切,NAFLD会增加T2DM慢性并发症的发生风险,而T2DM则会增加NAFLD进展为晚期肝纤维化、肝硬化甚至肝癌的风险。因此,在T2DM患者中早期识别NAFLD及其纤维化进展并进行治疗干预至关重要。NAFLD及其纤维化与肝内胰岛素抵抗(insulin resistance,IR)、脂代谢异常、炎症反应、氧化应激等多种机制密切相关。有研究[1]表明,血小板/白细胞比值(platelet to white blood cell ratio,PWR) 与乙型肝炎患者肝脏纤维化密切相关,本研究旨在探讨PWR与T2DM合并NAFLD患者肝纤维化的相关性,为临床提供更便捷的早期筛查指标。
1 材料与方法 1.1 研究对象选取2018年7月至2020年11月于中国医科大学附属第四医院内分泌代谢内科就诊的T2DM患者440例,其中,男258例,女182例,年龄23~94岁,平均年龄为(58.5±11.9) 岁。纳入标准:(1) T2DM符合1999年世界卫生组织糖尿病诊断标准[2];(2) NAFLD符合《非酒精性脂肪性肝病防治指南(2018年更新版) 》 [3]的诊断标准。排除标准:(1) T2DM伴有急性代谢紊乱、严重肝功能不全[谷丙转氨酶(alanine aminotransferase,ALT) > 8~10正常值上限(upper limit of normal,ULN) 或者ALT > 3 ULN且总胆红素(total bilirubin,TBIL) > 2 ULN] [4]、严重肾功能不全[估算的肾小球滤过率(estimated glomerular filtration rate,eGFR) < 30 mL/min]、心功能Ⅲ~Ⅳ级、风湿免疫系统疾病、慢性炎性疾病或肿瘤、血液系统疾病、处于妊娠或哺乳期;(2) 嗜酒,乙醇量男 > 140 g/周,女 > 70 g/周;(3) 合并病毒性肝炎、药物性肝损伤、胆汁淤积性肝损伤、肝豆状核变性及药物依赖患者。
1.2 分组纳入研究对象被分为单纯T2DM组(120例) 和T2DM合并NAFLD组(320例)。将T2DM合并NAFLD组根据NAFLD纤维化评分(NAFLD fibrosis score,NFS) [5]分为T2DM+F0 (除外肝纤维化亚组,NFS < -1.455,120例)、T2DM+F1 (可疑肝纤维化亚组,-1.455≤NFS < 0.676,120例) 和T2DM+F2 (肝纤维化亚组,NFS≥0.676,80例) 3个亚组。其中,F0表示无肝纤维化,F1表示可疑肝纤维化,F2表示肝纤维化。
1.3 方法 1.3.1 一般资料收集记录患者性别、年龄、T2DM病程、近半年用药情况等,由专人测量并记录患者的身高、体质量,并计算体质量指数(body mass index,BMI),BMI=体质量(kg) /身高2 (m2)。
1.3.2 实验室检查患者夜间禁食≥8 h,并于次日清晨6时抽取肘部静脉血。检测白细胞计数(white blood cell,WBC)、中性粒细胞计数(neutrophil,N)、单核细胞计数(monocyte,M)、淋巴细胞计数(lymphocyte,L),血小板(platelet,PLT)、糖化血红蛋白(hemoglobin A1C,HbA1c)、空腹血糖(fasting plasma glucose FPG)、白蛋白(albumin,ALB)、ALT、谷草转氨酶(aspartate aminotransferase,AST)、碱性磷酸酶(alkaline phosphatase,ALP)、总胆固醇(total cholesterol,TC)、三酰甘油(triacylglycerol,TG)、高密度脂蛋白(high density lipoprotein,HDL)、低密度脂蛋白(low density lipoprotein,LDL)、尿酸(uric acid,UA)、肌酐(creatinine,Cr),空腹C肽(fasting C-peptide,FCP)和空腹胰岛素(fasting insulin,FINS)。计算PWR,PWR=PLT/WBC。计算单核细胞与HDL比值(monocyte to high density lipoprotein ratio,MHR)、中性粒细胞/淋巴细胞比值(neutrophil to lymphocyte ratio,NLR)、血小板/淋巴细胞比值(platelet to lymphocyte ratio,PLR)、淋巴细胞/单核细胞比值(lymphocyte to monocyte ratio,LMR),MHR=M/HDL,NLR=N/L,PLR=PLT/L,LMR=L/M。计算胰岛素抵抗指数(homeostatic model assessment for insulin resistance,HOMA-IR),HOMA-IR=FINS×FPG/22.5。计算NFS,NFS=-1.675+0.037×年龄(岁) +0.094×BMI (kg/m2) +1.13×糖耐量异常或糖尿病(是=1,否=0) +0.99×AST/ALT-0.013×PLT (109/L)-0.66×Alb (g/dL)。
1.3.3 肝脏超声检查患者行超声检查前需禁食≥8 h,由超声科专业技师行肝脏超声检查,根据《超声医学》诊断标准[6]诊断脂肪肝。
1.4 统计学分析采用SPSS 25.0软件进行统计学分析,符合正态分布的计量资料以x±s表示,2组间比较采用t检验,多组间比较采用单因素方差分析,非正态分布的计量资料以M (P25~P75) 表示,2组间比较采用Mann-Whitney U检验,多组间比较采用Kruskal-Wallis H检验;计数资料以相对数表示,多组间比较采用χ2检验;采用二分类logistic回归分析探讨T2DM患者合并NAFLD的影响因素、T2DM合并NAFLD患者肝纤维化进展的影响因素;采用受试者操作特征(receiver operating characteristic,ROC) 曲线评价PWR对T2DM合并NAFLD患者肝纤维化进展的诊断价值。P < 0.05为差异有统计学意义。
2 结果 2.1 单纯T2DM组与T2DM合并NAFLD组患者临床资料和实验室检查指标比较2组患者的性别、PLT、ALB、ALP、LDL、Cr、NLR、LMR比较,差异均无统计学意义(P > 0.05);2组患者的年龄、病程、胰岛素应用史、BMI、WBC、N、M、L、HbA1c、FCP、FINS、FPG、ALT、AST、TC、TG、HDL、UA、HOMA-IR、MHR、PLR、PWR比较,差异均有统计学意义(P < 0.05),见表 1。
| Index | Simple T2DM group | T2DM combined with NAFLD group | χ2/t/Z | P |
| Sex (male/female) | 67/53 | 191/129 | 0.535 | 0.465 |
| Age (x±s,year) | 61.38±9.43 | 57.38±12.55 | 3.603 | < 0.001 |
| Course of disease [M (P25-P75),year] | 10.00(5.00-16.00) | 6.00(2.00-10.00) | -5.597 | < 0.001 |
| History of insulin use [n (%)] | 60(50.00) | 117(36.56) | 6.554 | 0.010 |
| BMI (x±s,kg/m2) | 23.32±2.70 | 26.41±3.40 | -9.946 | < 0.001 |
| WBC [M (P25-P75),×109/L] | 5.19(4.65-6.29) | 6.27(5.32-7.23) | -6.460 | < 0.001 |
| N [M (P25-P75),×109/L] | 3.05(2.55-3.89) | 3.69(3.13-4.49) | -4.881 | < 0.001 |
| M [M (P25-P75),×109/L] | 0.37(0.30-0.44) | 0.44(0.37-0.51) | -6.008 | < 0.001 |
| PLT (x±s,×109/L) | 214.95±56.56 | 218.32±63.15 | -0.524 | 0.601 |
| L (x±s,×109/L) | 1.62±0.53 | 1.91±0.55 | -5.016 | < 0.001 |
| HbA1c [M (P25-P75),%] | 7.25(6.40-9.10) | 8.30(6.90-9.80) | -3.578 | < 0.001 |
| FCP [M (P25-P75),ng/mL] | 0.92(0.65-1.35) | 1.56(1.08-2.15) | -7.579 | < 0.001 |
| FINS [M (P25-P75),μU/mL] | 9.48(5.90-14.93) | 12.06(8.29-17.75) | -4.054 | < 0.001 |
| FPG [M (P25-P75),mmol/L] | 8.01(6.12-10.88) | 8.85(7.27-11.21) | -2.439 | 0.015 |
| ALB [M (P25-P75),g/L] | 44.90(41.23-46.28) | 44.80(41.73-47.18) | -1.565 | 0.118 |
| ALT [M (P25-P75),U/L] | 18.00(14.00-24.00) | 25.50(18.00-42.00) | -6.321 | < 0.001 |
| AST [M (P25-P75),U/L] | 19.50(16.00-23.00) | 21.50(17.00-29.00) | -3.563 | < 0.001 |
| ALP [M (P25-P75),U/L] | 76.00(64.00-87.75) | 77.50(63.00-92.00) | -0.865 | 0.387 |
| TC (x±s,mmol/L) | 4.71±0.95 | 5.00±1.16 | -2.686 | 0.008 |
| TG [M (P25-P75),mmol/L] | 1.05(0.82-1.45) | 1.99(1.40-3.14) | -9.683 | < 0.001 |
| HDL [M (P25-P75),mmol/L] | 1.33(1.14-1.51) | 1.03(0.91-1.18) | -9.273 | < 0.001 |
| LDL (x±s,mmol/L) | 3.01±0.93 | 3.08±1.09 | -0.740 | 0.460 |
| UA [M (P25-P75),μmol/L] | 282.00(233.25-347.00) | 338.00(288.25-401.75) | -6.162 | < 0.001 |
| Cr [M (P25-P75),μmol/L] | 63.00(53.25-73.50) | 66.00(56.00-75.00) | -1.375 | 0.169 |
| HOMA-IR [M (P25-P75)] | 3.31(1.87-6.05) | 4.79(3.19-7.46) | -4.259 | < 0.001 |
| MHR [M (P25-P75)] | 0.29(0.21-0.35) | 0.39(0.30-0.50) | -8.024 | < 0.001 |
| NLR [M (P25-P75)] | 2.10(1.68-2.62) | 1.98(1.53-2.62) | -0.965 | 0.334 |
| PLR [M (P25-P75)] | 138.15(108.41-170.04) | 115.16(89.66-146.88) | -4.446 | < 0.001 |
| LMR (x±s) | 4.41±1.35 | 4.46±1.38 | -0.308 | 0.758 |
| PWR (x±s) | 40.08±10.35 | 35.04±11.33 | 4.252 | < 0.001 |
2.2 T2DM患者发生NAFLD的影响因素分析
以发生NAFLD为因变量,以表 1中差异有统计学意义的指标为自变量进行二分类logistic回归分析,结果显示,BMI、TG、UA、MHR是T2DM患者发生NAFLD的危险因素,病程是T2DM患者发生NAFLD的保护因素(P < 0.05),见表 2。
| Index | β | SE | Wald χ2 | P | OR | 95%CI |
| Course of disease | -0.607 | 0.223 | 7.427 | 0.006 | 0.545 | 0.352-0.843 |
| BMI | 0.550 | 0.150 | 13.430 | < 0.001 | 1.734 | 1.292-2.328 |
| TG | 0.883 | 0.208 | 18.069 | < 0.001 | 2.418 | 1.609-3.633 |
| UA | 0.513 | 0.198 | 6.736 | 0.009 | 1.670 | 1.134-2.459 |
| MHR | 0.757 | 0.263 | 8.263 | 0.004 | 2.132 | 1.272-3.571 |
2.3 T2DM合并NAFLD各纤维化亚组临床资料和实验室检查指标比较
3组患者的性别、M、HbA1c、FPG、AST、UA比较,差异均无统计学意义(P > 0.05);3组患者的年龄、病程、胰岛素应用史、BMI、WBC、N、PLT、L、FCP、FINS、ALB、ALT、ALP、TC、TG、HDL、LDL、Cr、HOMA-IR、MHR、NLR、PLR、LMR、PWR比较,差异均有统计学意义(P < 0.05),见表 3。
| Index | T2DM+F1(n = 120) | T2DM+F0(n = 120) | T2DM+F2(n = 80) | χ2/F/H | P |
| Sex (male/female) | 77/43 | 67/53 | 47/33 | 1.771 | 0.413 |
| Age (x±s,year) | 57.33±9.47 | 49.73±11.00 | 68.96±9.66 | 86.788 | < 0.001 |
| Course of disease [M (P25-P75),year] | 6.50(2.00-10.00) | 5.00(2.00-8.00) | 10.00(4.00-13.50) | 17.974 | < 0.001 |
| History of insulin use [n (%)] | 35(29.17) | 45(37.50) | 37(46.25) | 13.419 | 0.047 |
| BMI (x±s,kg/m2) | 25.91±3.07 | 25.82±2.91 | 28.05±4.01 | 6.112 | < 0.001 |
| WBC [M (P25-P75),×109/L] | 5.84(5.18-6.87) | 6.47(5.74-7.33) | 6.32(5.41-7.41) | 7.693 | 0.021 |
| N [M (P25-P75),×109/L] | 3.56(2.95-4.29) | 3.70(3.19-4.41) | 3.85(3.28-4.88) | 6.081 | 0.048 |
| M [M (P25-P75),×109/L] | 0.44(0.37-0.50) | 0.44(0.38-0.50) | 0.47(0.36-0.55) | 1.742 | 0.419 |
| PLT (x±s,×109/L) | 201.31±40.11 | 276.21±45.29 | 156.63±34.70 | 221.425 | < 0.001 |
| L (x±s,×109/L) | 1.85±0.52 | 2.09±0.54 | 1.74±0.53 | 12.127 | < 0.001 |
| HbA1c [M (P25-P75),%] | 8.15(6.90-9.20) | 8.20(6.93-9.90) | 8.75(6.93-9.90) | 4.257 | 0.119 |
| FCP [M (P25-P75),ng/mL] | 1.67(1.24-2.88) | 1.44(1.03-2.04) | 1.50(0.95-1.94) | 7.475 | 0.024 |
| FINS [M (P25-P75),μU/mL] | 12.00(8.02-15.76) | 10.89(8.07-15.83) | 14.75(10.00-21.96) | 11.503 | 0.003 |
| FPG [M (P25-P75),mmol/L] | 9.03(7.13-10.80) | 8.83(7.48-11.88) | 8.58(7.24-11.08) | 0.792 | 0.673 |
| ALB [M (P25-P75),g/L] | 44.95(42.70-46.90) | 46.75(44.30-48.58) | 40.95(39.40-43.35) | 85.370 | < 0.001 |
| ALT [M (P25-P75),U/L] | 27.00(19.00-46.25) | 30.00(20.50-51.00) | 38.00(13.00-28.00) | 34.920 | < 0.001 |
| AST [M (P25-P75),U/L] | 22.00(18.00-32.00) | 22.00(18.00-28.00) | 20.00(16.00-26.75) | 3.734 | 0.155 |
| ALP [M (P25-P75),U/L] | 76.00(62.25-92.00) | 82.00(68.25-92.00) | 72.00(59.00-86.00) | 6.783 | 0.034 |
| TC (x±s,mmol/L) | 4.91±1.02 | 5.24±1.10 | 4.77±1.36 | 4.653 | 0.010 |
| TG [M (P25-P75),mmol/L] | 1.98(1.33-3.27) | 2.34(1.50-3.25) | 1.65(1.29-2.63) | 10.176 | 0.006 |
| HDL [M (P25-P75),mmol/L] | 1.03(0.91-1.19) | 1.06(0.96-1.23) | 0.98(0.87-1.11) | 9.759 | 0.008 |
| LDL (x±s,mmol/L) | 3.01±1.02 | 3.32±1.04 | 2.84±1.22 | 5.078 | 0.007 |
| UA [M (P25-P75),μmol/L] | 351.00(301.25-420.75) | 319.00(283.00-394.75) | 336.00(290.25-392.75) | 3.858 | 0.145 |
| Cr [M (P25-P75),μmol/L] | 64.00(57.00-73.00) | 63.50(54.00-72.00) | 72.00(60.75-82.00) | 16.812 | < 0.001 |
| HOMA-IR [M (P25-P75)] | 4.50(3.11-7.01) | 4.64(3.21-6.42) | 5.85(3.21-10.35) | 6.812 | 0.033 |
| MHR [M (P25-P75)] | 0.42(0.33-0.53) | 0.41(0.33-0.49) | 0.48(0.36-5.78) | 45.964 | < 0.001 |
| NLR [M (P25-P75)] | 1.96(1.54-2.62) | 1.82(1.48-2.24) | 2.28(1.81-3.16) | 20.223 | < 0.001 |
| PLR [M (P25-P75)] | 111.21(89.36-139.46) | 135.80(108.10-166.74) | 92.82(73.49-116.92) | 56.496 | < 0.001 |
| LMR (x±s) | 4.34±1.22 | 4.88±1.34 | 4.00±1.49 | 11.078 | < 0.001 |
| PWR [M (P25-P75)] | 33.84(27.34-39.55) | 42.22(35.83-49.32) | 24.01(20.66-28.30) | 142.164 | < 0.001 |
2.4 T2DM合并NAFLD肝纤维化进展影响因素的logistic回归分析
以发生肝纤维化为因变量,以表 3中差异有统计学意义的指标为自变量进行二分类logistic回归分析,结果显示,HOMA-IR与T2DM合并NAFLD肝纤维化进展呈正相关,TG、PWR与T2DM合并NAFLD肝纤维化进展呈负相关(P < 0.05),见表 4。
| Index | β | SE | Wald χ2 | P | OR | 95%CI |
| TG | -0.612 | 0.252 | 5.900 | 0.015 | 0.542 | 0.331-0.888 |
| HOMA-IR | 0.385 | 0.195 | 3.890 | 0.049 | 1.470 | 1.002-2.156 |
| PWR | -0.893 | 0.221 | 16.320 | < 0.001 | 0.410 | 0.266-0.632 |
2.5 PWR对T2DM合并NAFLD患者肝纤维化的预测价值
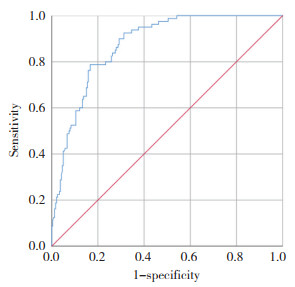
PWR可较好地预测T2DM合并NAFLD患者肝纤维化的发生风险,ROC曲线下面积为0.874,当PWR=28.61时,灵敏度为78.8%,特异度为83.3%,约登指数为0.621 (图 1)。可见PWR对T2DM合并NAFLD患者肝纤维化具有一定的预测价值。
|
| 图 1 PWR对T2DM合并NAFLD患者肝纤维化的预测价值 Fig.1 Predictive value of PWR for liver fibrosis in patients with T2DM and NAFLD |
3 讨论
NAFLD进一步发展可出现肝纤维化、肝硬化甚至肝癌。如果能早期识别肝纤维化并及时干预,可能会阻止失代偿期肝硬化的发生发展。PWR作为一个简单易测的指标,已被发现与乙型肝炎患者肝脏纤维化密切相关[1]。
本研究结果显示,MHR是T2DM合并NAFLD的危险因素,单核细胞的募集是肝脏纤维化的关键事件,其参与诱导肝内主要纤维细胞-肝星状细胞的活化过程[7]。MHR可以作为T2DM合并NAFLD的预测因子。研究[8]表明,NAFLD中脂质摄取升高和脂肪从头合成发生率增加导致TG增高,而本研究结果相反,可能与T2DM患者大多长期应用调脂药物,但本研究未纳入药物应用情况有关。
本研究结果显示,PWR与T2DM合并NAFLD患者肝纤维化程度呈负相关,当PWR为28.61时,灵敏度为78.8%,特异度为83.3%,具有较好的临床应用价值。多个研究[9-11]发现WBC计数与NAFLD呈正相关,这可能与IR、氧化应激及全身低度炎症有关。在没有肝硬化的情况下,NAFLD患者的PLT可能会减少,但一般 > 40×109/L[12]。LIU等[13]认为,肝脏是产生血小板生成素(thrombopoietin,TPO) 的重要器官,在NAFLD病程中,过度的脂质沉积和氧化应激可能损害线粒体功能,影响TPO合成,导致PLT减少。也有研究[14]认为PLT减少与脾功能亢进有关,也可能与外周血细胞寿命缩短、维生素缺乏等相关。此外,IR所导致的肝损伤,也可能引发PLT减少,其减少程度与肝组织的脂肪浸润程度有关[15]。本研究发现PWR随纤维化程度的加重而逐渐降低。
本研究未纳入非糖尿病患者,未能在基线水平上进行比较,作为回顾性研究,代表了单中心试验,未排除调脂药物的影响,研究结果具有一定的局限性。综上所述,MHR更适用于NAFLD的早期筛查,而PWR则更适用于纤维化的早期评估,在T2DM人群的筛查中,若MHR增高,应建议患者完善肝脏超声。已有NAFLD的患者发现PWR降低,则应高度警惕肝脏纤维化,及时进行肝纤维化分期,开展多学科诊疗,早期治疗干预,加强临床随访,以降低T2DM合并NAFLD患者肝硬化甚至肝癌的发生风险,提高患者的生活及生存质量。
| [1] |
郭向东, 郭伟, 阿曼古, 等. 血小板与白细胞比值在预测慢性乙型肝炎肝纤维化中的临床意义[J]. 中国现代医生, 2014, 52(33): 149-151. |
| [2] |
ALBERTI KG, ZIMMET PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1:diagnosis and classification of diabetes mellitus provisional report of a WHO consultation[J]. Diabet Med, 1998, 15(7): 539-553. DOI:10.1002/(SICI)1096-9136(199807)15:7<539:AID-DIA668>3.0.CO;2-S |
| [3] |
中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI:10.3969/j.issn.1001-5256.2018.05.007 |
| [4] |
朱珠, 曹运莉, 孙钢, 等. 肝功能不全分级方法概述[J]. 中国药师, 2012, 15(3): 418-421. DOI:10.3969/j.issn.1008-049X.2012.03.053 |
| [5] |
ANGULO P, HUI JM, MARCHESINI G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD[J]. Hepatology, 2007, 45(4): 846-854. DOI:10.1002/hep.21496 |
| [6] |
郭万学. 超声医学[M]. 6版. 北京: 人民军医出版社, 2011.
|
| [7] |
PASTORE M, CALIGIURI A, RAGGI C, et al. Macrophage MerTK promotes profibrogenic cross-talk with hepatic stellate cells via solu-ble mediators[J]. JHEP Rep, 2022, 4(4): 100444. DOI:10.1016/j.jhepr.2022.100444 |
| [8] |
KASPER P, MARTIN A, LANG S, et al. NAFLD and cardiovascular diseases: a clinical review[J]. Clin Res Cardiol, 2021, 110(7): 921-937. DOI:10.1007/s00392-020-01709-7 |
| [9] |
YU YY, CAI JT, SONG ZY, et al. The associations among Helicobacter pylori infection, white blood cell count and nonalcoholic fatty liver disease in a large Chinese population[J]. Medicine, 2018, 97(46): e13271. DOI:10.1097/MD.0000000000013271 |
| [10] |
WANG SK, ZHANG CQ, ZHANG G, et al. Association between white blood cell count and non-alcoholic fatty liver disease in urban Han Chinese: a prospective cohort study[J]. BMJ Open, 2016, 6(6): e010342. DOI:10.1136/bmjopen-2015-010342 |
| [11] |
CHUNG GE, YIM JY, KIM D, et al. Associations between white blood cell count and the development of incidental nonalcoholic fatty liver disease[J]. Gastroenterol Res Pract, 2016, 2016: 7653689. DOI:10.1155/2016/7653689 |
| [12] |
OLIVARES-GAZCA JC, NUÑEZ-CORTES AK, MENDEZ-HUERTA MA, et al. More on the thrombocytopenia of the non-alcoholic fatty liver disease[J]. Hematology, 2017, 22(5): 316-319. DOI:10.1080/10245332.2016.1266435 |
| [13] |
LIU F, ZHOU H, CAO L, et al. Risk of reduced platelet counts in patients with nonalcoholic fatty liver disease (NAFLD): a prospective cohort study[J]. Lipids Health Dis, 2018, 17(1): 221. DOI:10.1186/s12944-018-0865-7 |
| [14] |
RIVERA-ÁLVAREZ M, CÓRDOVA-RAMÍREZ AC, ELÍAS-DE-LA-CRUZ GD, et al. Non-alcoholic fatty liver disease and thrombocytopenia Ⅳ: its association with granulocytopenia[J]. Hematol Transfus Cell Ther, 2021. DOI:10.1016/j.htct.2021.06.004 |
| [15] |
LÓPEZ-TRUJILLO MA, OLIVARES-GAZCA JM, CANTERO-FORTIZ Y, et al. Nonalcoholic fatty liver disease and thrombocytopeniaⅢ: its association with insulin resistance[J]. Clin Appl Thromb Hemost, 2019, 25: 1076029619888694. DOI:10.1177/1076029619888694 |
 2022, Vol. 51
2022, Vol. 51