文章信息
- 喻言, 钟红珊
- YU Yan, ZHONG Hongshan
- 环状RNA FGGY在肝癌中的表达及其对HepG2细胞铁死亡的影响
- Expression of FGGY circular RNA in liver cancer and its effect on ferroptosis in HepG2 cells
- 中国医科大学学报, 2022, 51(12): 1057-1061
- Journal of China Medical University, 2022, 51(12): 1057-1061
-
文章历史
- 收稿日期:2022-03-30
- 网络出版时间:2022-12-08 17:54:01
铁死亡在肝癌的发生发展中起关键作用,是一种受调节的细胞死亡形式,与其他已知的细胞死亡模式(如细胞凋亡、坏死和自噬)不同,具有独特的形态、生化和遗传特征,如线粒体萎缩、膜密度增加、铁和活性氧(reactive oxygen species,ROS)超载[1-6]。铁死亡抑制蛋白1(ferroptosis-suppressor-protein 1,FSP1)在包括肝癌在内的多种肿瘤细胞中高表达,发挥抑制肿瘤细胞铁死亡的作用[7]。
musahi RNA结合蛋白2(musashi RNA binding pro-tein 2,MSI2)是一种RNA结合蛋白,可通过高表达调控Wnt/β-catenin通路,促进肝癌细胞生长[8-10]。MSI2高表达还能增强人肺腺癌细胞的化疗药物耐药性,发挥促癌作用[11]。RNA结合蛋白能与环状RNA(circular RNA,circRNA)两端侧翼序列的Alu元件结合,诱导其5’端和3’端的共价结合,促进circRNA的形成[12]。本研究组前期通过生物信息学研究发现,circFGGY的两端也存在Alu元件,可能与MSI2结合。FGGY可在多种肿瘤中发挥作用[13],但circFGGY在肝癌细胞中的表达和功能目前尚不明确。
m6A甲基化是指发生在腺嘌呤第6个氮原子上的甲基化修饰,广泛存在于许多真核生物的RNA中[14]。circRNA也存在m6A修饰,由去甲基化酶FTO和甲基转移酶METTL3等相关酶共同调控[15]。METTL3在肝癌细胞中高表达,并发挥促癌基因作用[16]。m6A甲基化修饰还可导致circRNA在细胞核和细胞质中的位置重分布。
本研究拟探讨circFGGY、MSI2、METTL3、FSP1对肝癌细胞铁死亡的调控,旨在明确肝癌细胞铁死亡的调控机制。
1 材料与方法 1.1 材料 1.1.1 细胞人正常肝细胞系WRL68及肝癌细胞系HepG2购自中国科学院上海生科院细胞资源中心。
1.1.2 主要试剂及材料Western blotting相关试剂(上海碧云天生物技术有限公司);FSP1抗体和GAPDH抗体,Lipofectamine3000,Opti-MEM®Ⅰ(美国Life公司);鼠、兔二抗(美国Santa cruz公司);TRIzol试剂(上海联迈生物公司);RPMI 1640培养基、RNase R、脂质过氧化物试剂盒(美国Sigma公司);TaqMan® MicroRNA Reverse Transcription Kit,TaqMan® Universal Master MixⅡ(美国Ambion公司);circFGGY引物设计及合成(中国上海闪晶公司);MSI2、METTL3及circFGGY沉默质粒(苏州吉玛公司);免疫荧光相关探针及试剂盒(中国迈新基因公司);CCK-8试剂盒(日本东仁公司);C11-BODIPY染色测定试剂盒(深圳子科生物科技有限公司);铁测定试剂盒,谷胱甘肽(glutathione,GSH)检测试剂盒(北京索莱宝科技有限公司)。
1.2 方法 1.2.1 实时荧光定量PCR应用TRIzol试剂提取HepG2细胞总RNA,加入RNase R降解线状RNA。应用TaqMan® MicroRNA Reverse Transcription Kit反转录,应用TaqMan® Universal Master MixⅡ和TaqMan MicroRNA Assay(circFGGY)行实时荧光定量PCR,以GAPDH为内参照。应用7500Fast Real-Time PCR System测定Ct值,以2-ΔΔCt表示circFGGY的相对表达量。
1.2.2 细胞转染及分组接种5×104个HepG2细胞至24孔板,待细胞生长至70% 融合时,按照Lipofec-tamine3000说明书,分别转染MSI2、METTL3及circFGGY的沉默质粒,用0.4 mg/mL G418筛选细胞。4周后,筛选出稳定转染的细胞系。将细胞分为阴性对照(sh-NC)组和沉默组。
1.2.3 CCK-8实验96孔板中加入5×105/μL细胞悬液100 μL,在37 ℃、5%CO2培养箱中孵育24 h。加入CCK-8溶液10 μL/孔,培养箱中孵育4 h。用酶标仪在450 nm处检测各孔吸光度。
1.2.4 脂质过氧化物检测按照试剂盒说明书,取细胞悬液至1.5 mL离心管中,加入700 μL检测试剂,45 ℃孵育60 min,4 000 r/min离心10 min,取上清液200 μL加入96孔板中,用酶标仪在586 nm波长下测定各孔吸光度。
1.2.5 C11-BODIPY染色4%多聚甲醛固定细胞15 min后,0.5% Triton X-100溶液处理20 min;普通山羊血清封闭30 min,4 ℃下与C11-BODIPY探针孵育1 h;与FITC标记的山羊抗兔IgG在37 ℃暗室中孵育1 h;DAPI染色5 min,拍照成像。
1.2.6 流式细胞仪检测ROS用无血清培养液稀释活性氧荧光探针(2,7-dichlorodihydrofluorescein diacetate,DCFH-DA)(1∶1 000),使终浓度为10 μmol/L。去除细胞培养液,加入适当体积稀释DCFH-DA。37 ℃培养箱内孵育20 min。用无血清细胞培养液洗涤细胞3次,以充分去除未进入细胞内的DCFH-DA。收集细胞并悬浮于DCFH-DA(1∶1 000稀释)中,培养箱内孵育20 min。每3~5 min混匀1次。用流式细胞仪检测。
1.2.7 细胞铁离子测定预冷PBS清洗细胞2次,加入裂解液(200 μL/孔),在摇床上裂解细胞2 h。加入铁离子检测试剂盒的检测试剂,混匀,60 ℃孵育1 h,冷却至室温并离心。加入30 μL铁离子检测剂并混匀,室温孵育30 min。取200 μL至96孔板,用酶标仪检测550 nm处吸光度。
1.2.8 细胞GSH检测裂解细胞后,加入GSH检测试剂,加至96孔板。用酶标仪检测340 nm处吸光值。
1.2.9 RNA免疫沉淀(immunoprecipitation,IP)实验获取细胞裂解液,加入与磁珠耦联的MSI2抗体(IgG作为对照),孵育,离心、漂洗后得到RNA/MSI2蛋白复合物,样品加入蛋白酶K,震荡孵育消化蛋白,然后提取沉淀的RNA。
1.2.10 甲基化RNA免疫沉淀(methylated RNA immunoprecipitation,MeRIP)实验获取细胞裂解液,加入与磁珠耦联的的METTL3甲基化抗体(IgG作为对照),孵育,离心、漂洗后得到RNA/METTL3蛋白复合物,样品加入蛋白酶K,震荡孵育消化蛋白,然后提取沉淀的RNA。
1.2.11 免疫印迹实验提取细胞中的总蛋白并进行定量。随后应用SDS-PAGE分离蛋白质,转移到聚偏二氟乙烯膜上。然后,将膜用含有0.1% Tween 20(TBST)和5%脱脂牛奶的Tris缓冲盐水封闭1 h,并在4 ℃下与相应的抗体孵育过夜。用TBST洗涤3次后,将膜与二抗在室温下孵育。孵育后,通过图像分析系统分析蛋白膜。
1.3 统计学分析采用SPSS 21.0软件行统计学分析。数据采用x±s表示,组间比较采用t检验。P < 0.05为差异有统计学意义。
2 结果 2.1 circFGGY在HepG2细胞中的表达及功能如图 1A所示,与正常肝细胞相比,肝癌HepG2细胞中circFGGY的表达水平显著上调,差异有统计学意义(P < 0.01)。如图 1B~1G所示,沉默circFGGY能显著抑制HepG2细胞活力,上调HepG2细胞的ROS水平、脂质含量、铁含量、线粒体超氧化物含量、GSH含量以及线粒体膜电位等铁死亡生物学效应,差异有统计学意义(P < 0.01)。
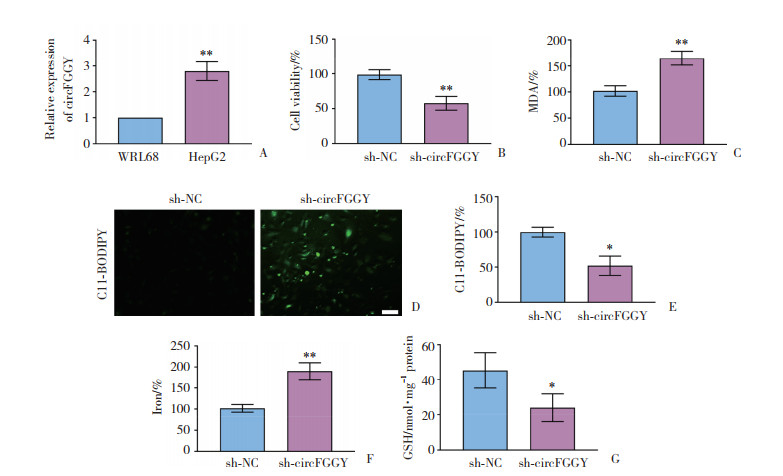
|
| A, real-time PCR was used to detect the expression of circFGGY in human normal hepatocytes (WRL68) and hepatoma cells (HepG2);B, CCK-8 assay was used to detect the effect of silencing circFGGY on the viability of HepG2 cells; C, lipid peroxidation silencing circFGGY was used to detect the peroxide end product MDA of HepG2 cells by a biological kit assay; D, C11-BODIPY staining was used to determine the production of ROS in HepG2 cells (scale bar = 50 μm); E, flow cytometry was used to detect the effect of silencing circFGGY on the production of ROS in HepG2 cells; F, the effect of silencing circFGGY on iron content in HepG2 cells detected with iron assay kit; G, the effect of silencing circFGGY on GSH content in HepG2 cells detected with GSH kit. *P < 0.05, **P < 0.01. 图 1 circFGGY在HepG2细胞中的表达和功能 Fig.1 Expression and function of circFGGY in HepG2 cells |
2.2 MSI2结合并促进circFGGY形成
如图 2所示,anti-MSI2洗脱液中的circFGGY较anti-IgG组显著增多;与sh-NC组相比,沉默MSI2能够显著下调circFGGY的表达水平,差异均有统计学意义(均P < 0.01)。
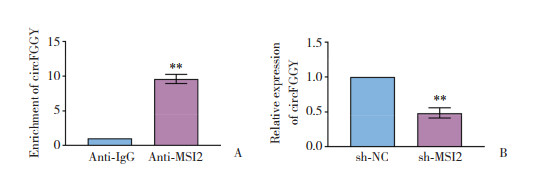
|
| A, the binding of MSI2 to circFGGY (n = 5);B, the expression level of circFGGY in sh-NC group and sh-MSI2 group (n = 3). **P < 0.01. 图 2 MSI2结合并促进circFGGY形成 Fig.2 MSI2 binds and promotes circFGGY formation |
2.3 METTL3促进circFGGY m6A甲基化修饰并促进circFGGY出细胞核
MeRIP结果显示,沉默METTL3可使circFGGY的m6A甲基化修饰水平显著下调(P < 0.01,图 3A)。进一步应用免疫荧光方法检测发现,沉默METTL3导致细胞质中circFGGY RNA的表达水平显著下调(图 3B)。
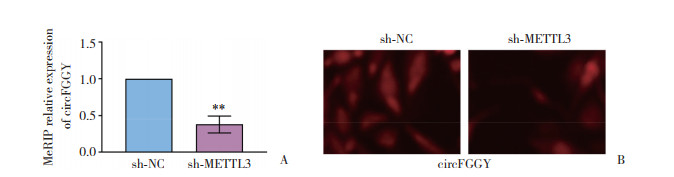
|
| A, MeRIP method was used to detect the expression level of circFGGY in sh-NC group and sh-METTL3 group (n = 3);B, immunofluorescence method was used to detect the nucleocytoplasmic distribution of circFGGY in sh-NC group and sh-METTL3 group (×100). **P < 0.01. 图 3 METTL3促进circFGGY m6A甲基化修饰并促进circFGGY出细胞核 Fig.3 METTL3 promotes circFGGY m6A methylation modification and promotes circFGGY out of the nucleus |
2.4 circFGGY间接调控FSP1的表达
沉默circFGGY后,肝癌HepG2细胞中FSP1蛋白表达水平显著下调(P < 0.01,图 4A)。进一步行RNA IP实验发现,anti-FSP1洗脱液中circFGGY的富集程度无明显变化(图 4B)。
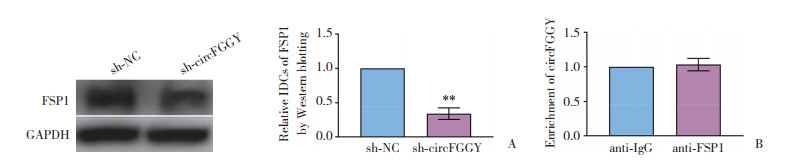
|
| A, Western blotting was used to detect the expression level of FSP1 protein in sh-NC group and sh-circFGGY group (n = 3);B, RNA IP was used to detect the binding effect of circFGGY and FSP1. ** P < 0.01. 图 4 circFGGY间接调控FSP1的表达 Fig.4 circFGGY indirectly regulates the expression of FSP1 |
3 讨论
研究显示,高表达的RNA结合蛋白MSI2通过与YAP mRNA结合调控mRNA降解,进而调控乳腺癌干细胞的干细胞性。人胃癌中,MSI2与c-MYC mRNA结合并增强其稳定性,提高胃癌细胞的化疗药物耐药性[17-18]。表明RNA结合蛋白MSI2具有与RNA结合的作用,而且MSI2的高表达发挥促癌作用。本研究组前期应用生物信息学软件发现circFGGY基因两端具有Alu元件,当Alu元件被结合激活后,可促进外显子的结合拼接。并进一步预测到其序列存在MSI2的结合位点,还证实了MSI2可与circFGGY结合并调控其表达。这说明RNA结合蛋白MSI2可通过促进circFGGY的形成发挥其影响肿瘤功能的作用。
RNA,特别是circRNA的甲基化修饰是近年的研究热点。RNA的m6A甲基化修饰参与细胞的多种分子调控过程。METTL14通过结合circORC5调控其m6A甲基化水平,进而调控其表达,发挥抑癌作用。本研究组前期应用生物信息学软件发现circFGGY存在m6A甲基化修饰位点。本研究发现,METTL3可结合circFGGY并增加其m6A甲基化修饰水平,促进其出细胞核。FSP1是铁死亡抑制因子,主要定位于细胞质,本研究结果显示,沉默circFGGY能下调FSP1的表达水平,但二者并不存在直接结合作用,具体机制还有待进一步探索。
本研究结果显示,MSI2、METTL3以及circFGGY在HepG2细胞中呈高表达,三者的沉默质粒转染HepG2细胞可促进细胞铁死亡,发挥促癌作用。circRNA及其甲基化修饰调控肿瘤细胞有氧糖酵解的机制极其复杂。MSI2、METTL3/circFGGY通过何种效应蛋白和途径影响肝癌细胞铁死亡的机制尚有待进一步研究探讨。
| [1] |
HOLCZBAUER Á, WANGENSTEEN KJ, SHIN S. Cellular origins of regenerating liver and hepatocellular carcinoma[J]. JHEP Rep, 2021, 4(4): 100416. DOI:10.1016/j.jhepr.2021.100416 |
| [2] |
MA HY, NIU YJ. Metabolomic profiling reveals new insight of fowl adenovirus serotype 4 infection[J]. Front Microbiol, 2022, 12: 784745. DOI:10.3389/fmicb.2021.784745 |
| [3] |
MOU YH, WANG J, WU JC, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer[J]. J Hematol Oncol, 2019, 12(1): 34. DOI:10.1186/s13045-019-0720-y |
| [4] |
BATTAGLIA AM, CHIRILLO R, AVERSA I, et al. Ferroptosis and cancer: mitochondria meet the "iron maiden" cell death[J]. Cells, 2020, 9(6): 1505. DOI:10.3390/cells9061505 |
| [5] |
CHEN GQ, BENTHANI FA, WU J, et al. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis[J]. Cell Death Differ, 2020, 27(1): 242-254. DOI:10.1038/s41418-019-0352-3 |
| [6] |
LI DS, LI YS. The interaction between ferroptosis and lipid metabolism in cancer[J]. Signal Transduct Target Ther, 2020, 5(1): 108. DOI:10.1038/s41392-020-00216-5 |
| [7] |
WANG Y. The inhibition of microRNA-15a suppresses hepatitis B virus-associated liver cancer cell growth through the Smad/TGF-β pathway[J]. Oncol Rep, 2017, 37(6): 3520-3526. DOI:10.3892/or.2017.5618 |
| [8] |
GUAN BG, LI GR, WAN BH, et al. RNA-binding protein RBM38 inhibits colorectal cancer progression by partly and competitively binding to PTEN 3' UTR with miR-92a-3p[J]. Environ Toxicol, 2021, 36(12): 2436-2447. DOI:10.1002/tox.23356 |
| [9] |
MONTALBANO M, MCALLEN S, PUANGMALAI N, et al. RNA-binding proteins Musashi and tau soluble aggregates initiate nuclear dysfunction[J]. Nat Commun, 2020, 11(1): 4305. DOI:10.1038/s41467-020-18022-6 |
| [10] |
WANG MH, QIN SY, ZHANG SG, et al. Musashi-2 promotes hepatitis B virus related hepatocellular carcinoma progression via the Wnt/β-catenin pathway[J]. Am J Cancer Res, 2015, 5(3): 1089-1100. |
| [11] |
YIMING R, TAKEUCHI Y, NISHIMURA T, et al. MUSASHI-2 confers resistance to third-generation EGFR-tyrosine kinase inhibitor osimertinib in lung adenocarcinoma[J]. Cancer Sci, 2021, 112(9): 3810-3821. DOI:10.1111/cas.15036 |
| [12] |
STAGSTED LVW, O'LEARY ET, EBBESEN KK, et al. The RNA-binding protein SFPQ preserves long-intron splicing and regulates circRNA biogenesis in mammals[J]. eLife, 2021, 10: e63088. DOI:10.7554/eLife.63088 |
| [13] |
ZHANG R, ZHANG F, SUN Z, et al. LINE-1 retrotransposition promotes the development and progression of lung squamous cell carcinoma by disrupting the tumor-suppressor gene FGGY[J]. Cancer Res, 2019, 79(17): 4453-4465. DOI:10.1158/0008-5472.CAN-19-0076 |
| [14] |
SUN T, WU RY, MING L. The role of m6A RNA methylation in cancer[J]. Biomed Pharmacother, 2019, 112: 108613. DOI:10.1016/j.biopha.2019.108613 |
| [15] |
PRATS AC, DAVID F, DIALLO LH, et al. Circular RNA, the key for translation[J]. Int J Mol Sci, 2020, 21(22): 8591. DOI:10.3390/ijms21228591 |
| [16] |
WANG Q, CHEN C, DING QQ, et al. METTL3-mediated m6A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance[J]. Gut, 2020, 69(7): 1193-1205. DOI:10.1136/gutjnl-2019-319639 |
| [17] |
ZOU HL, LUO J, GUO YB, et al. RNA-binding protein complex LIN28/MSI2 enhances cancer stem cell-like properties by modulating Hippo-YAP1 signaling and independently of Let-7[J]. Oncogene, 2022, 41(11): 1657-1672. DOI:10.1038/s41388-022-02198-w |
| [18] |
FAN HN, CHEN ZY, CHEN XY, et al. METTL14-mediated m6A modification of circORC5 suppresses gastric cancer progression by regulating miR-30c-2-3p/AKT1S1 axis[J]. Mol Cancer, 2022, 21(1): 51. DOI:10.1186/s12943-022-01521-z |
 2022, Vol. 51
2022, Vol. 51




