文章信息
- 姜红, 姜红堃
- JIANG Hong, JIANG Hongkun
- 血清胱抑素C对川崎病及其合并冠状动脉损害的诊断价值
- The diagnostic value of serum cystatin C in Kawasaki disease and its concomitant coronary artery lesion
- 中国医科大学学报, 2021, 50(9): 769-773
- Journal of China Medical University, 2021, 50(9): 769-773
-
文章历史
- 收稿日期:2020-11-13
- 网络出版时间:2021-09-09 12:04
川崎病(Kawasaki disease,KD) 临床上又称为皮肤黏膜淋巴结综合征,是一种发生于5岁以下婴幼儿的急性自限性全身性血管炎,是发达国家儿童急性血管炎及获得性心脏病的主要病因[1]。KD在世界各地不同种族的儿童中均有发现,以东北亚国家的发病率最高。研究[2]显示我国KD发病率呈上升趋势。KD最主要的后遗症为冠状动脉损害(coronary artery lesion,CAL) 与冠状动脉瘤形成,是导致患儿死亡的首要原因[3]。KD的诊断依据非特异性临床表现,因此易漏诊误诊,若患儿未得到及时治疗,可导致CAL的发生风险增加[4]。胱抑素C (cystatin C,Cys-C) 属于半胱氨酸蛋白酶抑制剂2型半胱氨酸蛋白酶超家族成员,是由122个氨基酸组成分子量为13.3×103的非糖基化的碱性蛋白质[5]。血清Cys-C是经典的监控肾功能的生物学标志物,可预测心血管疾病、癌症、肝脏损伤等[6-7]。本研究分析KD患儿血清Cys-C水平,探讨血清Cys-C对KD及KD合并CAL的诊断价值。
1 材料与方法 1.1 临床资料及分组收集2010年1月至2019年10月间于中国医科大学附属第一医院儿科就诊的157例KD急性期患儿(KD组) 的临床资料,包括年龄、性别、血清Cys-C、尿素氮(blood urea nitrogen,BUN)、肌酐(creatinine,Cr)、丙氨酸转氨酶(alanine transaminase,ALT)、天冬氨酸转氨酶(aspartate aminotransferase,AST)。KD组纳入标准:(1) 综合患儿病史、临床症状指征、影像学检查等资料,符合KD诊断标准[8];(2) 患儿发热1~2周内;(3) 临床资料完整。排除标准:(1) 合并低血流量休克;(2) 合并明确由其他疾病所导致的肝肾损害;(3) 合并先天性心脏病、心肌病;(4) 入院前已接受静脉注射丙种球蛋白、阿司匹林等治疗;(5) 病例资料缺失。其中男101例,女56例,年龄2个月~8岁,中位年龄为[2.00 (1.00~4.00)]岁。依据超声心动图CAL诊断标准[9],具体包括:年龄 < 36个月,冠状动脉直径≥2.5 mm;年龄36个月~ < 108个月,冠状动脉直径≥3 mm;年龄≥108个月,冠状动脉直径≥3.5 mm。合并CAL患儿为CAL组(n = 34),其中,冠状动脉扩张(coronary artery ectasia,CAE) 31例,冠状动脉瘤3例;未合并CAL患儿为NCAL组(n = 123)。又根据Cys-C水平(参考区间0.53~0.95 mg/L)分组:>0.95 mg/L患儿为Cys-C高值组(n = 23);≤0.95 mg/L患儿为Cys-C正常组(n = 134)。
同时选取此期间门诊就诊的健康体检儿童200例为对照组,其中男105例,女95例,年龄2个月~8岁,中位年龄[5.00 (3.00~6.00)]岁。
1.2 血清Cys-C、BUN、Cr、ALT、AST测定受试者空腹至少3~4 h后采集静脉血(2~3 mL),应用日立7600-110全自动生化分析仪采用颗粒增强透射免疫比浊法测定血清Cys-C浓度;尿素酶法测血清BUN浓度;肌氨酸氧化酶法测定血清Cr浓度。应用Olympus AU-5400型全自动生化分析仪检测ALT、AST水平。
1.3 超声心动图检查应用GE公司Vivid Dimension彩色超声诊断仪行超声心动图检查,重点显示左冠状动脉主干、左前降支、左回旋支、右冠状动脉及后降支冠状动脉图像。KD病程45 d内,每周1次超声心动图检查,然后每月检查1次,至第3个月末。
1.4 统计学分析采用SPSS 25.0软件进行统计学分析,正态分布计量资料采用x±s表示,组间比较采用t检验;不符合正态分布的计量资料用M (P25~P75) 表示,组间比较采用Mann-Whitney U检验。绘制Cys-C预测KD及其合并CAL的受试者工作特征(receiver operator characteristic,ROC) 曲线,分析曲线下面积(area under the curve,AUC) 及不同界点的诊断价值,确定血清Cys-C值最佳界值,P < 0.05为差异有统计学意义。
2 结果 2.1 KD组与对照组临床指标比较结果显示,与对照组比较,KD组血清Cys-C值明显增高(P < 0.05)。BUN与Cr水平明显降低(P < 0.01)。2组年龄差异有统计学意义(P < 0.01),表 1。
| Group | n | Age (year) | Cys-C (mg/L) | BUN (mmol/L) | Cr (mmol/L) |
| Control | 200 | 5.00(3.00-6.00) | 0.73(0.67-0.79) | 4.15(3.32-4.74) | 29.00(24.00-33.75) |
| KD | 157 | 2.00(1.00-4.00) | 0.78(0.69-0.87) | 2.61(1.96-3.18) | 23.00(19.00-28.00) |
| Z | -8.618 | -3.245 | -11.535 | -7.041 | |
| P | < 0.001 | 0.010 | < 0.001 | < 0.001 | |
| Cys-c, cystatin C, BUN, blood urea nitrogen; Cr, creatine. | |||||
2.2 Cys-C高值组与Cys-C正常组ALT、AST比较
结果显示,2组ALT、AST水平比较无统计学差异(均P > 0.05)。见表 2。
| Group | n | ALT | AST |
| High Cys-C | 23 | 31.00(14.00-43.00) | 26.00(16.00-44.00) |
| Normal Cys-C | 134 | 21.00(11.00-64.25) | 26.00(19.00-36.00) |
| Z | -0.236 | -0.084 | |
| P | 0.814 | 0.933 | |
| ALT, alanine transaminase; AST, aspartate aminotransferase. | |||
2.3 CAL组与NCAL组血清Cys-C、BUN、Cr水平比较
结果显示,CAL组血清Cys-C明显高于NCAL组(P < 0.001);而2组血清BUN和Cr比较无统计学差异(均P > 0.05),见表 3。
| Group | n | Cys-C (mg/L) | BUN (mmol/L) | Cr (mmol/L) |
| CAL | 34 | 0.95±0.18 | 2.79±0.97 | 24.00(19.00-27.00) |
| NCAL | 123 | 0.75±0.11 | 2.62±0.93 | 23.00(19.00-28.00) |
| t/Z | 12.334 | 0.632 | -0.400 | |
| P | < 0.001 | 0.363 | 0.968 |
2.4 血清Cys-C预测KD及KD合并CAL的ROC曲线分析
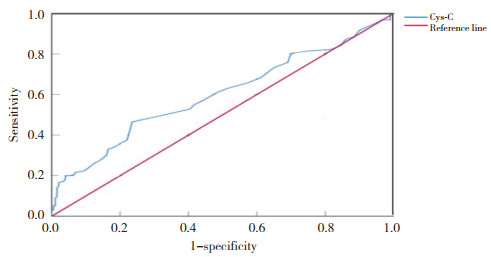
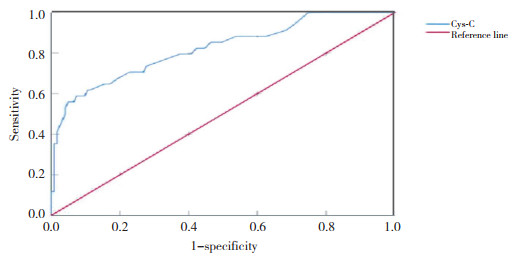
结果显示,Cys-C预测KD及KD合并CAL的ROC曲线AUC分别为0.600和0.821;预测的最佳界值分别为0.795 mg/L、0.845 mg/L。见表 4,图 1、2。
| Item | AUC | SE | P | 95% CI | Cut-off (mg/L) | Sensitivity (%) | Specificity (%) |
| KD | 0.600 | 0.031 | 0.001 | 0.540-0.660 | 0.795 | 46.5 | 76.5 |
| CAL | 0.821 | 0.044 | < 0.001 | 0.735-0.907 | 0.845 | 61.8 | 89.4 |
|
| 图 1 血清Cys-C预测KD的ROC曲线 Fig.1 Curve of serum Cys-C predicting KD |
|
| 图 2 血清Cys-C预测KD合并CAL的ROC曲线 Fig.2 Curve of serum Cys-C predicting KD combined with CAL |
3 讨论
自1967年川崎首次报道KD以来,学者们对KD的认识日益完善[1-3]。KD属于感染性疾病与自身免疫性疾病,疾病进展依赖于感染触发、遗传易感性、宿主及环境影响等多种因素[2, 10]。目前,KD确切病理生理机制尚未明确[10-11]。KD初期治疗主要为静脉注射免疫球蛋白及口服阿司匹林。研究[11-12]显示,若未接受常规治疗,25%KD患儿会发生CAL,包括心肌梗死、CAE、冠状动脉瘘及冠状动脉瘤等[11-12]。因此,找到早期诊断KD及其合并CAL的生物学标志物非常重要。
Cys-C的编码基因是CST3管家基因,位于20 pl1.21,在有核细胞中均有表达。Cys-C以相对恒定的速率产生并分泌到各种生物体液中,通过肾小球的滤过作用从血浆中去除,再由近端小管重新吸收降解[13]。Cys-C的功能与其靶酶密切相关,它既可特异性调节半胱氨酸蛋白酶活性,也可调节其他重要的生物学功能(细胞增殖、细胞分化、细胞迁移、免疫调节等) [14]。
本研究结果显示,KD组血清Cys-C值明显高于对照组,而血清BUN与Cr明显低于对照组(均P < 0.01)。研究[15-16]显示血清BUN、Cr受年龄因素影响,本研究中对照组年龄高于KD组,推测为KD组血清BUN与Cr明显低于对照组的原因。AL MUSAIMII等[17]研究发现年龄、性别等因素对血清Cys-C无影响,本研究中KD组血清Cys-C值明显高于对照组,因此推测血清Cys-C可能对KD具有诊断价值。JIA等[18]发现组织蛋白酶B在KD的组织炎症过程中发挥关键作用。本研究中KD组血清Cys-C高于对照组,可能与组织蛋白酶B、H等参与KD炎症反应,导致Cys-C与半胱氨酸蛋白酶平衡失调,继而使血清Cys-C水平升高有关。
有研究[7, 19]表明,肝功能损伤是KD急性期的常见并发症,且肝功能损伤可能导致血清Cys-C升高。为排除肝功能异常对Cys-C的影响,本研究比较了Cys-C高值组与Cys-C正常组肝功能相关指标,结果发现2组ALT、AST无统计学差异(P > 0.05),表明本研究中Cys-C水平增高与肝功能损伤无关。绘制血清Cys-C预测KD的ROC曲线发现AUC为0.600,最佳界值为0.795 mg/L,认为Cys-C可能对KD具有一定的预测价值。
本研究结果显示,KD患儿CAL组较NCAL组血清Cys-C水平增高(P < 0.05),推测KD患儿急性期血清Cys-C水平增高对KD合并CAL具有预测价值。本研究34例CAL患儿中31例CAE,这与YETKIN等[20]提出冠状动脉粥样硬化性心脏病合并CAE患者血清Cys-C水平明显增高的结论相似。GUPTA-MALHOTRA等[21]对17例KD急性期血清Cys-C研究发现,KD血清Cys-C在急性期低于对照组,考虑可能的原因包括:(1) 种族差异;(2) 样本量不同;(3) 研究对象不同,本研究对照组为儿童,而GUPTA-MALHOTRA等研究的对照组包括儿童及成人。ROC曲线分析结果显示,当血清Cys-C浓度为0.845 mg/L时,约登指数达到峰值,灵敏度和特异度分别为61.8%、89.4%,可见血清Cys-C对CAL具有较高的预测效能。
研究[10-11]表明,KD的两个重要病理机制是炎症反应及内皮细胞损伤,并涉及先天免疫及适应性免疫的激活。另有研究[22-23]表明,血清Cys-C与炎症及血管重建过程密切相关,在先天性免疫和自身免疫性疾病中发挥重要作用。因此推测血清Cys-C在KD的组织炎症及免疫机制中同时发挥作用,但具体作用机制尚需进一步研究论证。
综上所述,血清Cys-C水平可能对于KD具有一定的辅助诊断价值,对KD合并CAL具有预测价值。今后将采取多中心、大样本研究以期更加准确找到可预测儿童KD及发生CAL的Cys-C界值。
| [1] |
PORRITT RA, MARKMAN JL, MARUYAMA D, et al. Interleukin-1 beta-mediated sex differences in Kawasaki disease vasculitis development and response to treatment[J]. Arterioscler Thromb Vasc Biol, 2020, 40(3): 802-818. DOI:10.1161/ATVBAHA.119.313863 |
| [2] |
ELAKABAWI K, LIN J, JIAO F, et al. Kawasaki disease: global burden and genetic background[J]. Cardiol Res, 2020, 11(1): 9-14. DOI:10.14740/cr993 |
| [3] |
KIM HJ, KIM JJ, YUN SW, et al. Association of the IL16 Asn1147Lys polymorphism with intravenous immunoglobulin resistance in Kawasaki disease[J]. J Hum Genet, 2020, 65(4): 421-426. DOI:10.1038/s10038-020-0721-2 |
| [4] |
MARRANI E, BURNS JC, CIMAZ R. How should we classify Kawasaki disease?[J]. Front Immunol, 2018, 9: 2974. DOI:10.3389/fimmu.2018.02974 |
| [5] |
FERNANDO S, POLKINGHORNE KR. Cystatin C: not just a marker of kidney function[J]. J Brasileiro De Nefrologia, 2020, 42(1): 6-7. DOI:10.1590/2175-8239-JBN-2019-0240 |
| [6] |
MAO Q, ZHAO N, WANG Y, et al. Association of cystatin C with metabolic syndrome and its prognostic performance in non-ST-segment elevation acute coronary syndrome with preserved renal function[J]. Biomed Res Int, 2019, 2019: 8541402. DOI:10.1155/2019/8541402 |
| [7] |
ICHIKAWA T, MIYAAKI H, MIUMA S, et al. Indices calculated by serum creatinine and cystatin C as predictors of liver damage, muscle strength and sarcopenia in liver disease[J]. Biomed Rep, 2020, 12(3): 89-98. DOI:10.3892/br.2020.1273 |
| [8] |
NEWBURGER JW, TAKAHASHI M, GERBER MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association[J]. Circulation, 2004, 110(17): 2747-2771. DOI:10.1161/01.CIR.0000145143.19711.78 |
| [9] |
FU S, GONG F, XIE C, et al. S100A12 on circulating endothelial cells surface in children with Kawasaki disease[J]. Pediatr Res, 2010, 68(2): 165-168. DOI:10.1203/pdr.0b013e3181e67ce8 |
| [10] |
BELKAIBECH S, POTTER BJ, KANG H, et al. Maternal autoimmune disorders and risk of Kawasaki disease in offspring[J]. J Pediatr, 2020, 222: 240-243. DOI:10.1016/j.jpeds.2020.02.016 |
| [11] |
QIAN BY, HUANG H, CHENG MY, et al. Mechanism of HMGB1-RAGE in Kawasaki disease with coronary artery injury[J]. Eur J Med Res, 2020, 25(1): 8. DOI:10.1186/s40001-020-00406-5 |
| [12] |
WANG Y, HU J, LIU J, et al. The role of Ca2+/NFAT in dysfunction and inflammation of human coronary endothelial cells induced by sera from patients with Kawasaki disease[J]. Sci Rep, 2020, 10(1): 4706. DOI:10.1038/s41598-020-61667-y |
| [13] |
MOHD TAHIR NA, MOHD SAFFIAN S, ISLAHUDIN FH, et al. Effects of CST3 gene G73A polymorphism on cystatin C in a prospective multiethnic cohort study[J]. Nephron, 2020, 144(4): 204-212. DOI:10.1159/000505296 |
| [14] |
CARLESSON E, SUPHARATTANASITTHI W, JACKSON M, et al. Increased rate of retinal pigment epithelial cell migration and pro-angiogenic potential ensuing from reduced cystatin C expression[J]. Invest Ophthalmol Vis Sci, 2020, 61(2): 9. DOI:10.1167/iovs.61.2.9 |
| [15] |
PENG X, LV Y, FENG G, et al. Algorithm on age partitioning for estimation of reference intervals using clinical laboratory database exemplified with plasma creatinine[J]. Clin Chem Lab Med, 2018, 56(9): 1514-1523. DOI:10.1515/cclm-2017-1095 |
| [16] |
LIU Q, WANG Y, CHEN Z, et al. Age- and sex-specific reference intervals for blood urea nitrogen in Chinese general population[J]. Sci Rep, 2021, 11(1): 10058. DOI:10.1038/s41598-021-89565-x |
| [17] |
AL MUSAIMI O, ABU-NAWWAS AH, AL SHAER D, et al. Influence of age, gender, smoking, diabetes, thyroid and cardiac dysfunctions on cystatin C biomarker[J]. Semergen, 2019, 45(1): 44-51. DOI:10.1016/j.semerg.2018.07.005 |
| [18] |
JIA C, ZHANG J, CHEN H, et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation[J]. Cell Death Dis, 2019, 10(10): 778. DOI:10.1038/s41419-019-2021-3 |
| [19] |
MAMMADOV G, LIU HH, CHEN WX, et al. Hepatic dysfunction secondary to Kawasaki disease: characteristics, etiology and predictive role in coronary artery abnormalities[J]. Clin Exp Med, 2020, 20(1): 21-30. DOI:10.1007/s10238-019-00596-1 |
| [20] |
YETKIN E, ACIKGOZ N, SIVRI N, et al. Increased plasma levels of cystatin C and transforming growth factor-beta1 in patients with coronary artery ectasia: can there be a potential interaction between cystatin C and transforming growth factor-beta1[J]. Coron Artery Dis, 2007, 18(3): 211-214. DOI:10.1097/MCA.0b013e328087bd98 |
| [21] |
GUPTA-MALHOTRA M, LEVINE DM, COOPER RS, et al. Decreased levels of cystatin C, an inhibitor of the elastolytic enzyme cysteine protease, in acute and subacute phases of Kawasaki disease[J]. Cardiology, 2003, 99(3): 121-125. DOI:10.1159/000070668 |
| [22] |
ZHANG W, ZI M, SUN L, et al. Cystatin C regulates major histocompatibility complex-Ⅱ-peptide presentation and extracellular signal-regulated kinase-dependent polarizing cytokine production by bone marrow-derived dendritic cells[J]. Immunol Cell Biol, 2019, 97(10): 916-930. DOI:10.1111/imcb.12290 |
| [23] |
LARSEN TR, GERKE O, DIEDERICHSEN ACP, et al. Lack of association between cystatin C and different coronary atherosclerotic manifestations[J]. Scand J Clin Lab Invest, 2017, 77(8): 574-581. DOI:10.1080/00365513.2017.1355980 |
 2021, Vol. 50
2021, Vol. 50




