文章信息
- 许乐鹏, 刘培慧, 盛伟伟, 王季堃
- XU Lepeng, LIU Peihui, SHENG Weiwei, WANG Jikun
- 胰腺癌组织中SMC1A表达的临床意义及对细胞增殖的影响
- The clinical significance of SMC1A expression in pancreatic cancer and its influence on cell proliferation
- 中国医科大学学报, 2021, 50(11): 1020-1025
- Journal of China Medical University, 2021, 50(11): 1020-1025
-
文章历史
- 收稿日期:2021-01-13
- 网络出版时间:2021-11-03 17:07
2. 葫芦岛市中心医院肿瘤日间病房, 辽宁 葫芦岛 125000;
3. 葫芦岛市中心医院介入血管外科, 辽宁 葫芦岛 125000;
4. 中国医科大学附属第一医院胃肠疝外科, 沈阳 110001
2. Oncology Day Ward, Huludao Central Hospital, Huludao 125000, China;
3. Department of Interventional Vascular Surgery, Huludao Central Hospital, Huludao 125000, China;
4. Department of Gastrointestinal Hernia Surgery, The First Hospital of China Medical University, Shenyang 110001, China
研究[1]显示,我国2000年至2011年男性胰腺癌发病率及年龄-标准化死亡率显著上升,分别为恶性肿瘤的第一位和第二位。胰腺癌死亡率高的主要原因为疾病早期检出率低,而且发病后肿瘤细胞的转移能力以及对周围组织的侵袭能力强,进而预后极差。因此,提高疾病的早期检出率,改善患者预后是目前急需解决的课题。
已有研究[2]发现染色体不稳定是恶性肿瘤的主要特征之一,其在恶性肿瘤发生进展中起着关键作用。在细胞对染色体稳定性进行维护的过程中,凝聚蛋白复合体是关键因子。染色体结构维持蛋白1A(structural maintenance of chromosomes protein 1A,SMC1A)是组成凝聚蛋白的主要亚基,其功能异常与恶性肿瘤(大肠癌、前列腺癌和肝癌)发生密切相关[3-5]。胰腺癌组织中SMC1A表达的临床意义及对细胞增殖的影响尚未见报道。本研究旨在检测胰腺癌组织中SMC1A表达情况,探讨其表达的临床意义及对胰腺癌细胞增殖的影响。
1 材料与方法 1.1 材料选取2010年1月至2015年12月锦州医科大学附属第一医院肿瘤内科行胰腺癌手术切除并经术后病理证实为腺癌的标本59例。纳入标准:(1)临床资料完整;(2)提供标本的患者可接受随访。胰腺癌细胞系,包括转移性胰腺癌细胞[AsPC-1(转移性腹水)、SW1990(脾转移)]和原发胰腺癌细胞(BxPC-3和PANC-1)均购自中科院上海细胞库,并用含10%胎牛血清1640培养液(美国HyClone公司)培养。主要试剂包括SMC1A单克隆抗体(美国Abcam公司),DAB显色液、免疫组化试剂盒(北京中杉金桥公司),转染试剂Opti-MEM及Oligofectamine2000(美国Invitrogen公司),ECL发光试剂盒、BCA定量蛋白裂解液及抑制剂(碧云天生物公司),SYBR Green Ⅱ实时荧光定量PCR试剂盒、逆转录试剂盒(DRR037S)、RNA分离试剂盒Trizol(日本TaKaRa公司)。SMC1A siRNA由上海吉玛公司设计合成。
1.2 方法 1.2.1 免疫组化标本均经甲醛固定、4 μm切片、二甲苯脱蜡、梯度乙醇水化后去除内源性过氧化酶。PBS清洗后将胰腺癌组织标本置于pH 6.0柠檬酸高压修复1 min,自然冷却。室温环境下封闭血清,再将一抗(1∶200)加入,更换至4 ℃环境下12 h孵育。第2天加入霉菌抗生物素蛋白-过氧化物酶,PBS清洗、DAB显色、苏木素复燃。盐酸乙醇分化后脱水、透明、封片后光学显微镜(400倍)下观察。
1.2.2 SMC1A表达判定参照文献[6],每张切片观察5个视野,每个视野记数100个细胞。(1)阳性细胞数0~10%,0分;> 10%~25%,1分;> 25%~50%,2分;> 50%~75%,3分;> 75%,4分。(2)染色强度:无着色,0分;浅黄色,1分;黄或深黄色,2分;褐或棕褐色,3分。2项评分乘积 > 4分为SMC1A高表达,≤4分为低表达。
1.2.3 Western blotting检测转染SMC1A胰腺癌细胞系接种至6孔板,48 h后加入裂解液和蛋白酶抑制剂提取总蛋白,超声裂解,冰上静置30 min后12 000 r/min 4 ℃离心10 min,收集上清液,采用二辛可宁酸法测定蛋白浓度,经10%十二烷基磺酸钠-聚丙烯酰胺凝胶电泳,80 V 100 min电转移至聚偏二氟乙烯膜。5%脱脂奶粉封闭2 h,加入SMC1A(1∶1 000)4 ℃孵育过夜。二抗室温孵育2 h,凝胶显像仪(以色列MF-chemibis 3.2 DNR公司)显像。
1.2.4 实时定量PCR(quantitative real-time PCR,qRT-PCR)采用RNA分离试剂盒Trizol提取并纯化组织总RNA,所有RNA样本浓度均稀释至1 μg/μL,根据逆转录和扩增试剂盒说明书进行逆转录和扩增。荧光实时PCR反应体系25 µL(2× SYBRR Premix ExTaqTM 12.5 µL,100 nmol/ L上下游引物各1 µL,cDNA 2 µL,H2O 8.5 µL)。SMC1A上游引物5’-AAGTGAGGAGGAGGAGGAG-3’,下游引物5’-ACTTTCTTCAGGGTCTTGTTC-3’;GAPDH上游引物5’-GGAGCGAGATCCCTCCAAAAT-3’,下游引物5’-GGCTGTTGTCATACTTCTCATGG-3’。反应条件:95 ℃ 5 min,保持5 s,60 ℃ 30 s,以此为1个循环,共循环45次,并配有复孔,焦碳酸二乙酯水为阴性对照。结果采用2-ΔΔCt计算。
1.2.5 SMC1A siRNA干扰实验将对数生长期Capan-2细胞加入6孔板,严格按照Oligofectamine2000说明进行瞬时转染,取对数生长期Capan-2胰腺癌细胞加入6孔板中68 h,8 μL脂质体与200 μL opti-MEM混合静置5 min后与含8 μL siRNA(20 μmol/L)200 μL的opti-MEM混合静置20 min,然后加入相应各孔中。6 h后换液,24~48 h后提取相应RNA、蛋白。
1.2.6 细胞增殖酶标定量仪检测SMC1A siRNA组(转染SMC1A siRNA)和对照组(转染空白对照)转染1~5 d后的细胞,经MTT噻唑蓝法(5 mg/mL)和二甲基亚砜(DMSO)处理后用酶标定量仪检测波长570 nm处的吸光度(optical density,OD)值。细胞增殖率=(OD实验组-OD调零孔)/(OD对照组-OD调零孔)×100%,实验重复3次,取平均值。
1.3 统计学分析利用SPSS 18.0软件进行统计学处理,计量资料采用x±s显示;组间比较采用t检验;计数资料采用率(%)表示,组间比较采用χ2检验。累积生存率采用Kaplan-Meier单因素分析,多因素分析采用Cox回归分析,P < 0.05为差异有统计学意义。
2 结果 2.1 胰腺癌组织中SMC1A的表达结果显示,癌组织中SMC1A主要在胞质和胞核中表达,59例胰腺癌组织中有37例(62.7%)高表达,22例(37.2%)低表达,见图 1。
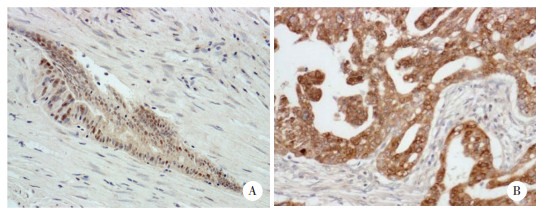
|
| A,low expression group;B,high expression group. 图 1 免疫组化检测胰腺癌中SMC1A的表达情况×200 Fig.1 Immunohistochemistry of SMC1A expression in pancreatic cancer tissue ×200 |
2.2 SMC1A表达与临床指标的关系
结果显示,SMC1A表达与患者肿瘤大小(χ2=4.515,P = 0.034)、T分期(χ2=10.277,P = 0.003)及UICC分期(χ2=3.940,P = 0.047)正相关,见表 1。
| Item | Low expression group(n=22) | High expression group(n=37) | χ2 | P |
| Age | 0.567 | 0.451 | ||
| < 65 years | 14(23.73) | 27(45.76) | ||
| ≥65 years | 8(13.56) | 10(16.95) | ||
| Sex | 0.002 | 0.961 | ||
| Male | 15(25.42) | 25(42.37) | ||
| Female | 7(11.86) | 12(20.34) | ||
| Tumor location | 0.101 | 0.750 | ||
| Pancreatic head | 8(13.56) | 15(25.42) | ||
| Pancreatic body and tail | 14(23.73) | 22(37.29) | ||
| Tumor size | 4.515 | 0.034 | ||
| < 3 cm | 14(23.73) | 13(22.03) | ||
| ≥3 cm | 8(13.56) | 24(40.68) | ||
| Differentiation | 0.975 | 0.380 | ||
| Well differentiated | 8(13.56) | 9(15.25) | ||
| Poorly differentiated | 14(23.73) | 28(47.46) | ||
| T stage | 10.277 | 0.003 | ||
| T1-T2 | 16(27.12) | 11(18.64) | ||
| T3-T4 | 6(10.17) | 26(44.07) | ||
| Lymph node metastasis | 2.571 | 0.133 | ||
| N0 | 19(32.20) | 25(42.37) | ||
| N1 | 3(5.08) | 12(20.34) | ||
| UICC stage | 3.940 | 0.047 | ||
| Ⅰ-ⅡA | 19(32.20) | 23(38.98) | ||
| ⅡB-Ⅲ | 3(5.08) | 14(23.72) | ||
| Vascular invasion | 1.904 | 0.168 | ||
| No | 13(22.03) | 15(25.42) | ||
| Yes | 9(15.25) | 22(37.29) | ||
| Preoperative CA19-9 level | 0.978 | 0.380 | ||
| < 37 U/mL | 8(13.56) | 9(15.25) | ||
| ≥37 U/mL | 14(23.73) | 28(47.46) |
2.3 患者各项临床指标与预后的关系
Kaplan-Meier生存曲线分析结果显示,SMC1A高表达胰腺癌患者较低表达者预后差,见图 2。单因素分析结果显示,SMC1A表达、血管浸润、UICC分期与患者预后相关(表 2)。将单因素分析有统计学意义(P < 0.05)指标进行多因素分析,结果显示,SMC1A高表达[95%CI:1.011~5.207,P = 0.047] 和UICC分期晚[95%CI:1.209~5.342,P = 0.014] 是患者预后的独立危险因素。
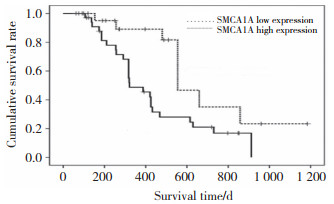
|
| 图 2 SMC1A低表达和高表达患者的Kaplan-Meier生存曲线分析 Fig.2 Kaplan-Meier survival curve analysis of patients with low and high SMC1A expression |
| Variable | Median survival time(d) | Univariate analysis(P,log rank) | Multi-factor analysis | ||
| OR | 95% CI | P | |||
| Age(< 60 years/≥60 years) | 555/431 | 0.181 | - | - | - |
| Sex(male/female) | 468/480 | 0.542 | - | - | - |
| Tumor location(head of pancreas/body and tail of pancreas) | 589/321 | 0.111 | - | - | - |
| Tumor size(< 3 cm/≥3 cm) | 678/458 | 0.075 | - | - | - |
| Differentiation(well/poorly) | 425/468 | 0.435 | - | - | - |
| T stage(T1-T2/T3-T4) | 559/415 | 0.176 | - | - | - |
| Lymph node metastasis(N0/N1) | 555/357 | 0.064 | - | - | - |
| UICC stage(Ⅰ+ⅡA/ⅡB+Ⅲ) | 555/317 | 0.015 | 2.537 | 1.209-5.342 | 0.014 |
| Vascular invasion(no/yes) | 555/387 | 0.003 | 1.995 | 0.976-4.079 | 0.058 |
| Preoperative CA19-9 level(< 37 U/mL/≥37 U/mL) | 468/432 | 0.420 | - | - | - |
| SMC1A(high/low expression) | 321/555 | 0.011 | 2.204 | 1.011-5.207 | 0.047 |
2.4 SMC1A在胰腺癌细胞系中表达以及SMC1A干扰效果验证
结果显示,SMC1A在转移性胰腺癌细胞AsPC-1及SW1990中蛋白和mRNA表达明显高于原发胰腺癌细胞(PANC-1和BxPC-3,均P < 0.01),见图 3。提示SMC1A表达与胰腺癌侵袭转移密切相关。AsPC-1和SW1990细胞中SMC1A干扰实验结果显示,siRNA组SMC1A蛋白和mRNA表达水平均显著低于其对照组,见图 4。

|
| 1,AsPC-1 cells;2,SW1990 cells;3,PANC-1 cells;4,BxPC-3 cells. A,SMC1A protein expression;B,SMC1A mRNA expression. ** P < 0.01 vs AsPC-1 group. 图 3 不同胰腺癌细胞中SMC1A蛋白及mRNA表达比较 Fig.3 Comparison of SMC1A protein and mRNA expression in different pancreatic cancer cells |
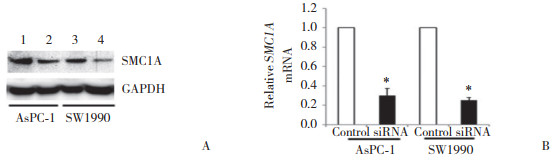
|
| 1,control group of AsPC-1 cells;2,siRNA of AsPC-1 cells;3,control group of SW1990 cells;4,siRNA of SW1990 cells.*P < 0.05 vs control group in the same cells. A,SMC1A protein expression;B,SMC1A mRNA expression. 图 4 AsPC-1和SW1990细胞中SMC1A干扰后表达情况 Fig.4 Expression of SMC1A after interference in AsPC-1 and SW1990 cells |
2.5 SMC1A干扰对胰腺癌细胞增殖的影响
结果显示,在AsPC-1和SW1990中与对照组比较,转染后3~5 d SMC1A siRNA组细胞增殖率显著降低(均P < 0.05),见图 5。
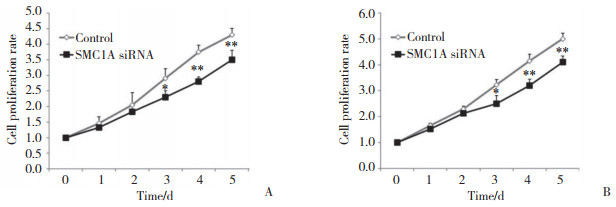
|
| A,AsPC-1 cells;B,SW1990 cells. *P < 0.05,** P < 0.01 vs control group. 图 5 SMC1A干扰对胰腺癌细胞增殖的影响 Fig.5 The effect of SMC1A interference on the proliferation of pancreatic cancer cells |
3 讨论
SMC1A是凝聚蛋白复合体重要亚基,在染色体凝聚过程中防止姐妹染色单体提前分裂,维护子细胞染色体稳定性。SMC1A表达失调可引起染色体不稳定,与肿瘤发生密切相关[6-7]。本研究结果显示,胰腺癌组织中SMC1A表达明显升高,且SMC1A高表达与肿瘤大小、T分期及UICC分期正相关(均P < 0.05);同时SMC1A表达是影响胰腺癌预后的独立危险因素。已有研究显示,SMC1A依据肿瘤类型不同发挥不同生物学作用。ZHANG等[5]研究显示在78例肝癌组织中SMC1A呈明显高表达,并且与肝癌患者临床分期及不良预后密切相关。WANG等[8]在427例大肠癌组织中同样发现SMC1A高表达,其高表达与患者淋巴结转移、TNM分期、远处转移及不良预后显著正相关。HÖMME等[9]在116例急性髓性白血病患者中发现SMC1A呈明显低表达,并与患者不良预后密切相关。本研究发现SMC1A表达与胰腺癌细胞转移密切相关,同时SMC1A干扰抑制了胰腺癌细胞增殖。与以往研究[3, 5]结果一致。已有研究[10]显示,前列腺癌中SMC1A的干扰抑制了PC-3和DU145细胞的生长、菌落形成和细胞迁移能力。可见,实体肿瘤中SMC1A扮演癌基因角色,其表达失调可促进肿瘤的进展。
综上所述,SMC1A在胰腺癌中呈高表达,且SMC1A表达与肿瘤大小、T分期、UICC分期及不良预后密切相关,其机制可能是SMC1A表达促进了胰腺癌细胞增殖。目前,胰腺癌恶性生物学行为的分子机制尚不明确,SMC1A可作为胰腺癌靶向治疗的有效靶点,但有待于进一步研究论证。
| [1] |
CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [2] |
RIBEIRO-SILVA C, VERMEULEN W, LANS H. SWI/SNF: complex complexes in genome stability and cancer[J]. DNA Repair (Amst), 2019, 77: 87-95. DOI:10.1016/j.dnarep.2019.03.007 |
| [3] |
SAROGNI P, PALUMBO O, SERVADIO A, et al. Overexpression of the cohesin-core subunit SMC1A contributes to colorectal cancer development[J]. J Exp Clin Cancer Res, 2019, 38(1): 108. DOI:10.1186/s13046-019-1116-0 |
| [4] |
YADAV S, KOWOLIK CM, LIN M, et al. SMC1A is associated with radioresistance in prostate cancer and acts by regulating epithelial-mesenchymal transition and cancer stem-like properties[J]. Mol Carcinog, 2019, 58(1): 113-125. DOI:10.1002/mc.22913 |
| [5] |
ZHANG Y, YI F, WANG L, et al. Phosphorylation of SMC1A promotes hepatocellular carcinoma cell proliferation and migration[J]. Int J Biol Sci, 2018, 14(9): 1081-1089. DOI:10.7150/ijbs.24692 |
| [6] |
盛伟伟, 董明, 周建平, 等. Numb、MDM2和p53在胰腺癌中表达的相关性及临床病理学意义[J]. 中华外科杂志, 2014, 1(9): 675-681. DOI:10.3760/cma.j.issn.0529-5815.2014.09.011 |
| [7] |
YANG Y, ZHANG ZX, WANG RZ, et al. siRNA-mediated knockdown of SMC1A expression suppresses the proliferation of glioblastoma cells[J]. Mol Cell Biochem, 2013, 381(1/2): 209-215. DOI:10.1007/s11010-013-1704-9 |
| [8] |
WANG J, YU S, CUI L, et al. Role of SMC1A overexpression as a predictor of poor prognosis in late stage colorectal cancer[J]. BMC Cancer, 2015, 15: 90. DOI:10.1186/s12885-015-1085-4 |
| [9] |
HÖMME C, KRUG U, TIDOW N, et al. Low SMC1A protein expression predicts poor survival in acute myeloid leukemia[J]. Oncol Rep, 2010, 24(1): 47-56. DOI:10.3892/or_00000827 |
| [10] |
YADAV S, KOWOLIK CM, LIN M, et al. SMC1A is associated with radioresistance in prostate cancer and acts by regulating epithelial-mesenchymal transition and cancer stem-like properties[J]. Mol Carcinog, 2019, 58(1): 113-125. DOI:10.1002/mc.22913 |
 2021, Vol. 50
2021, Vol. 50




