文章信息
- 张晓琳, 邵秀丽, 朱荣利, 张瑞佳, 郝丽英, 封瑞
- ZHANG Xiaolin, SHAO Xiuli, ZHU Rongli, ZHANG Ruijia, HAO Liying, FENG Rui
- 基于长QT综合征的钙调蛋白突变体CaMD130G与心肌钠通道NaV1.5 IQ基序的结合作用
- Binding between the calmodulin mutant CaMD130G and the cardiac sodium channel NaV1.5 IQ motif in the context of long QT syndrome
- 中国医科大学学报, 2021, 50(3): 193-197
- Journal of China Medical University, 2021, 50(3): 193-197
-
文章历史
- 收稿日期:2020-06-30
- 网络出版时间:2021-03-18 12:28
长QT综合征(long QT syndrome,LQTs)是一种心律失常综合征,心电图检查具有QT间期延长、T波或U波异常等表征,临床易产生恶性心律失常[1-2]。LQTs不仅患病率较高,猝死率也很高[3]。目前,研究[4-5]已经明确LQTs与某些离子通道有关,例如L型钙通道,钠通道和钾通道。此外,许多调节通道的蛋白也被证实与LQTs的发生相关,如钙调蛋白(calmodulin,CaM)[6-8]。
心肌细胞上主要表达的钠通道为NaV1.5通道,由1个α亚基和1个或多个β亚基组成。心肌NaV1.5受细胞内CaM等多种细胞因子的调节[9-12]。CaM某些位点发生突变,导致NaV1.5的调节异常,最终可引发心脏疾病[13]。本课题组早期的研究已经成功制备出CaMD130G(第130位天冬氨酸突变为甘氨酸)突变体蛋白,但其在LQTs发病过程中与心肌NaV1.5通道的结合及调节作用仍然未知。
本研究拟采用同源建模和分子对接的方法对CaM、CaMD130G与心肌NaV1.5通道IQ基序对接,初步探索结合可能性。同时拟构建、表达、制备与纯化IQ蛋白,并初步探索其与CaMD130G的结合活性,为相关疾病的研究提供新思路。
1 材料与方法 1.1 材料pGEX-6P-1/GST-IQ质粒由北京擎科新业生物技术有限公司合成;胰蛋白胨和酵母提取物购自美国Oxid公司;异丙基硫代-β-D半乳糖苷(isopropyl-beta-D-thiogalactopyranoside,IPTG)、二硫苏糖醇(dithiothreitol,DTT)、氨苄西林(ampicillin,Amp)、溶菌酶(lysozyme,Lys)以及N-月桂酰肌氨酸(N-lauroylsarcosine sodium,N-lau)均购自美国Sigma公司;Triton X-100和磷酸盐缓冲液(phosphate bufer,PBS)粉末购自北京Solarbio公司;Glutathione-Sepharose 4B beads(GS-4B beads)购自英国GE Healthcare公司。
1.2 同源建模与蛋白对接获得心肌NaV1.5通道IQ基序和CaM及其突变体CaMD130G氨基酸序列(图 1),采用SWISS MODEL数据库进行同源建模与分子对接。通过PyMOL软件读取,修饰并分析对接结果。
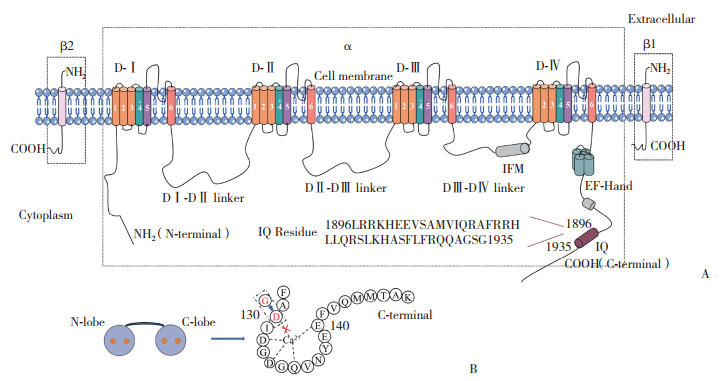
|
| A, cardiac NaV1.5 channel; B, CaMD130G. 图 1 心肌Nav1.5通道及CaMD130G的模式图 Fig.1 Schematic diagram of the cardiac NaV1.5 channel and CaMD130G |
1.3 融合蛋白GST-IQ的制备 1.3.1 诱导与表达
将重组pGEX-6P-1/GST-IQ质粒转化入大肠杆菌感受态细胞并放于含有Amp的1×LB培养液中进行培养,在90 r/min、37 ℃水浴的条件中振荡培养12~16 h。当光密度(optical density,OD)600处于0.6~1.2时向培养基中加入IPTG(终浓度为1 mmol/L),37 ℃、110 r/min振荡4 h,以诱导重组蛋白的表达。
1.3.2 蛋白提取收集诱导表达重组IQ蛋白的菌液,4 000 r/min离心10 min,收集沉淀。向其中加入PBS缓冲液重悬沉淀,依次加入20 mg/mL Lys(终浓度为0.1 mg/mL)、N-Lau(终浓度为1.5%)和1 mol/L DTT(终浓度为5 mmol/L)并混匀,于冰上静置30 min。然后于冰上超声破碎30 min,加入30%TritonX-100至1%混匀后再次静置30 min,此后得到的细菌裂解液放入4 ℃恒温离心机中15 000 g高速离心10 min,得到IQ蛋白粗提液。
1.4 Pull-down结合实验在15 mL EP管中用PBS清洗GS-4B beads 3次(每次800 r/min,3 min离心),将提取的IQ蛋白加入到洗好的beads中,于4 ℃条件下过夜孵育。次日,用Tris buffer清洗3次,获得GST-IQ蛋白。在2 mL EP管中加入GST-IQ蛋白40 μL,再依次加入相应浓度的CaM/CaMD130G,使终浓度分别为2.1,3.5,7.0,10.0 μmol/L,然后向其中加入CaCl2至钙离子(Ca2+)终浓度为10 μmol/L,再加入Tris Buffer至300 μL,然后于4 ℃的条件中缓慢旋转孵育4 h。孵育后,清洗2遍。最后加入loading buffer,煮沸5 min,将获得的结合蛋白进行15% SDS-PAGE电泳。
2 结果 2.1 CaMD130G蛋白与心肌NaV1.5通道IQ的同源建模与蛋白对接在SWISS-MODEL网站中对心肌NaV1.5通道的IQ蛋白、CaM及其突变体CaMD130G蛋白成功建模,其中IQ蛋白和CaM的模型与目标序列完全一致,同源性高达100%。而CaMD130G因为第130位点突变,只有1个氨基酸序列发生变化,所以模型同源性为99.45%。虽然CaMD130G在建模时空间结构与CaM一致,但其结合Ca2+的数量减少。本研究结果显示,正常CaM结合4个Ca2+,但突变体CaMD130G只结合3个Ca2+,在靠近C末端第130位氨基酸位点处丢失了1个Ca2+的结合。同时,研究发现无论是CaM还是CaMD130G在蛋白对接中都能与IQ蛋白对接上,预测CaMD130G可与心肌NaV1.5有结合活性。见图 2。

|
| A, CaM-IQ docking diagram; B, CaMD130G-IQ docking diagram; C, sequences of CaM, CaMD10130G, and IQ, 4ovn.1.A, CaM; 4ovn.1.B, IQ. 图 2 CaM/CaMD130G-IQ蛋白对接图 Fig.2 CaM/CaMD130G-IQ protein docking diagram |
2.2 重组心肌NaV1.5通道IQ质粒和蛋白的制备
质粒载体pGEX-6P-1碱基长约4 900 bp,酶切位点BamHⅠ和XhoⅠ之间的碱基全长约20 bp,IQ的碱基序列大小为135 bp,经3’端和5’端双酶切并插入IQ基因后整个重组质粒pGEX-6P-1/IQ全长约5 015 bp。重组质粒EcoRⅤ和XhoⅠ双酶切后获得1个大分子量DNA(约为3 100 bp)和1个小分子量DNA(约为1 900 bp)。琼脂糖凝胶电泳(图 3A)结果显示,泳道1中约5 000 bp处的条带是重组质粒pGEX-6P-1/IQ的碱基大小。泳道2中3 100 bp和2 900 bp处均可看到明显的条带,质粒制备结果与理论值一致,证明心肌NaV1.5通道IQ质粒构建成功。将质粒转化入大肠杆菌,再由IPTG诱导IQ蛋白的表达,采用超声破碎法提取IQ蛋白并进行15% SDS-PAGE电泳,得到纯度和浓度较高的IQ蛋白片段,见图 3B。

|
| A, plasmid preparation; B, protein preparation.1, plasmid DNA; 2, plasmid digested by EcoR V/Xho I; M, DNA marker. 图 3 重组心肌NaV1.5通道IQ质粒与蛋白制备 Fig.3 Recombinant cardiac NaV1.5 channel IQ plasmid and protein |
2.3 心肌NaV1.5通道IQ蛋白与CaMD130G结合作用的鉴定
Pull-down结合实验结果显示,在10.0 μmol/L Ca2+情况下,CaM和CaMD130G与心肌NaV1.5通道IQ蛋白均能结合,并且这种结合作用具有CaM和CaMD130G浓度依赖性,证明CaM和CaMD130G与心肌NaV1.5通道IQ蛋白具有很好的结合活性,见图 4。

|
| 1, CaM 2.1 μmol/L; 2, CaMD130G 2.1 μmol/L; 3, CaM 3.5 μmol/L; 4, CaMD130G 3.5 μmol/L; 5, CaM 7.0 μmol/L; 6, CaMD130G 7.0 μmol/L; 7, CaM.10.0 μumol/L; 8, CaMD130G 10.0 pumol/L. 图 4 CaM及CaMD130G与GST-IQ结合的SDS-PAGE电泳图 Fig.4 Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of the binding between CaM/CaMD130G and GST-IQ |
3 讨论
CaM作为一类胞内高度保守的Ca2+结合蛋白,不仅参与体内多种信号传导,在Ca2+依赖性的信号转导途径中更是起着至关重要的作用。Ca2+对CaM具有重要的调节作用,CaM对Ca2+具有依赖性,是动态多功能Ca2+传感器[14]。CaM有2个球型末端,每个末端含有2个Ca2+结构域,每个结构域能结合1个Ca2+,因此,每个CaM可以结合4个Ca2+。当CaM与Ca2+结合后自身构象发生改变,从而与目标蛋白结合,调控靶蛋白的活性使其发挥相应的生物学功能[15]。最近的研究[16-18]发现CaM基因的某些位点突变在多种心血管系统疾病中扮演着重要角色,严重威胁人类生命健康。
NaV1.5通道由SCN5A基因编码,结构复杂,是决定心肌细胞兴奋的关键因素,主要参与动作电位上升支形成和电冲动在心肌细胞间的传导。研究[10, 16, 19]报道,CaM主要与NaV1.5通道的IQ基序相互作用,从而对通道功能进行调节。CaM某些位点发生突变,破坏了NaV1.5失活状态的电压依赖性和稳定性,导致钠电流异常,引起LQTs等疾病,可导致心脏骤停甚至出现心源性猝死等不良事件。目前,与LQTs有关的CaM的多个突变位点已经被报道,如CaMD96V、CaME141G和CaMD130G等。有研究[20]证明CaME141G对心肌NaV1.5的调节的影响在LQTs中发挥重要作用。而对于CaMD130G与心肌NaV1.5的相互作用研究较少,探寻CaMD130G与心肌NaV1.5的结合作用对于研究LQTs等心脏疾病具有重要意义。本研究采用同源建模的方法对CaMD130G突变体进行建模,结果显示,CaMD130G模型可能因为第130位氨基酸突变导致结合的Ca2+减少了1个,此结果提示该位点的突变可能会影响CaMD130G的Ca2+依赖性,改变CaMD130G对Ca2+的亲和力,推测CaMD130G对心肌NaV1.5通道IQ基序的调节作用发生改变,最终可能导致LQTs等心脏疾病的发生。
以往研究[20]提示CaM与Ca2+结合特性的变化可能是CaM突变体引发LQTs的病理机制之一。本研究结果证明CaMD130G与心肌NaV1.5的IQ基序具有很好的结合活性,但是对其结合的Ca2+浓度依赖性和CaM浓度依赖性还不了解,需要进一步的实验明确其详细的结合模式,从而深入探讨LQTs发病可能的详细分子机制。本研究初步探索了CaMD130G与心肌NaV1.5的IQ的相互结合作用,为未来的深入研究奠定了坚实的基础,并为探讨LQTs的病理机制提供理论依据。
| [1] |
SHAH SR, PARK K, ALWEIS R. Long QT syndrome: a comprehensive review of the literature and current evidence[J]. Curr Probl Cardiol, 2019, 44(3): 92-106. DOI:10.1016/j.cpcardiol.2018.04.002 |
| [2] |
ADAMOS G, IACOVIDOU N, XANTHOS T. Medical therapy for long QT syndrome[J]. Mini-Rev Med Chem, 2018, 18(6): 495-506. DOI:10.2174/1389557517666170707110000 |
| [3] |
GIUDICESSI JR, ACKERMAN MJ. Genotype-and phenotype-guided management of congenital long QT syndrome[J]. Curr Probl Cardiol, 2013, 38(10): 417-455. DOI:10.1016/j.cpcardiol.2013.08.001 |
| [4] |
BOHNEN MS, PENG G, ROBEY SH, et al. Molecular pathophysiology of congenital long QT syndrome[J]. Physiol Rev, 2017, 97(1): 89-134. DOI:10.1152/physrev.00008.2016 |
| [5] |
BOHANNON BM, CRUZ ADL, WU XA, et al. Polyunsaturated fatty acid analogues differentially affect cardiac NaV, CaV, and KV channels through unique mechanisms[J]. Elife, 2020, 9: e51453. DOI:10.7554/eLife.51453 |
| [6] |
PIPILAS DC, JOHNSON CN, WEBSTER G, et al. Novel calmodulin mutations associated with congenital long QT syndrome affect calcium current in human cardiomyocytes[J]. Heart Rhythm, 2016, 13(10): 2012-2019. DOI:10.1016/j.hrthm.2016.06.038 |
| [7] |
REED GJ, BOCZEK NJ, ETHERIDGE SP, et al. CALM3 mutation associated with long QT syndrome[J]. Heart Rhythm, 2015, 12(2): 419-422. DOI:10.1016/j.hrthm.2014.10.035 |
| [8] |
CHAZIN WJ, JOHNSON CN. Calmodulin mutations associated with heart arrhythmia: a status report[J]. Int J Mol Sci, 2020, 21(4): 1418. DOI:10.3390/ijms21041418 |
| [9] |
PITT GS, LEE SY. Ca2+/CaM interaction with voltage-gated Na+ channels[J]. PNAS, 2019, 116(52): 26150-26151. DOI:10.1073/pnas.1909835116 |
| [10] |
BOCZEK NJ, GOMEZ-HURTADO N, YE D, et al. Spectrum and prevalence of CALM1-, CALM2-, and CALM3-encoded calmodulin variants in long QT syndrome and functional characterization of a novel long QT syndrome-associated calmodulin missense variant, E141G[J]. Circ Cardiovasc Genet, 2016, 9(2): 136-146. DOI:10.1161/circgenetics.115.001323 |
| [11] |
GARDILL BR, RIVERA-ACEVEDO RE, TUNG CC, et al. Crystal structures of Ca2+-calmodulin bound to NaV C-terminal regions suggest role for EF-hand domain in binding and inactivation[J]. Proc Natl Acad Sci USA, 2019, 116(22): 10763-10772. DOI:10.1073/pnas.1818618116 |
| [12] |
BALSE E, EICHEL C. The cardiac sodium channel and its protein partners[J]. Handb Exp Pharmacol, 2018, 246: 73-99. DOI:10.1007/164_2017_45 |
| [13] |
胡金柱, 洪葵. 心脏Nav1.5相互作用蛋白与心律失常[J]. 中华心血管病杂志, 2011, 39(7): 682-685. DOI:10.3760/cma.j.issn.0253-3758.2011.07.019 |
| [14] |
SHAIK NA, AWAN ZA, VERMA PK, et al. Protein phenotype diagnosis of autosomal dominant calmodulin mutations causing irregular heart rhythms[J]. J Cell Biochem, 2018, 119(10): 8233-8248. DOI:10.1002/jcb.26834 |
| [15] |
WANG KQ, HOLT C, LU J, et al. Arrhythmia mutations in calmodulin cause conformational changes that affect interactions with the cardiac voltage-gated calcium channel[J]. PNAS, 2018, 115(45): E10556-E10565. DOI:10.1073/pnas.1808733115 |
| [16] |
CROTTI L, JOHNSON CN, GRAF E, et al. Calmodulin mutations associated with recurrent cardiac arrest in infants[J]. Circulation, 2013, 127(9): 1009-1017. DOI:10.1161/CIRCULATIONAHA.112.001216 |
| [17] |
JIMÉNEZ-JÁIMEZ J, PALOMINO DOZA J, ORTEGA Á, et al. Calmodulin 2 mutation N98S is associated with unexplained cardiac arrest in infants due to low clinical penetrance electrical disorders[J]. PLoS One, 2016, 11(4): e0153851. DOI:10.1371/journal.pone.0153851 |
| [18] |
LIMPITIKUL WB, DICK IE, JOSHI-MUKHERJEE R, et al. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca2+ currents and promote proarrhythmic behavior in ventricular myocytes[J]. J Mol Cell Cardiol, 2014, 74: 115-124. DOI:10.1016/j.yjmcc.2014.04.022 |
| [19] |
SU JY, GAO QH, YU LF, et al. The LQT-associated calmodulin mutant E141G induces disturbed Ca2+-dependent binding and a flickering gating mode of the CaV1.2 channel[J]. Am J Physiol-Cell Physiol, 2020, 318(5): C991-C1004. DOI:10.1152/ajpcell.00019.2020 |
| [20] |
苏敬阳, 王蓉蓉, 袁媛, 等. CaM突变体质粒的构建、表达纯化及活性鉴定[J]. 中国医科大学学报, 2018, 47(2): 97-101. DOI:10.12007/j.issn.02584646.2018.02.001 |
 2021, Vol. 50
2021, Vol. 50




