文章信息
- 陈芳萍, 王琪, 郑丹, 官志忠, 楼迪栋
- CHEN Fangping, WANG Qi, ZHENG Dan, GUAN Zhizhong, LOU Didong
- 二十二碳六烯酸对甲基苯丙胺诱导的人神经母细胞瘤细胞神经毒性的影响
- Effects of docosahexaenoic acid on neurotoxicity induced by methamphetamine in human neuroblastoma cells
- 中国医科大学学报, 2021, 50(10): 904-909
- Journal of China Medical University, 2021, 50(10): 904-909
-
文章历史
- 收稿日期:2020-11-16
- 网络出版时间:2021-09-29 19:29
2. 贵阳市妇幼保健院围产期保健科, 贵阳 550003;
3. 贵州医科大学地方病与少数民族疾病教育部重点实验室, 贵州省医学分子生物学重点实验室, 贵阳 550004;
4. 贵州中医药大学基础医学院法医学教研室, 贵阳 550025
2. Department of Perinatal Health Care, Guiyang Maternal and Child Health Care Hospital, Guiyang 550003, China;
3. Key Laboratory of Endemic and Ethnic Diseases, Ministry of Education, Guizhou Medical University, Key Laboratory of Medical Molecular Biology, Guizhou Province, Guiyang 550004, China;
4. Department of Forensic Medicine, College of basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, China
甲基苯丙胺(methamphetamine,METH)俗称“冰毒”,能够引起强烈的精神兴奋,是一种极易成瘾的新型合成毒品[1]。研究证实,METH作用于多个存在奖励机制的大脑区域(边缘系统、海马体、伏隔核),主要通过促进单胺类神经递质的释放及抑制其再摄取导致成瘾[2]。长期吸食METH可导致意识障碍、幻听、幻视、抑郁等临床症状,显示了METH的神经毒性作用[3-5]。METH诱导的神经毒性主要表现为多种神经细胞的细胞炎症、细胞凋亡和氧化应激。METH精神依赖性强、药效持久且价格相对低廉,因此成为世界范围内的主要毒品之一。而METH长期滥用导致的神经毒性及成瘾性给公共健康带来沉重负担[6-7]。
二十二碳六烯酸(docosahexaenoic acid,DHA)是一种ω-3不饱和脂肪酸,俗称“脑黄金”,是生物膜中磷脂的重要成分,对人类神经细胞的生长及功能维持起着重要作用。DHA在机体内代谢可衍生成炎症抑制介质消退素和保护素。消退素和保护素调控各种黏附分子和炎症介质的表达和释放,不仅具有强效的抗炎促消退效应,还具有神经保护作用[8]。本研究主要采用蛋白质组学方法研究并鉴定人神经母细胞瘤SH-SY5Y细胞蛋白表达和相关信号通路的变化,探讨DHA在METH诱导的神经毒性中的作用及其作用机制。
1 材料与方法 1.1 材料SH-SY5Y细胞株购自美国Sigma公司。主要试剂包括METH(美国Cerilliant公司,标准品:M-009,纯度99.9 %)、DHA(美国Sigma公司)、DMEM∶F12细胞培养基(美国Gibco公司)、annexin V-FITC双染凋亡试剂盒(江苏凯基生物公司)。主要仪器包括流式细胞仪(美国Beckman公司)、LC-20AB液相系统(日本Shimadzu公司)、UltiMate 3000液相色谱仪、Q-Exactive HF X串联质谱仪(美国Thermo Fisher Scientific公司)。
1.2 方法 1.2.1 细胞培养与分组配制含89 % DMEM∶F12、10 % 胎牛血清、1 % 双抗的细胞培养液,1~2 d换液1次,取对数生长期的细胞进行传代。根据前期实验[9]结果,设置METH的作用浓度为2.0 mmol·L-1,作用时间为24 h,将同时段培养的处于对数生长期的SH-SY5Y细胞分为METH(2.0 mmol·L-1)组、METH(2.0 mmol·L-1)+DHA(50 μmol·L-1)组、Control组(不含METH与DHA)[10-11]。用相应的含METH和DHA的培养基处理各组细胞,37 ℃、5 % CO2、饱和湿度的培养箱中培养24 h。
1.2.2 Tandem Mass Tags(TMT)标记定量蛋白组学收集每个样本中的细胞液,加入终浓度10 mmol·L-1的二硫苏糖醇(DL-dithiothreitol,DTT),利用超声仪破碎裂解,4 ℃ 25 000 g离心15 min,取上清。上清中加入终浓度55 mmol·L-1的碘代乙酰胺(iodoacetamide,IAA),暗室放置45 min。4 ℃ 25 000 g离心15 min,取上清得到蛋白液。将蛋白溶液加入Trypsin酶,涡旋振荡后,低速离心1 min,37 ℃孵育2 h。
将酶切并除盐后的肽段与TMT溶液(0.8 mg)快速混合均匀,震荡离心,室温放置2 h;使用LC-20AB液相系统,对样品进行液相分离。经过液相分离的肽段通过nanoESI源离子化后进入串联质谱仪Q-Exactive HF X进行data-dependent acquisition(DDA)模式检测。
1.2.3 生物信息学分析在Mascot Percolator算法的基础上对标记的多肽与同位素标签进行定量分析,使FDR≤0.01。蛋白质相互作用网络应用String(https://string-db.org)进行分析,置信度为0.7。
1.2.4 形态学观察与细胞长短轴比值计算显微镜下观察SH-SY5Y的形态,记录各组图像;测量各组细胞的长轴与短轴,计算比值[12]。
1.2.5 SH-SY5Y细胞凋亡水平检测用不含乙二胺四乙酸(ethylenediamine tetraacetic acid,EDTA)的胰酶消化培养板中的细胞1 min,温和吹打并收集,磷酸盐缓冲溶液(phosphate-buffered saline,PBS)漂洗3次后悬浮于结合缓冲液中,加入annexin V-FITC/PI双染料,避光染色15 min,采用流式细胞仪检测细胞凋亡水平。
1.3 统计学分析应用SPSS 24.0软件进行统计分析,计量资料以x±s表示,2组间比较采用t检验;多组间比较采用单因素方差分析,组间两两比较采用Bonferroni校正。P < 0.05为差异有统计学意义。
2 结果 2.1 DHA可改善METH引起的SH-SY5Y细胞形态学变化结果显示,Control组SH-SY5Y细胞呈长梭形,具有较长的神经突。METH组SH-SY5Y细胞神经突缩短,胞体萎缩,呈圆形;METH+DHA组SH-SY5Y细胞神经突皱缩情况有所改善,见图 1。不同组SH-SY5Y细胞长轴与短轴比值的分析结果显示,METH组(1.48±0.09)较对照组(3.16±0.17)减小(P < 0.05),METH+DHA组(2.09±0.13)较METH组增大(P < 0.05)。
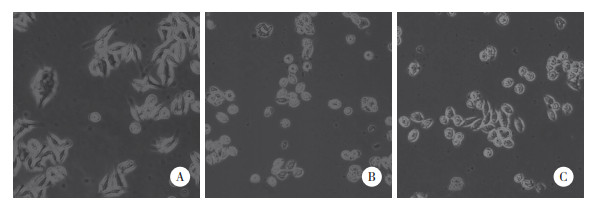
|
| A, Control group; B, METH group; C, METH+DHA group. 图 1 各组SH-SY5Y细胞形态学比较×200 Fig.1 Comparison of the morphology of SH-SY5Y cells in each group ×200 |
2.2 DHA缓解METH诱导的SH-SY5Y细胞凋亡
流式细胞术结果显示,与对照组[Q2:(0.91±0.23)%;Q3:(3.09±0.32)%]比较,METH组[Q2:(3.24±0.27)%;Q3:(5.72±0.74)%]早期凋亡细胞(Q3)和晚期凋亡细胞(Q2)比例升高,且总凋亡细胞(Q2+Q3)与对照组[(4.01±0.53)%]比较,METH组[(8.96±0.60)%]比例也明显升高,差异有统计学意义(P < 0.05)。与METH组比较,METH+DHA组晚期凋亡细胞[(1.53±0.20)%]和总凋亡细胞[(5.63±0.51)%]细胞比例降低,差异有统计学意义(P < 0.05);而早期凋亡细胞[(4.10±0.52)%]比例改变没有统计学差异(P > 0.05),但仍表现出了降低的趋势,见图 2。可见METH具有神经毒性作用,同时也表明DHA对经METH处理的SH-SY5Y细胞具有神经保护作用。
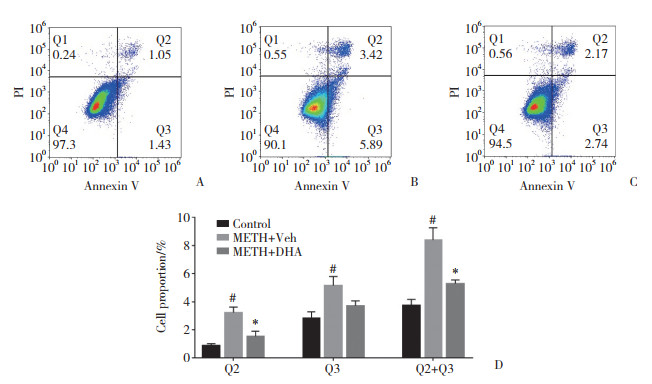
|
| A, Control group; B, METH group; C, METH+DHA group; D, statistical analysis of the cell proportion. # P < 0.05 vs Control group, * P < 0.05 vs METH group. 图 2 流式细胞术分析SH-SY5Y细胞凋亡 Fig.2 Analysis of apoptosis in SH-SY5Y cells by flow cytometry |
2.3 差异表达蛋白(differentially expressed proteins,DEPs)分析
定量质谱共检测到的767 173个肽段,以1%为标准的肽段匹配率(peptide-spectrum matches,PSM)和假阳性率(false discovery rate,FDR)判断,共识别出6 543个蛋白。以倍数变化 > 1.200视为上调蛋白,倍数变化 < 0.833视为下调蛋白。与对照组比较,METH组蛋白谱发生了显著变化,上调蛋白1 249个(19.09%),下调蛋白1 503个(22.97%)。在METH+DHA组和对照组的比较中,共识别出2 754个DEPs,其中1 243个上调,1 511个下调。在METH+DHA组和METH组的比较中,共识别出70个DEPs,其中36个上调,34个下调,见图 3。
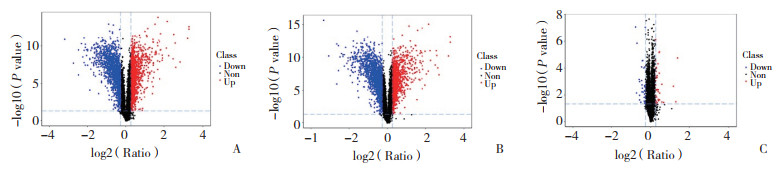
|
| A, METH group vs Control group; B, METH+DHA group vs Control group; C, METH+DHA group vs METH group. Each dot presented one single protein: blue dots for down-regulated protein, red dots for up-regulated protein. 图 3 Control组、METH组和METH+DHA组的蛋白全谱比较 Fig.3 Comparison of the protein profiles in the Control, METH, and METH+DHA groups |
2.4 DHA反向调节METH诱导的DEPs(图 4)
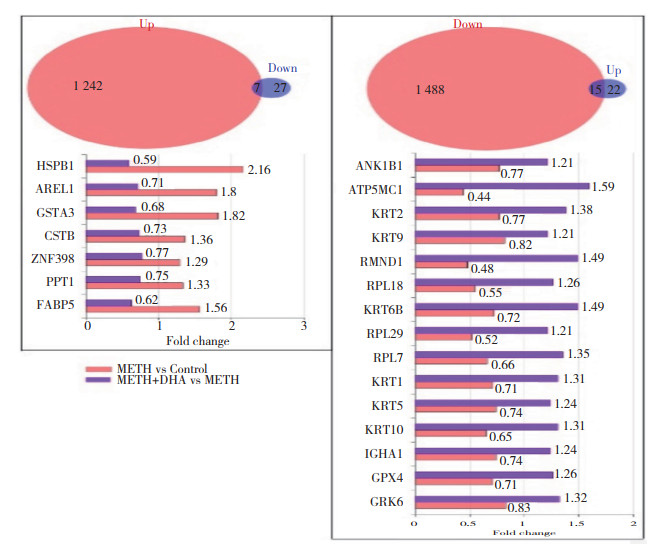
|
| The left panel showed the proteins which were both upregulated in METH group vs Control group and downregulated in METH+DHA group vs METH group. The right panel showed the proteins which were both downregulated in METH group vs Control group and upregulated in METH+DHA group vs METH group. 图 4 DEPs的联合分析 Fig.4 Combination analysis for DEPs |
为进一步了解DHA对METH神经毒性的缓解作用,在METH组与对照组相比得到的DEPs和METH+DHA组与METH组相比得到的DEPs基础上,筛选出两者间调控相反的DEPs。如图 4所示,有7个蛋白既在METH组与对照组比较中上调,又在METH+DHA组与METH组比较中下调;METH组与对照组比较中15个下调蛋白在METH+DHA组与METH组中重新上调。提示此22个反向调节的蛋白可能与DHA缓解METH神经毒性有关。对此22个反向调节蛋白进行相互作用的网络分析结果显示,11个蛋白存在相互作用,其作用分别涉及细胞骨架、核糖体以及谷胱甘肽代谢,见图 5。
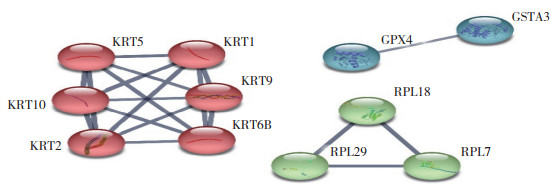
|
| 图 5 反向调节蛋白的互作网络构建 Fig.5 Interaction network of the reversely regulated proteins |
3 讨论
本研究从形态学和细胞凋亡的角度揭示了DHA可缓解METH引起的神经毒性,并运用蛋白质组学技术发现了METH引起的SH-SY5Y蛋白全谱的变化,揭示了DHA在METH神经毒性作用方面的潜在有效靶点。
研究[13-15]发现METH可诱导神经元和内皮细胞的骨架重排,可能诱发神经结构的改变和血脑屏障的破坏。此外,在METH的诱导下,大鼠C6星形胶质细胞也呈现为圆形。本研究结果显示,加入METH后,镜下可见SH-SY5Y细胞质突起明显缩短,趋向于圆形,提示METH可引起神经细胞的形态变化,与以往研究[16]结果一致。另一方面已有研究显示,反复暴露于METH可导致纹状体多巴胺能轴突末梢发生神经退行性病变[17]。在METH作用下小胶质细胞也经历由C/EBP-β活化诱导的凋亡过程[18-19]。本研究中流式细胞术显示了METH显著增加了早期凋亡和晚期凋亡细胞比例,说明了其诱导神经细胞凋亡的神经毒性作用。
DHA是大脑中主要的ω-3多不饱和脂肪酸,由于其独特的神经保护和抗炎特性而备受关注。研究[20]表明,DHA可增加靶标微管蛋白的表达,而微管蛋白是细胞骨架的结构单位。本研究观察到DHA增加了SH-SY5Y细胞神经突的长度,同时使6种角蛋白的表达重新上调。角蛋白作为保持细胞形态稳定的基本单位,可能是DHA缓解METH神经毒性的作用靶标。谷胱甘肽通过其还原性巯基具有抗氧化和解毒作用。研究[21]表明,DHA增加总谷胱甘肽过氧化物酶(glutathione peroxidase,GPX)活性,对于H2O2诱导的氧化应激、凋亡具有保护作用。GPX蛋白家族,尤其是GPX1和GPX4,主要功能是清除H2O2和减少过氧化物的生成,对神经元的活性和功能至关重要[22-23]。本研究结果显示METH降低了SH-SY5Y细胞中GPX4的表达,加入DHA可以显著回调其表达,并缓解神经细胞凋亡,表明DHA可能通过调节GPX4,进一步改善谷胱甘肽代谢,进而缓解METH引起的神经细胞凋亡。
综上所述,DHA可以缓解METH引起的SH-SY5Y细胞的形态及功能的改变,其机制可能与调控角蛋白和GPX4等信号通路密切相关。本研究为戒毒治疗药物研发奠定了基础。然而定量蛋白组学只能宏观检测蛋白质量的变化,并不能反应出具体功能的改变,因此,DHA减弱METH诱导的神经毒性作用的具体机制仍需进一步阐明。
| [1] |
COURTNEY KE, RAY LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature[J]. Drug Alcohol Depend, 2014, 143: 11-21. DOI:10.1016/j.drugalcdep.2014.08.003 |
| [2] |
IMEH-NATHANIEL A, ADEDEJI A, HUBER R, et al. The rewarding properties of methamphetamine in an invertebrate model of drug addiction[J]. Physiol Behav, 2016, 153: 40-46. DOI:10.1016/j.physbeh.2015.10.017 |
| [3] |
杨根梦, 陈逊, 曾晓锋. 甲基苯丙胺诱导神经细胞自噬的研究进展[J]. 2016, 32(10): 1341-1344. DOI: 10.3969/j.issn.1001-1978.2016.10.003.
|
| [4] |
SHAERZADEH F, STREIT WJ, HEYSIEATTALAB S, et al. Methamphetamine neurotoxicity, microglia, and neuroinflammation[J]. J Neuroinflammation, 2018, 15(1): 341. DOI:10.1186/s12974-018-1385-0 |
| [5] |
FOROUGHI K, KHAKSARI M, RAHMATI M, et al. Apelin-13 protects PC12 cells against methamphetamine-induced oxidative stress, autophagy and apoptosis[J]. Neurochem Res, 2019, 44(9): 2103-2112. DOI:10.1007/s11064-019-02847-9 |
| [6] |
SHAO XT, LIU YS, TAN DQ, et al. Methamphetamine use in typical Chinese cities evaluated by wastewater-based epidemiology[J]. Environ Sci Pollut Res, 2020, 27(8): 8157-8165. DOI:10.1007/s11356-019-07504-w |
| [7] |
TAIT RJ, WHETTON S, SHANAHAN M, et al. Quantifying the societal cost of methamphetamine use to Australia[J]. Int J Drug Policy, 2018, 62: 30-36. DOI:10.1016/j.drugpo.2018.08.015 |
| [8] |
JOFFRE C, REY C, LAYÉ S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation[J]. Front Pharmacol, 2019, 10: 1022. DOI:10.3389/fphar.2019.01022 |
| [9] |
牟登峰, 郑丹, 王琪, 等. 甲基苯丙胺对体外培养SH-SY5Y细胞线粒体膜电位、超微结构及Mfn1、Fis1蛋白表达的影响[J]. 中国药理学通报, 2019, 35(7): 935-939. DOI:10.3969/j.issn.1001-1978.2019.07.010 |
| [10] |
PITAKSALEE R, SANVARINDA Y, SINCHAI T, et al. Autophagy inhibition by caffeine increases toxicity of methamphetamine in SH-SY5Y neuroblastoma cell line[J]. Neurotox Res, 2015, 27(4): 421-429. DOI:10.1007/s12640-014-9513-9 |
| [11] |
ZHANG YP, BROWN RE, ZHANG PC, et al. DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells[J]. Prostaglandins Leukot Essent Fat Acids, 2018, 136: 85-94. DOI:10.1016/j.plefa.2017.07.003 |
| [12] |
KAWA A, STAHLHUT M, BEREZIN A, et al. A simple procedure for morphometric analysis of processes and growth cones of neurons in culture using parameters derived from the contour and convex hull of the object[J]. J Neurosci Methods, 1998, 79(1): 53-64. DOI:10.1016/S0165-0270(97)00165-9 |
| [13] |
FERNANDES S, SALTA S, SUMMAVIELLE T. Methamphetamine promotes α-tubulin deacetylation in endothelial cells: the protective role of acetyl-l-carnitine[J]. Toxicol Lett, 2015, 234(2): 131-138. DOI:10.1016/j.toxlet.2015.02.011 |
| [14] |
XUE Y, HE JT, ZHANG KK, et al. Methamphetamine reduces expressions of tight junction proteins, rearranges F-actin cytoskeleton and increases the blood brain barrier permeability via the RhoA/ROCK-dependent pathway[J]. Biochem Biophys Res Commun, 2019, 509(2): 395-401. DOI:10.1016/j.bbrc.2018.12.144 |
| [15] |
YOUNG EJ, BRIGGS SB, MILLER CA. The actin cytoskeleton as a therapeutic target for the prevention of relapse to methamphetamine use[J]. CNS Neurol Disord Drug Targets, 2015, 14(6): 731-737. DOI:10.2174/1871527314666150529145531 |
| [16] |
BADISA RB, WILEY C, RANDELL K, et al. Identification of cytotoxic markers in methamphetamine treated rat C6 astroglia-like cells[J]. Sci Rep, 2019, 9(1): 1-12. DOI:10.1038/s41598-019-45845-1 |
| [17] |
ZHU JP, XU W, ANGULO JA. Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice[J]. Brain Res, 2005, 1049(2): 171-181. DOI:10.1016/j.brainres.2005.04.089 |
| [18] |
HUANG EP, HUANG HY, GUAN TS, et al. Involvement of C/EBPβ-related signaling pathway in methamphetamine-induced neuronal autophagy and apoptosis[J]. Toxicol Lett, 2019, 312: 11-21. DOI:10.1016/j.toxlet.2019.05.003 |
| [19] |
XU X, HUANG EP, LUO BY, et al. Methamphetamine exposure triggers apoptosis and autophagy in neuronal cells by activating the C/EBPβ-related signaling pathway[J]. FASEB J, 2018, 32(12): 6737-6759. DOI:10.1096/fj.201701460rrr |
| [20] |
WANG PY, CHEN JJ, SU HM. Docosahexaenoic acid supplementation of primary rat hippocampal neurons attenuates the neurotoxicity induced by aggregated amyloid beta protein (42) and up-regulates cytoskeletal protein expression[J]. J Nutrition Biochem, 2010, 21(4): 345-350. DOI:10.1016/j.jnutbio.2009.01.012 |
| [21] |
ZHU W, DING Y, KONG W, et al. Docosahexaenoic acid (DHA) provides neuroprotection in traumatic brain injury models via activating Nrf2-ARE signaling[J]. Inflammation, 2018, 41(4): 1182-1193. DOI:10.1007/s10753-018-0765-z |
| [22] |
SU YW, ZHAO B, ZHOU LF, et al. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs[J]. Cancer Lett, 2020, 483: 127-136. DOI:10.1016/j.canlet.2020.02.015 |
| [23] |
BARAYUGA SM, PANG X, ANDRES MA, et al. Methamphetamine decreases levels of glutathione peroxidases 1 and 4 in SH-SY5Y neuronal cells: protective effects of selenium[J]. Neurotoxicology, 2013, 37: 240-246. DOI:10.1016/j.neuro.2013.05.009 |
 2021, Vol. 50
2021, Vol. 50




