文章信息
- 郭庭维, 杨召铭, 李峰, 林建贞, 张城硕, 孙宁, 李晓航, 张佳林
- GUO Tingwei, YANG Zhaoming, LI Feng, LIN Jianzhen, ZHANG Chengshuo, SUN Ning, LI Xiaohang, ZHANG Jialin
- 人羊膜提取物对小鼠胰岛缺氧损伤的保护作用及其分子机制
- Protective effect and molecular mechanism of human amniotic membrane extract against hypoxic pancreatic islet injury in mice
- 中国医科大学学报, 2021, 50(12): 1128-1133, 1137
- Journal of China Medical University, 2021, 50(12): 1128-1133, 1137
-
文章历史
- 收稿日期:2021-04-12
- 网络出版时间:2021-12-07 12:46
自Edmonton方案问世以来,胰岛移植在过去的几十年间取得了显著进展,目前被认为是治疗1型糖尿病最有前景的方法之一[1]。胰岛移植可以恢复糖尿病患者胰岛素的生理性分泌,最大限度降低因严重低血糖导致的死亡风险。目前临床胰岛移植尚存在许多不足,主要包括供胰短缺、需要终身使用免疫抑制药物及胰岛移植物体内长期存活率低等。其中,缺氧是导致移植前培养胰岛及移植后胰岛移植物活性及功能丧失的关键因素之一[2-6]。因此,避免胰岛受到缺氧损伤具有重要的临床意义。
人羊膜提取物(human amniotic membrane extract,hAME)是一种天然的生物材料,含有丰富的生物活性分子,目前已被临床应用于眼部疾病的治疗。研究[7-10]表明,hAME能促进组织再生和伤口愈合,具有减轻炎症反应和减少氧化应激损伤的功能。
本研究通过建立小鼠肾被膜下胰岛移植模型,拟探讨hAME对小鼠胰岛缺氧损伤的保护作用及相关分子机制,进一步通过体内移植研究验证在缺氧状态下经hAME预处理的胰岛治疗糖尿病的效果。
1 材料与方法 1.1 动物C57BL/6小鼠由辽宁长生生物科技有限公司提供。选择6~8周龄、体质量18~22 g的雄性C57BL/6小鼠作为胰岛供体和糖尿病受体。所有手术均采用异氟醚吸入麻醉(RWD,中国瑞沃德公司)。本研究按照国家卫生研究院实验动物指导与使用指南进行,并经中国医科大学动物福利伦理委员会批准。
1.2 hAME的制备本研究通过中国医科大学附属第一医院医学科学研究伦理委员会批准([2019]2019-219-3),选取在中国医科大学附属第一医院产科进行剖宫产手术的健康产妇,经受试者同意且签署知情同意书后,收集剖宫产孕妇羊膜。将人羊膜用含1 000U/mL青霉素和0.1 mg/mL链霉素双抗的PBS清洗3次,洗去残余的血块。羊膜切成碎块后置于液氮中研磨成细粉。称重后按质量体积比1∶1加入PBS混合,在冰上用超声匀浆机以10 000 r/min的参数超声匀浆1 h。然后在4 ℃离心机中以4 000 g的离心力离心10 min,收集上清,以15 000 g的离心力离心5 min。收集最终上清液并通过0.22 μm滤器滤菌,即得hAME,所得hAME置于-80 ℃保存备用。
1.3 小鼠胰岛的分离和缺氧培养颈椎脱臼法处死小鼠,充分暴露腹腔,用6-0丝线结扎胆总管汇入十二指肠处后,用胶原酶Ⅴ(1 mg/mL)2~3 mL充分灌注小鼠胰腺,在37 ℃恒温水浴锅中静置消化20 min。然后,用含10% FBS的预冷Hanks平衡盐溶液终止消化。使用Ficoll密度梯度离心法纯化小鼠胰岛(密度1.108、1.096、1.069和1.037 g/mL),并通过体视显微镜手动挑选,进一步纯化小鼠胰岛。将纯化后的小鼠胰岛随机分为4组:1%O2缺氧培养组、缺氧培养加hAME实验组、正常氧气培养组、正常氧气培养加hAME组。各组胰岛置于37 ℃湿润的细胞培养箱中培养12 h。胰岛基础培养基为含10%FBS、100 U/mL青霉素和0.1 mg/mL链霉素的RPMI 1640。
1.4 胰岛活性检测缺氧培养12 h后,用吖啶橙(acridine orange,AO,美国Sigma公司)和溴化乙锭(ethidium bromide,EB,美国Sigma公司)染色评估胰岛的活性,其中活细胞在荧光显微镜下呈绿色。采集荧光图像并使用Image J软件分析胰岛细胞的活性。小鼠胰岛的活性通过以下公式计算:胰岛活性(%)=绿色荧光所占面积/(绿色荧光所占面积+红色荧光所占面积)×100。
1.5 葡萄糖刺激胰岛素分泌试验缺氧培养12 h后,收集胰岛进行葡萄糖刺激胰岛素释放试验。每组随机选取10个胰岛置于含2.8 mmol/L葡萄糖的克-林二氏重碳酸盐缓冲液(krebs-ringer-bicarbonate buffer,KRBH)中预孵育30 min。预孵育后,将胰岛分别在含糖2.8 mmol/L(低)和16.8 mmol/L(高)葡萄糖的KRBH中孵育1 h。收集上清液,采用小鼠超敏胰岛素酶联免疫吸附检测试剂盒(10-1249-01,瑞典Mercodia公司)测定各个样品中胰岛素浓度。刺激指数(stimulation index,SI)按以下公式计算:SI=高糖刺激下胰岛分泌的胰岛素/低糖环境下胰岛分泌的胰岛素。
1.6 胰岛细胞核因子相关因子2(nuclear erythroid 2-related factor 2,Nrf2)和血红素加氧酶1(heme oxygenase-1,HO-1)蛋白表达检测Western blotting检测胰岛细胞内Nrf2和HO-1蛋白表达的水平。探讨加入hAME培养的胰岛细胞,相较于不加入hAME培养的胰岛细胞,在缺氧和正常培养环境下抵抗氧化应激的能力是否增强。
1.7 小鼠肾被膜下胰岛移植 1.7.1 糖尿病小鼠模型的诱导及分组进行移植:采用链脲佐菌素(S0130,美国Sigma公司)诱导小鼠糖尿病模型。将糖尿病小鼠随机分为4组,分别为缺氧培养对照组(n = 10)、缺氧培养+hAME实验组(n = 10)、假手术组(Sham组,n = 6)、正常对照组(Naive组,n = 6)。在体外培养12 h后,随机挑选200个胰岛移植到受体小鼠肾被膜下。假手术组和正常对照组用于与实验组和对照组进行比较。移植术后每3 d相同时间段测量并记录小鼠的非禁食血糖值。连续2次测量血糖值< 11.1 mmol/L视为糖尿病小鼠血糖恢复至正常。在观察6周后,将受体小鼠含胰岛移植物的左肾切除后,监测非禁食血糖7 d。
1.7.2 腹腔葡萄糖耐量测试(intraperitoneal glucose tolerance test,IPGTT):用IPGTT评估移植后小鼠体内胰岛移植物的功能,在移植术后第30天,将胰岛移植后糖尿病逆转的小鼠禁食不禁水过夜,然后腹腔注射葡萄糖(2 g/kg)。在注射后0、15、30、60、90、120 min测量血糖,绘制血糖变化趋势曲线并计算曲线下面积(area under the curve,AUC)。
1.7.3 移植物组织病理学观察:在移植术后第42天,切取胰岛移植物,并进行固定、石蜡包埋、切片及苏木素-伊红(hematoxylin/eosin,HE)染色、胰岛素免疫组化染色及胰岛素和胰高血糖素荧光双标染色,于荧光显微镜下观察并采集图像。
1.8 统计学分析采用Graph-Pad Prism 6软件(GraphPad Software,lnc.,USA)进行数据统计分析及作图,所有数据以x±s表示。采用One-way ANOVA、t检验及Tukey’s multiple comparisons test进行统计学分析。P < 0.05为差异有统计学意义。
2 结果 2.1 hAME对缺氧培养胰岛活性的影响经过12 h的体外培养,缺氧组相较于正常氧气培养组胰岛活性明显降低(P < 0.000 1)。与缺氧组相比,加入不同浓度(0.25~1.0 mg/mL)hAME的胰岛活性明显增高,差异有统计学意义(P < 0.05),且在hAME浓度为0.75 mg/mL时对缺氧胰岛的保护作用达到峰值,见图 1。
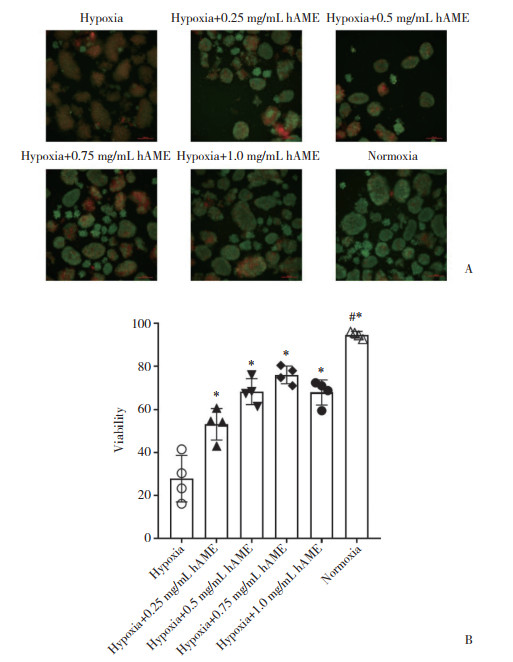
|
| A, AO/EB staining pictures of islets in each group after 12 hours of culture, viable cells are shown in green (scale bar=100 μm); B, after 12 hours of hypoxia culture, the percentage of islet activity added with hAME significantly increased. * P < 0.01 vs hypoxia group; # P < 0.01 vs hypoxia+0.75 mg/mL hAME group. 图 1 hAME提高缺氧培养胰岛的活性 Fig.1 hAME improves the activity of hypoxic cultured islets |
2.2 hAME对缺氧培养胰岛的胰岛素分泌功能的影响
如图 2所示,在低糖(2.8 mmol/L)刺激下各组胰岛之间的胰岛素分泌量无明显差异;在高糖(16.8 mmol/L)刺激下,缺氧对照组与hAME浓度为0.25 mg/mL时胰岛的胰岛素分泌量相比无明显差异(P > 0.05)。但添加浓度为0.5、0.75及1.0 mg/mL hAME时,缺氧胰岛的胰岛素分泌量均明显增多,且在hAME浓度为0.75 mg/mL时达到峰值。综上所述,hAME在浓度为0.75 mg/mL时对缺氧胰岛的保护作用最明显,故选择浓度为0.75 mg/mL的hAME用于后续研究。
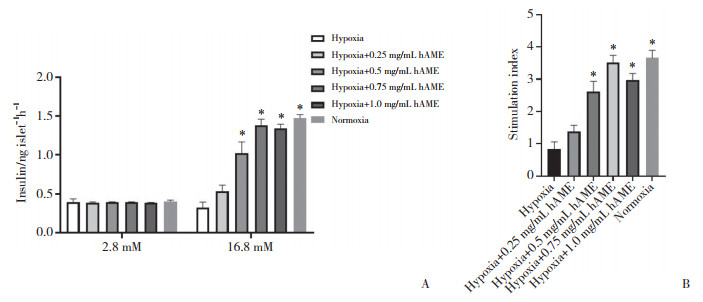
|
| A, the differences in the amount of insulin secretion in each group in the glucose-stimulated insulin secretion test; B, the difference in stimulation index of each group in the glucose-stimulated insulin secretion test. * P < 0.01 vs hypoxia group. 图 2 葡萄糖刺激下的胰岛素分泌实验和刺激指数分析 Fig.2 Insulin secretion experiment and stimulation index analysis under glucose stimulation |
2.3 hAME对缺氧状态下的胰岛Nrf2和HO-1蛋白表达的影响
如图 3所示,缺氧培养组与正常氧气培养组相比,胰岛蛋白中Nrf2和HO-1的表达明显增加(P < 0.000 1)。与缺氧培养组相比,正常氧气培养+hAME组胰岛蛋白中Nrf2(P < 0.001)和HO-1(P < 0.000 1)的表达增加,差异有统计学意义。此外,正常氧气培养+hAME组相比正常氧气培养组,Nrf2(P < 0.001)和HO-1(P < 0.000 1)的表达增加,差异有统计学意义。
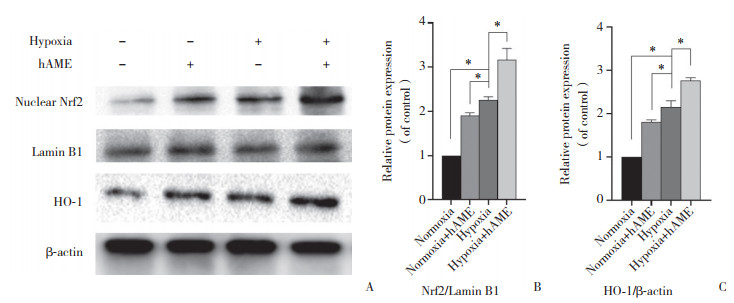
|
| A, the protein bands of each group obtained by Western blotting; B, the difference of Nrf2 protein expression in each experimental group; C, the difference of HO-1 expression in each experimental group. * P < 0.01. 图 3 Western blotting检测胰岛Nrf2和HO-1蛋白表达的变化 Fig.3 Western blotting to detect Nrf2 and HO-1 protein expression changes in the pancreatic islets |
2.4 缺氧培养后胰岛移植治疗小鼠糖尿病的效果 2.4.1 糖尿病受体小鼠非禁食血糖及体质量监测
为了进一步确定缺氧培养后胰岛移植治疗小鼠糖尿病的效果,在缺氧培养12 h后,将等量的胰岛移植到糖尿病受体小鼠肾被膜下。如图 4所示,与缺氧培养组相比,缺氧培养+hAME组的血糖AUC显著降低[(936.6±43.94)vs(673.4±29.53)mmol/L,P < 0.000 1];图 4C显示在42 d的血糖检测期间,缺氧培养组和缺氧培养+hAME组中个体的非禁食血糖变化;图 4D小鼠体质量监测表明,与缺氧培养组相比,缺氧培养+ hAME组的小鼠体质量明显增高[AUC(383.1±10.47)vs(298.8±10.94)g/42 d,P < 0.000 1]。
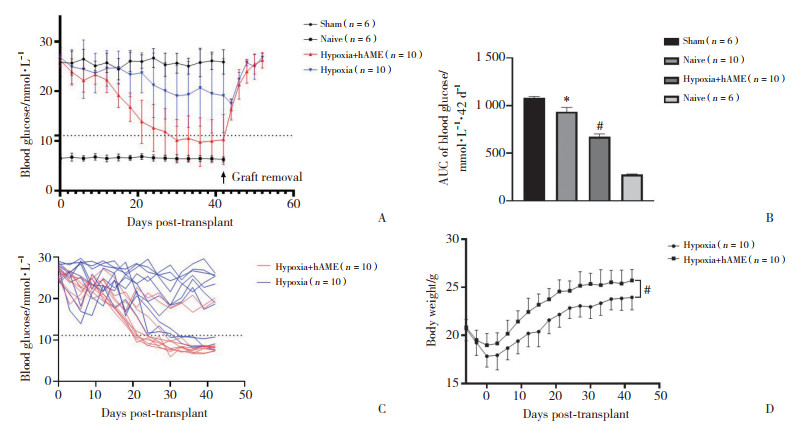
|
| A, the blood glucose curve change of each group after graft removal; B, differences in the areas under the blood glucose curve of each group; C, the non-fasting blood glucose changes of the hypoxia and hypoxia +hAME groups during the 42-day blood glucose test; D, recipient mouse body weight monitoring. * P < 0.01 vs sham group; # P < 0.01 vs hypoxia group. 图 4 胰岛移植后糖尿病受体小鼠血糖及体质量监测 Fig.4 Blood glucose and body weight monitoring of diabetic recipient mice after islet transplantation |
2.4.2 IPGTT
缺氧培养+hAME组的腹腔葡萄糖耐量试验的AUC明显低于缺氧培养组[(1 553±90.96)vs(1 836±48.78)mmol/L(120 min),P < 0.001],表明缺氧培养+hAME组的胰岛移植物比缺氧培养组有更好的血糖调节能力。见图 5。
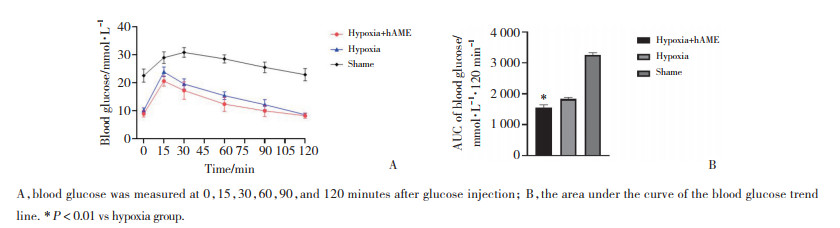
|
| A, blood glucose was measured at 0, 15, 30, 60, 90, and 120 minutes after glucose injection; B, the area under the curve of the blood glucose trend line. * P < 0.01 vs hypoxia group. 图 5 腹腔葡萄糖耐量试验 Fig.5 Intraperitoneal glucose tolerance test |
2.4.3 胰岛移植物的组织病理学染色
将受体小鼠的胰岛移植物所在肾脏取出并进行组织学染色。图 6为胰岛移植物的HE染色、胰岛素的免疫组织化学染色以及胰岛素和胰高血糖素的免疫荧光双标染色的图像。结果显示,Hypoxia+hAME组相较于Hypoxia组胰岛移植物面积更大。
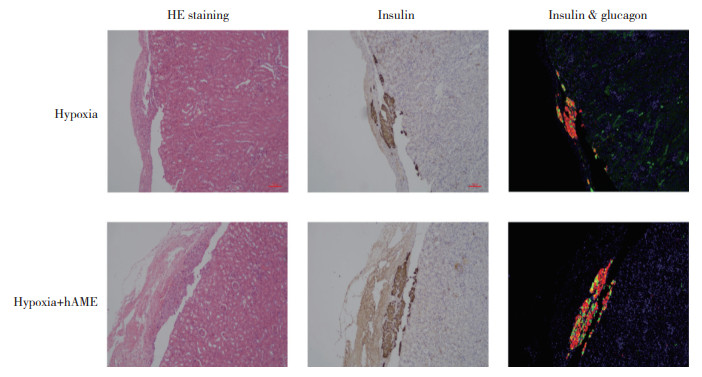
|
| Hematoxylin-esin staining, immunohistochemical staining (scale bar=100 um)and immunofluorescence slaining of islet grafits. 图 6 胰岛移植物组织学染色 Fig.6 Histological islet graft staining |
3 讨论
胰岛对缺氧十分敏感,在移植前胰腺保存、消化以及胰岛纯化过程中,胰岛的活性和分泌功能受到缺氧影响,最终导致移植效果不理想。为减少缺氧造成胰岛活性和功能的损伤,科学家进行了许多研究,例如在缺氧培养中充入适量的CO[11]、降低培养时的温度[12]、使用胶原蛋白构建的生物支架等以改善缺氧环境中胰岛的活性和功能[13]。羊膜作为一种天然的生物材料,含有丰富的生物活性分子且免疫原性低,目前被应用于临床烧伤和眼科疾病的治疗[14-16]。研究[17]表明,hAME在胰岛培养中作为培养基添加剂改善胰岛活性及分泌功能。以上结果提示hAME具有细胞保护作用,并可能在胰岛移植缺氧环境中充当保护剂。此外,在胰岛受到氧化应激损伤时,Nrf2作为抗氧化应激的转录因子[18],可通过诱导HO-1表达来应对氧化应激和炎症[19]。有研究[10]表明hAME通过调节Nrf2/HO-1通路来保护H9c2细胞免受低氧导致的氧化应激,因此,笔者推断hAME对缺氧状态下胰岛的保护作用可能涉及Nrf2/HO-1通路。在本研究中,添加hAME可以在缺氧状态下明显改善胰岛活力和胰岛素分泌功能,同时增加了缺氧状态胰岛细胞内Nrf2和HO-1的表达,这与之前的推断一致。在42 d的移植观察期内,与对照组相比,加入hAME的实验组胰岛移植效果有显著改善,这表明在缺氧环境下加入hAME培养的胰岛具有更高的活性和调节血糖的能力。本研究对hAME于缺氧状态下胰岛的活性及分泌功能的保护作用进行了初步研究,不良反应和hAME的具体成分有待进一步探讨。
综上所述,本研究证明hAME可改善缺氧状态下胰岛的活性及葡萄糖刺激胰岛素分泌功能,提高缺氧培养胰岛细胞内Nrf2/HO-1蛋白的表达,同时改善胰岛移植物的功能。hAME是一种天然的生物材料,富含生物活性分子及细胞外基质成分,且易获取,有望作为胰岛缺氧保护剂应用于胰岛移植中。
| [1] |
SHAPIRO AMJ, POKRYWCZYNSKA M, RICORDI C. Clinical pancreatic islet transplantation[J]. Nat Rev Endocrinol, 2017, 13(5): 268-277. DOI:10.1038/nrendo.2016.178 |
| [2] |
DE SOUZA BM, BOUÇAS AP, OLIVEIRA FD, et al. Effect of co-culture of mesenchymal stem/stromal cells with pancreatic islets on viability and function outcomes: a systematic review and meta-analysis[J]. Islets, 2017, 9(2): 30-42. DOI:10.1080/19382014.2017.1286434 |
| [3] |
VELMURUGAN K, BALAMURUGAN AN, LOGANATHAN G, et al. Antiapoptotic actions of exendin-4 against hypoxia and cytokines are augmented by CREB[J]. Endocrinology, 2012, 153(3): 1116-1128. DOI:10.1210/en.2011-1895 |
| [4] |
KESHTKAR S, KAVIANI M, JABBARPOUR Z, et al. Significant reduction of apoptosis induced via hypoxia and oxidative stress in isolated human islet by resveratrol[J]. Nutr Metab Cardiovasc Dis, 2020, 30(7): 1216-1226. DOI:10.1016/j.numecd.2020.04.011 |
| [5] |
KESHTKAR S, KAVIANI M, JABBARPOUR Z, et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis[J]. Sci Rep, 2019, 9(1): 11701. DOI:10.1038/s41598-019-48262-6 |
| [6] |
WANG H, STRANGE C, NIETERT PJ, et al. Autologous mesenchymal stem cell and islet cotransplantation: safety and efficacy[J]. Stem Cells Transl Med, 2018, 7(1): 11-19. DOI:10.1002/sctm.17-0139 |
| [7] |
WU MF, STACHON T, LANGENBUCHER A, et al. Effect of amniotic membrane suspension (AMS) and amniotic membrane homogenate (AMH) on human corneal epithelial cell viability, migration and proliferation in vitro[J]. Curr Eye Res, 2017, 42(3): 351-357. DOI:10.1080/02713683.2016.1192193 |
| [8] |
SHAYAN ASL N, NEJAT F, MOHAMMADI P, et al. Amniotic membrane extract eye drop promotes limbal stem cell proliferation and corneal epithelium healing[J]. Cell J, 2019, 20(4): 459-468. DOI:10.22074/cellj.2019.5423 |
| [9] |
DUDOK DV, NAGDEE I, CHEUNG K, et al. Effects of amniotic membrane extract on primary human corneal epithelial and limbal cells[J]. Clin Exp Ophthalmol, 2015, 43(5): 443-448. DOI:10.1111/ceo.12480 |
| [10] |
FARIDVAND Y, NOZARI S, VAHEDIAN V, et al. Nrf2 activation and down-regulation of HMGB1 and MyD88 expression by amnion membrane extracts in response to the hypoxia-induced injury in cardiac H9c2 cells[J]. Biomed Pharmacother, 2019, 109: 360-368. DOI:10.1016/j.biopha.2018.10.035 |
| [11] |
KIM DS, SONG LL, WANG JJ, et al. Carbon monoxide inhibits islet apoptosis via induction of autophagy[J]. Antioxid Redox Signal, 2018, 28(14): 1309-1322. DOI:10.1089/ars.2016.6979 |
| [12] |
ITOH T, SUGIMOTO K, TAKITA M, et al. Low temperature condition prevents hypoxia-induced islet cell damage and HMGB1 release in a mouse model[J]. Cell Transplant, 2012, 21(7): 1361-1370. DOI:10.3727/096368912x637514 |
| [13] |
ZBINDEN A, URBANCZYK M, LAYLAND SL, et al. Collagen and endothelial cell coculture improves β-cell functionality and rescues pancreatic extracellular matrix[J]. Tissue Eng Part A, 2021, 27(13/14): 977-991. DOI:10.1089/ten.tea.2020.0250 |
| [14] |
OBA J, OKABE M, YOSHIDA T, et al. Hyperdry human amniotic membrane application as a wound dressing for a full-thickness skin excision after a third-degree burn injury[J]. Burns Trauma, 2020, 8: tkaa014. DOI:10.1093/burnst/tkaa014 |
| [15] |
MURRI MS, MOSHIRFAR M, BIRDSONG OC, et al. Amniotic membrane extract and eye drops: a review of literature and clinical application[J]. Clin Ophthalmol, 2018, 12: 1105-1112. DOI:10.2147/opth.s165553 |
| [16] |
ZHAO X, ZUO X, ZHONG J, et al. Heparin-modified amniotic membrane combined with growth factors for promoting corneal wound healing after alkali burn[J]. Front Bioeng Biotechnol, 2020, 8: 599800. DOI:10.3389/fbioe.2020.599800 |
| [17] |
YANG ZM, LI XH, ZHANG CS, et al. Amniotic membrane extract protects islets from serum-deprivation induced impairments and improves islet transplantation outcome[J]. Front Endocrinol, 2020, 11: 587450. DOI:10.3389/fendo.2020.587450 |
| [18] |
SCHULTHEIS J, BECKMANN D, MULAC D, et al. Nrf2 activation protects mouse beta cells from glucolipotoxicity by restoring mitochondrial function and physiological redox balance[J]. Oxid Med Cell Longev, 2019, 2019: 7518510. DOI:10.1155/2019/7518510 |
| [19] |
ZHONG ZY, TANG Y. Upregulation of periostin prevents high glucose-induced mitochondrial apoptosis in human umbilical vein endothelial cells via activation of Nrf2/HO-1 signalin[J]. Cell Physiol Biochem, 2016, 39(1): 71-80. DOI:10.1159/000445606 |
 2021, Vol. 50
2021, Vol. 50




