文章信息
- 梁彗莉, 王涛, 陈昕, 李潭, 杨军, 马春燕
- LIANG Huili, WANG Tao, CHEN Xin, LI Tan, YANG Jun, MA Chunyan
- Libman-Sacks心内膜炎的超声心动图表现及临床特征分析
- The echocardiographic and clinical characteristics of patients with Libman-Sacks endocarditis
- 中国医科大学学报, 2021, 50(6): 540-543
- Journal of China Medical University, 2021, 50(6): 540-543
-
文章历史
- 收稿日期:2021-01-20
- 网络出版时间:2021-05-26 16:36
Libman-Sacks心内膜炎又称非细菌性血栓性心内膜炎,是系统性红斑狼疮(systemic lupus erythematosus,SLE)及抗磷脂抗体综合征(antiphospholipid syndrome,APS)的典型心脏表现,其特征为瓣膜非感染性疣状赘生物形成[1],主要累及二尖瓣[2],其次为主动脉瓣,三尖瓣及肺动脉瓣发生率最低。超声心动图是Libman-Sacks心内膜炎的首选检查方法,在其诊断及鉴别中发挥重要作用。此外,Libman-Sacks心内膜炎的发生率及瓣膜病变的严重程度与抗磷脂抗体阳性或继发性APS有关[3]。本研究回顾性分析总结Libman-Sacks心内膜炎患者超声表现及临床特征,并根据是否合并APS进行分组,比较其超声表现及临床特征,探讨超声心动图在Libman-Sacks心内膜炎诊断中的应用价值,以期为临床诊断及早期干预提供参考价值。
1 材料与方法 1.1 一般资料选择2016年1月至2020年12月于我院就诊经胸超声(transthoracic echocardiography,TTE)或经食管三维超声(three-dimensional transesophageal echocar- diography,3D-TEE)诊断为Libman-Sacks心内膜炎的SLE患者23例,均为女性患者,年龄13~62岁,平均年龄为(39.32±12.34)岁。抗核抗体、抗双链DNA抗体均为阳性,符合1997年美国风湿病学会SLE诊断标准[4]。反复血培养结果均为阴性。按照2006年悉尼国际APS会议诊断标准[5],进一步根据是否合并APS进行分组,比较其超声表现及临床特点。
1.2 仪器与方法采用Philips iE33及Philips EPIQ7C超声诊断仪进行TTE或3D-TEE图像采集,观察赘生物位置、累及范围、数量、回声、形态、活动度等,测量并记录其长度、宽度,观察并评估受累瓣膜的情况,有无狭窄、反流、破坏及其严重程度。比较单纯SLE组与合并APS组超声表现及临床特点的异同。
1.3 统计学分析采用SPSS 25.0软件进行统计学分析。计数资料采用n(%)表示,组间比较采用Fisher精确概率法,P < 0.05为差异有统计学意义。
2 结果 2.1 超声心动图表现23例患者均行TTE检查,其中12例行3D-TEE检查。TTE检查中,1例瓣膜未见确切附加回声,2例提示瓣膜轻微增厚,后经TEE检查可见瓣尖疣状赘生物形成。
Libman-Sacks心内膜炎的超声主要表现为瓣尖或瓣叶对合缘一个或多个簇状、桑葚状、颗粒样或结节样赘生物形成,回声不均,表面粗糙,体积较小,基底较宽,固定于瓣膜表面,无明显自主活动度,累及二尖瓣前后叶时,瓣叶对合处呈“对吻征”。其中,累及二尖瓣15例(65.2%),主动脉瓣5例(21.7%),三尖瓣1例(4.3%),同时累及二尖瓣及主动脉瓣2例(8.7%)。合并瓣膜轻度及以下反流17例(73.9%),中度反流5例(21.7%),重度反流1例(4.3%),轻度狭窄2例(8.7%)。3D-TEE检查的患者中,累及二尖瓣7例,其中6例前后叶均可见赘生物;累及主动脉瓣4例,其中无冠瓣2例,右冠瓣1例,同时累及左、无冠瓣1例;累及三尖瓣1例。同时测得赘生物平均长度为(7.23±2.76)mm,平均宽度为(5.16±1.83)mm。见图 1、2。
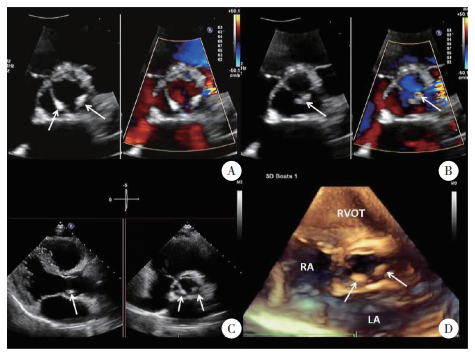
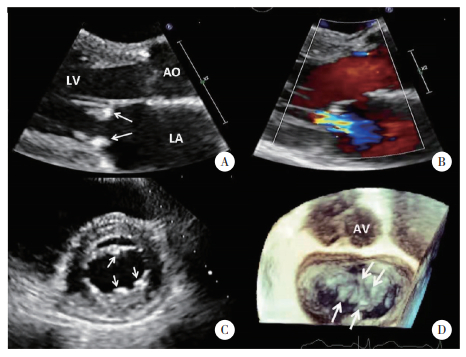
|
| A, TTE aotic valve short axis Zoom view showed Libman-Sackes vegetations on the left and non-coronary cusps during systole (arrow); B, trace aortic regurgitation was demonstrated by color Doppler during diastole (arrow); C, X-plane view showed vegetations on the surface of aortic valve from multi-angle (arrow); D, 3D-TTE view showed two small granular vegetations on the aortic side and tip portions of left and non-coronary cusps (arrow). 图 1 单纯SLE组Libman-Sacks心内膜炎累及主动脉瓣TTE图像 Fig.1 Libman-Sacks endocarditis involving the aortic valve in the SLE group on TTE |
|
| A, TTE parasternal long axis zoom view showed Libman-Sackes vegetations on the atrial surface of anterior and posterior of mitral leaflets during diastole (arrow); B, moderate mitral regurgitation was demonstrated by color Doppler during systole (arrow); C, TTE mitral valve short axis view showed multiple vegetations on the surface of anterior and posterior mitral leaflets (arrow); D, 3D-TEE left atral view showed multiple small cluster vegetations (arrow). 图 2 SLE合并APS组Libman-Sacks心内膜炎累及二尖瓣TTE及3D-TEE图像 Fig.2 Libman-Sacks endocarditis involving the mitral valve in the SLE+APS group on TTE and 3D-TEE |
2.2 单纯SLE与SLE合并APS伴Libman-Sacks心内膜炎患者对比
23例患者中,单纯SLE组14例,并发狼疮性肾炎3例,抗中性粒细胞胞浆抗体(anti-neutrophilic cytoplasmic antibodies,ANCA)相关性血管炎2例;赘生物累及二尖瓣8例,主动脉瓣4例,同时累及二尖瓣及主动脉瓣2例,其中2例(14.3%)合并瓣膜中度反流,其余反流程度均为轻度及以下。SLE合并APS组9例,并发狼疮性肾炎3例,ANCA相关性血管炎3例,脑血管栓塞3例;赘生物累及二尖瓣7例,主动脉瓣1例,三尖瓣1例,其中合并瓣膜轻度及以下反流5例(55.5%),中度反流3例(33.3%),重度反流1例(11.1%),轻度狭窄2例(22.2%),其中2例由于瓣膜病变严重,行机械瓣置换手术,术后病理证实为Libman-Sacks心内膜炎,见表 1。
| Item | SLE group(n = 14) | SLE+APS group(n = 9) | P |
| Laboratory examination | |||
| Lupus anticoagulant(+) | 0(0.0) | 6(66.7) | 0.001 |
| Anticardiolipin antibody(+) | 0(0.0) | 4(44.4) | 0.014 |
| Anti-β2 glycoprotein-1 antibody(+) | 0(0.0) | 9(100.0) | < 0.001 |
| Location of vegetations | |||
| Mitral valve | 10(71.4) | 7(77.8) | 0.999 |
| Aortic valve | 6(42.9) | 1(11.1) | 0.176 |
| Tricuspid valve | 0(0.0) | 1(11.1) | 0.391 |
| Valvular regurgitation | |||
| Trace or mild | 12(85.7) | 5(55.6) | 0.162 |
| Moderate | 2(14.3) | 3(33.3) | 0.343 |
| Severe | 0(0.0) | 1(11.1) | 0.391 |
| Complications | |||
| Lupus nephritis | 3(21.4) | 3(33.3) | 0.643 |
| ANCA associated vasculitis | 2(14.3) | 3(33.3) | 0.343 |
| Ischemic cerebrovascular disease | 0(0.0) | 3(33.3) | 0.047 |
3 讨论
Libman-Sacks心内膜炎又称为非细菌性血栓性心内膜炎[6],最常见于SLE。近年来,有少数原发性APS或SLE合并APS患者伴发Libman-Sacks心内膜炎的相关报道[7-9]。由于多数患者无明显症状,早期诊断难度大,随着病情进展,可能会导致脑血管或全身血管栓塞、瓣膜功能障碍、心力衰竭等严重并发症[2]。因此,早期、准确诊断可以及时进行治疗干预,预防疾病进展[10]。
超声心动图是Libman-Sacks心内膜炎首选的检查方法。TTE诊断Libman-Sacks心内膜炎发生率约为11%,而TEE约为53%~74%[2, 11]。该病典型超声表现为大小、形状各异的疣状赘生物,体积较小,直径均 < 1 cm,边界不规则,回声具有异质性,基底较宽,牢固附着于瓣膜表面,常位于瓣膜上游,也可累及腱索及房室心内膜表面,无自主活动度[10]。最常累及二尖瓣,约占63%[2],瓣叶对合处呈典型的“对吻征”,其次为主动脉瓣,三尖瓣及肺动脉瓣少见。多数患者无明显瓣膜功能障碍,仅合并轻度及以下反流[2]。本研究有3例患者TTE检查未见异常或仅见瓣膜轻微增厚,行3D-TEE检查后见瓣叶表面赘生物形成,结合临床诊断为Libman-Sacks心内膜炎,表明3D-TEE在Libman-Sacks心内膜炎的诊断中具有更高的灵敏度,作为TTE的有效补充,可以提高诊断的准确性。
近年抗磷脂抗体在Libman-Sacks心内膜炎中的作用引发关注[12]。抗磷脂抗体能够激活内皮细胞,导致单核细胞及血小板聚集,促进已经被免疫复合物沉积损害的瓣膜的血栓形成,加剧瓣膜损伤和炎症改变[2]。ZUILY等[3]研究表明,SLE患者中抗磷脂抗体阳性组Libman-Sacks心内膜炎发病率为阴性组的3倍,并更易出现中重度的二尖瓣反流。本研究中,与单纯SLE组相比,合并APS患者瓣膜反流程度更重,其中2例由于瓣膜病变严重,药物治疗效果不佳,最终行瓣膜置换术。但有研究[3]显示,由于抗磷脂抗体引起瓣膜血栓的风险增加,合并APS的患者预后不佳。
APS组有3例患者发生脑血管栓塞,可能与赘生物的脱落及抗磷脂抗体引起的高凝状态有关。研究[13]发现,Libman-Sacks心内膜炎是SLE及APS患者脑血管栓塞的潜在栓子来源,是脑血管事件的独立危险因素。对于合并脑血管栓塞的SLE及APS患者,Libman-Sacks心内膜炎的早期诊断至关重要。
综上所述,超声心动图是Libman-Sacks心内膜炎的首选检查方法,TTE结合3D-TEE能够早期发现并精确显示赘生物的位置、大小及形态。此外,合并APS的SLE患者,瓣膜病变程度更重,脑血管栓塞事件发生率更高。因此,在超声检查中应提高对该疾病的认识,尽可能减少漏诊及误诊,以便指导临床早期干预。
| [1] |
YOO BW, LEE SW, SONG JJ, et al. Clinical characteristics and long-term outcomes of Libman-Sacks endocarditis in patients with systemic lupus erythematosus[J]. Lupus, 2020, 29(9): 1115-1120. DOI:10.1177/0961203320930097 |
| [2] |
MOYSSAKIS I, TEKTONIDOU MG, VASILLIOU VA, et al. Libman-Sacks endocarditis in systemic lupus erythematosus:prevalence, associations, and evolution[J]. Am J Med, 2007, 120(7): 636-642. DOI:10.1016/j.amjmed.2007.01.024 |
| [3] |
ZUILY S, HUTTIN O, MOHAMED S, et al. Valvular heart disease in antiphospholipid syndrome[J]. Curr Rheumatol Rep, 2013, 15(4): 1-7. DOI:10.1007/s11926-013-0320-8 |
| [4] |
HOCHBERG MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997, 40(9): 1725. DOI:10.1002/art.1780400928 |
| [5] |
MIYAKIS S, LOCKSHIN MD, ATSUMI T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS)[J]. J Thromb Haemost, 2006, 4(2): 295-306. DOI:10.1111/j.1538-7836.2006.01753.x |
| [6] |
黄毅, 陈昕, 王晓冰, 等. 超声心动图诊断Libman-Sacks心内膜炎1例[J]. 中国临床医学影像杂志, 2020, 31(1): 72. |
| [7] |
KOTKAR KD, SAID SM. Libman-sacks endocarditis in a patient with antiphospholipid syndrome[J]. Ann Thorac Surg, 2016, 102(1): e31-e32. DOI:10.1016/j.athoracsur.2015.11.004 |
| [8] |
YAMASHITA G, KANEMITSU N, NAKASHIMA Y, et al. Hypertrophic obstructive cardiomyopathy and mitral regurgitation in Libman-Sacks endocarditis[J]. Gen Thorac Cardiovasc Surg, 2020, 68(2): 181-184. DOI:10.1007/s11748-018-1042-7 |
| [9] |
MURTAZA G, ISKANDAR J, HUMPHREY T, et al. Lupus-negative libman-sacks endocarditis complicated by catastrophic antiphospholipid syndrome[J]. Cardiol Res, 2017, 8(2): 57-62. DOI:10.14740/cr534e |
| [10] |
ROLDAN CA, TOLSTRUP K, MACIAS L, et al. Libman-sacks endocarditis:detection, characterization, and clinical correlates by three-dimensional transesophageal echocardiography[J]. J Am Soc Echocardiogr, 2015, 28(7): 770-779. DOI:10.1016/j.echo.2015.02.011 |
| [11] |
ISHIZU K, ISOTANI A, YAMAJI K, et al. Immunosuppressive therapy to reduce mitral regurgitation in Libman-Sacks endocarditis:a case report[J]. Eur Heart J-Case Rep, 2019, 3(3): ytz133. DOI:10.1093/ehjcr/ytz133 |
| [12] |
卢丽娟, 黄勤. SLE继发抗磷脂抗体综合征合并Libman-Sacks心内膜炎一例[J]. 新医学, 2021, 52(1): 70-73. DOI:10.3969/j.issn.0253-9802.2021.01.014 |
| [13] |
ROLDAN CA, SIBBITT WL, QUALLS CR, et al. Libman-Sacks endocarditis and embolic cerebrovascular disease[J]. JACC Cardiovasc Imaging, 2013, 6(9): 973-983. DOI:10.1016/j.jcmg.2013.04.012 |
 2021, Vol. 50
2021, Vol. 50




