文章信息
- 李润洲, 周欣
- LI Runzhou, ZHOU Xin
- ACAP1在卵巢浆液性囊腺癌中的表达及其意义
- Expression and significance of ACAP1 in ovarian serous carcinoma
- 中国医科大学学报, 2021, 50(10): 865-872
- Journal of China Medical University, 2021, 50(10): 865-872
-
文章历史
- 收稿日期:2021-05-19
- 网络出版时间:2021-09-30 11:20
卵巢癌是女性生殖系统常见的恶性肿瘤之一,致死率居妇科恶性肿瘤首位[1-2]。卵巢癌患者早期无明显症状,约3/4的卵巢癌患者首次确诊即为晚期,预后不良[3]。因此,深入研究卵巢癌的发生发展机制,并在此基础上寻找卵巢癌相关的分子标志物,对提高卵巢癌的诊治水平及改善患者的预后具有重要意义。
ACAP1(ADP-ribosylation factor GTPase-activating proteins with coiled-coil,ankyrin repeat and PH domains 1)是ACAP家族成员之一,属于GTP酶激活蛋白(GTPase-activating proteins,GAPs),可调节GTP结合蛋白ADP核糖基化因子-6(ADP-ribosylation factor 6,ARF6)的功能。ARF6在内吞、分泌、吞噬、细胞黏附和迁移中起调节作用[4-8]。ACAP1是ARF6特异性的GAP,可以与ARF6共同参与多种细胞功能的调节。ACAP1还能调节整合素内循环,从而调节细胞迁移[9-14]。研究[15]表明,泛素特异性蛋白酶6(ubiquitin-specific protease 6,USP6)表达下调可能通过影响ARF6/ACAP1依赖的途径抑制细胞增殖和胞质分裂。ACAP1可能与多种疾病相关[16-23],但它与卵巢恶性肿瘤关系的研究较少。因此,本研究应用免疫组化方法检测ACAP1在卵巢浆液性囊腺癌组织及正常卵巢组织中的表达情况,分析其与卵巢癌患者临床病理因素之间的关联,并初步探讨ACAP1在卵巢恶性肿瘤发生发展过程中可能的作用机制。
1 材料与方法 1.1 生物信息学分析通过Oncomine网站(http://www.oncomine.org)分析ACAP1 mRNA水平与卵巢癌的相关性[24];通过cBioportal网站(http://www.cbioportal.org)分析卵巢癌中ACAP1基因突变的类型[25];通过Genemania网站(http://www.genemania.org)分析ACAP1相关信号通路以及相互作用蛋白[26]。
1.2 免疫组化染色收集中国医科大学附属盛京医院2012年9月至2015年9月手术切除且病理诊断为卵巢浆液性囊腺癌的癌组织88例,其中,配对卵巢癌原发及转移灶组织67例,因良性病变切除的卵巢组织16例。纳入标准:术前未经任何治疗;术后病理确诊为卵巢浆液性囊腺癌。排除标准:术前经过放化疗或靶向治疗;转移性卵巢癌;有其他肿瘤病史。本研究获得中国医科大学附属盛京医院伦理委员会批准。
主要试剂包括抗ACAP1多克隆抗体(武汉三鹰生物技术有限公司),兔SP试剂盒(北京中杉金桥生物技术有限公司)。取标本蜡块制5 μm厚连续切片,应用EliVision二步法进行免疫组化染色,依次行烤片、二甲苯脱腊、梯度乙醇水化、抗原修复、PBS缓冲液冲洗、抗体孵育、DAB显色、流水冲洗和苏木素复染等步骤,最终以中性树胶封片。根据着色强度及阳性细胞百分比进行半定量评分。光镜下每张切片随机选取10个高倍视野。先根据着色强度进行评分:无色为0分,淡黄色为1分,棕黄色为2分,棕褐色为3分。再根据阳性细胞百分比评分:阳性细胞百分比≤25%为1分,> 25%~50%为2分,> 50%~75%为3分,> 75%为4分。2项评分相乘,每张切片所有视野评分取平均值,1~2分为(-),3~4分为(+),5~8分为(++),9~12分为(+++)。将5~12分定义为高表达。
1.3 统计学分析采用SPSS 19.0软件进行统计学分析。采用t检验比较ACAP1表达的组间差异。采用χ2检验进行ACAP1表达水平与临床病理因素间的关系分析。采用Kaplan-Meier分析探讨ACAP1在卵巢癌原发灶中的表达水平与总生存期的关系。采用Cox回归对影响卵巢癌预后的临床病理因素进行单因素及多因素分析。P < 0.05为差异有统计学意义。
2 结果 2.1 ACAP1 mRNA在不同癌症中的表达水平应用Oncomine网站分析ACAPs mRNA在不同癌症中的表达情况(图 1,表 1),比较卵巢癌中的7个数据集[27-32],其中2个数据集结果具有统计学意义。ADIB等[27]比较16例卵巢浆液性囊腺癌与4例卵巢癌组织,发现ACAP1在卵巢浆液性囊腺癌组织中的表达上调(p =0.019,fold change=1.389)。BONOME等[30]比较185例卵巢癌组织与10例卵巢组织,发现ACAP1在卵巢癌组织中表达上调(P =1.9e-4,fold change=1.268)。
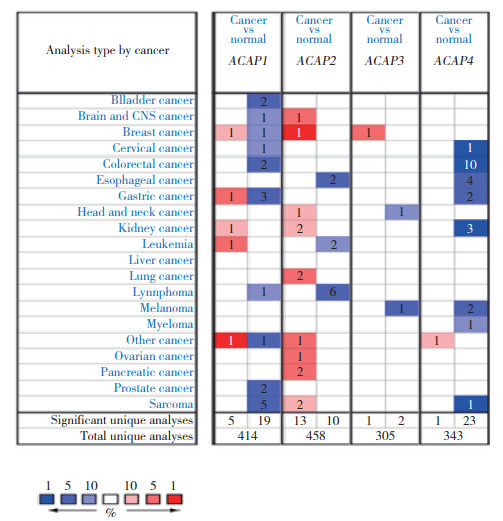
|
| The intensity of color(red or blue) in the figure is proportional to the significant level of upregulation or downregulation of ACAPs mRNA expression, respectively. The threshold parameter was designed with fold change=2 and P = 0.01. The number in the cell represents the number of datasets that meets the threshold. 图 1 ACAPs mRNA在不同癌症中的表达情况 Fig.1 Expression levels of ACAPs mRNA in various cancers |
| Tumor | n | Normal | n | Fold change | t | P | Dataset |
| Ovarian serous adenocarcinoma | 16 | Ovary | 4 | 1.389 | 2.630 | 0.019 | ADIB Ovarian |
| Ovarian serous surface papillary carcinoma | 28 | Ovary | 4 | 2.834 | 1.024 | 0.181 | WELSH Ovarian |
| Ovarian serous adenocarcinoma | 20 | Ovarian surface epithelium | 5 | 1.040 | 0.563 | 0.295 | LU Ovarian |
| Ovarian carcinoma | 185 | Ovarian surface epithelium | 10 | 1.268 | 4.569 | 1.90e-4 | BONOME Ovarian |
| Ovarian serous adenocarcinoma | 25 | Peritoneum | 8 | 1.598 | 1.625 | 0.058 | YOSHIHARA Ovarian |
| Ovarian serous adenocarcinoma | 41 | Ovary | 4 | -1.039 | -0.330 | 0.619 | HENDRIX Ovarian |
| Ovarian serous cystadenocarcinoma | 586 | Ovary | 8 | -1.003 | -0.134 | 0.552 | TCGA Ovarian |
2.2 ACAPs在卵巢癌中的基因变异
应用cBioportal网站检测4个ACAP家族成员,发现存在不同程度的基因变异,ACAP1、ACAP2、ACAP3、ACAP4均存在基因的重复及缺失,ACAPs总变异率为29.85%,其中最多的基因变异为重复,变异率为28.13%(数据集:TCGA,Firehose Legacy)。见图 2。
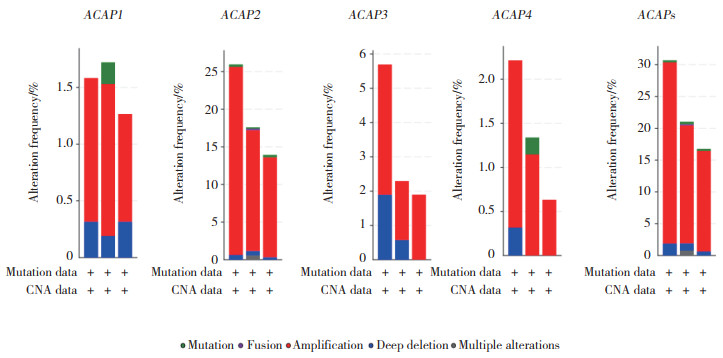
|
| 图 2 ACAPs的基因变异率分析 Fig.2 The analyses of genetic variations of ACAPs |
2.3 ACAP家族基因互作网络的构建。
应用GeneMANIA网站绘制ACAP家族基因相互作用图,同时分析互作蛋白的功能,见图 3。与ACAP家族关系最密切的5个基因是GULP1(engulfment adaptor PTB domain containing 1)、ARF6、RAB11FIP3(RAB11 family interacting protein 3)、SLC2A4(solute carrier family 2 member 4)、VAMP3(vesicle associated membrane protein 3)。GULP1与ACAP1及ARF6存在通路上的联系。ARF6与ACAP1、ACAP2存在通路关系,与ACAP4存在物理互作。这些互作蛋白主要参与肌动蛋白细胞骨架的调节及胞内体回收等功能。
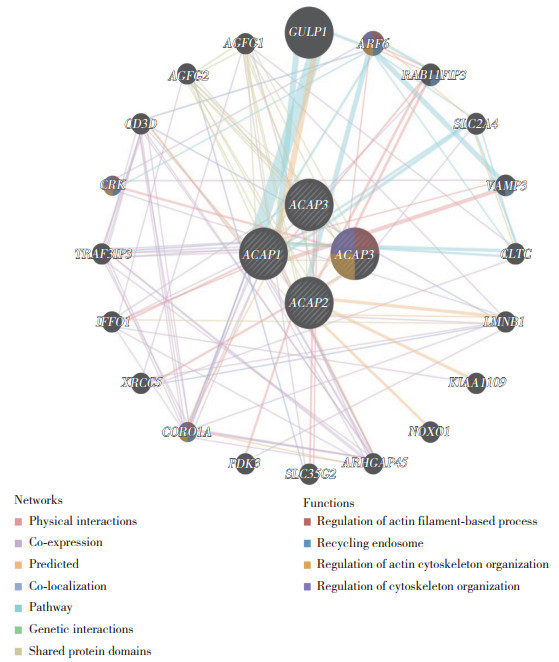
|
| ACAP4 also known as ASAP3. Each node represents a gene, the lines between nodes represent gene-gene interactions, and the color of the lines represents the type of gene-gene interactions. The node color represents the possible function of each gene. 图 3 ACAPs基因互作网络构建 Fig.3 The gene-gene interaction network of ACAPs |
2.4 ACAP1在卵巢浆液性囊腺癌组织和正常卵巢组织中的表达
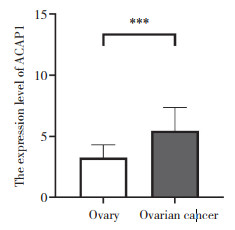
免疫组化结果表明,ACAP1蛋白主要在细胞质中表达,它在卵巢浆液性囊腺癌组织中的表达水平(5.4±1.9)高于正常卵巢组织(3.3±1.1),差异有统计学意义(P < 0.001),见图 4、图 5。
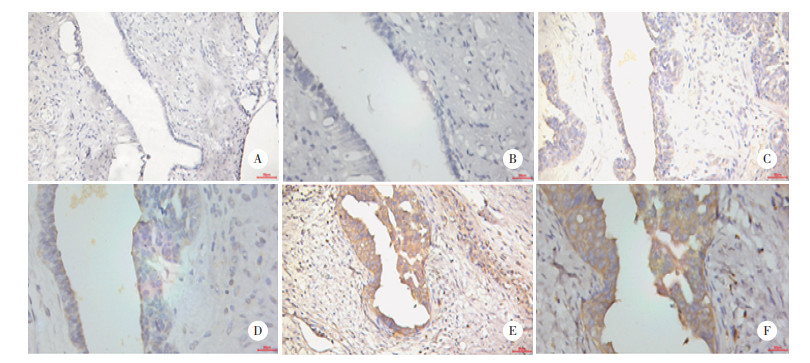
|
| A, B, the expression of ACAP1 in normal ovarian tissues; C, D, the expression of ACAP1 in primary ovarian carcinoma; E, F, the expression of ACAP1 in metastatic lesion of ovarian carcinoma; A, C, E: ×200; B, D, F: ×400. 图 4 ACAP1在正常卵巢组织、卵巢癌原发灶及卵巢癌转移灶中的表达情况 Fig.4 The expression of ACAP1 in normal ovarian tissues, primary ovarian carcinoma and metastatic lesion of ovarian cancer |
|
| *** P < 0.001. 图 5 ACAP1在正常卵巢组织与卵巢癌组织中的表达情况 Fig.5 The expression of ACAP1 in normal ovarian tissues and ovarian carcinoma |
2.5 ACAP1在卵巢癌组织中的表达水平与临床病理因素的关系
根据免疫组化结果评分,将88例卵巢癌分为ACAP1低表达组(-/+)及ACAP1高表达组(++/+++),应用SPSS 19.0软件进行统计分析,结果显示,在年龄、分期、淋巴结转移、大网膜转移、腹膜转移、腹水量等方面,ACAP1高表达组与低表达组间差异均无统计学意义(P > 0.05)。见表 2。
| Characteristics | Low | High | High positive rate(%) | P | |||
| - | + | ++ | +++ | ||||
| Age(year) | 0.715 | ||||||
| < 53 | 3 | 12 | 19 | 5 | 61.5 | ||
| ≥53 | 1 | 16 | 30 | 2 | 65.3 | ||
| FIGO stage | 0.885 | ||||||
| Ⅰ-Ⅱ | 2 | 5 | 11 | 2 | 65.0 | ||
| Ⅲ-Ⅳ | 2 | 23 | 38 | 5 | 63.3 | ||
| CA125(U/mL) | 0.838 | ||||||
| < 1 554 | 3 | 18 | 32 | 6 | 64.4 | ||
| ≥1 554 | 0 | 9 | 17 | 1 | 66.7 | ||
| Lymph node metastasis | 0.562 | ||||||
| No | 2 | 16 | 29 | 5 | 65.4 | ||
| Yes | 1 | 7 | 10 | 1 | 57.9 | ||
| Omentum metastasis | 0.564 | ||||||
| No | 3 | 11 | 19 | 2 | 60.0 | ||
| Yes | 1 | 17 | 30 | 5 | 66.0 | ||
| Peritoneal metastasis | 0.307 | ||||||
| No | 1 | 7 | 9 | 0 | 52.9 | ||
| Yes | 3 | 21 | 40 | 7 | 66.2 | ||
| Ascites(mL) | 0.410 | ||||||
| < 500 | 3 | 13 | 19 | 2 | 56.8 | ||
| ≥500 | 1 | 13 | 25 | 2 | 65.9 | ||
| Some information of CA125,lymph node metastasis and ascites of the patients was missing. | |||||||
2.6 ACAP1在原发灶中的表达与预后的关系
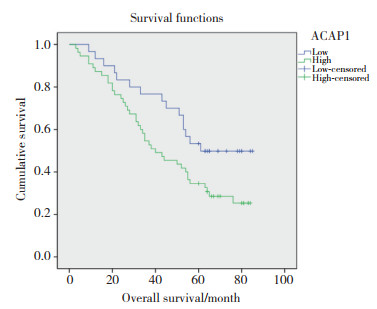
本研究对88例卵巢浆液性癌患者进行随访,随访时间截止至2020年4月30日,实际随访85例,失访3例,随访率为97%。应用IBM SPSS 19.0软件进行统计分析,结果显示,ACAP1在卵巢癌原发灶中的高表达与患者的不良预后相关(P = 0.037)。见图 6。
|
| 图 6 ACAP1在卵巢浆液性囊腺癌原发灶中的表达水平与患者预后的关系 Fig.6 Correlations of ACAP1 expression with the prognosis of patients with serous ovarian carcinoma |
2.7 ACAP1在卵巢浆液性囊腺癌原发灶和转移灶中的表达
免疫组化结果显示,ACAP1在卵巢癌转移灶中的表达水平(7.7±2.0)明显高于原发灶(5.5±1.8),差异有统计学意义(P < 0.001)。
2.8 卵巢浆液性囊腺癌预后相关因素分析以随访死亡或随访结束为观察终点,计算患者总生存期(overall survival OS),应用Cox回归模型进行卵巢癌预后相关因素的单因素及多因素分析。单因素分析结果提示,ACAP1的表达(P = 0.041)、腹水量(P = 0.003)、淋巴结转移(P = 0.001)、FIGO分期(P = 0.001)与患者OS显著相关。多因素分析结果提示,FIGO分期(P = 0.031)是影响卵巢浆液性囊腺癌预后的独立危险因素。见表 3。
| Subgroup | Univariate analysis | Multivariate analysis | |||
| HR(95% CI) | P | HR(95% CI) | P | ||
| ACAP1 expression | 1.858(1.025-3.367) | 0.041 | 1.886(0.926-3.839) | 0.080 | |
| CA125 | 1.637(0.933-2.872) | 0.086 | 1.422(0.668-3.025) | 0.361 | |
| Ascites | 2.492(1.360-4.567) | 0.003 | 1.226(0.566-2.653) | 0.605 | |
| Lymph node metastasi | 2.834(1.504-5.340) | 0.001 | 1.790(0.880-3.639) | 0.108 | |
| FIGO stage | 4.986(1.978-12.568) | 0.001 | 3.245(1.116-9.435) | 0.031 | |
3 讨论
卵巢癌是女性生殖系统常见的恶性肿瘤,致死率居女性生殖系统肿瘤之首[1]。大多数卵巢癌患者一经确诊即为晚期,晚期(Ⅲ~Ⅳ期)患者5年生存率仅为30%~40%,而早期(Ⅰ~Ⅱ期)患者5年生存率可达70%[33-34]。因此,深入研究卵巢癌的发生发展机制,寻找新的诊治靶点,对改善卵巢癌患者预后具有重要意义。本研究通过生物信息学分析及免疫组化实验,探讨了ACAP1在卵巢癌组织中的表达情况,并对其在卵巢癌发生发展中的作用机制进行初步探讨。
免疫组化结果显示,ACAP1在卵巢浆液性囊腺癌组织中的表达水平明显高于正常卵巢组织,提示ACAP1过表达可能与卵巢癌的发生有关。有研究[15]表明,敲除ACAP1可抑制细胞增殖及胞质分裂,提示ACAP1可能参与胞质分裂过程,结合本研究结果,提示ACAP1过表达可能通过促进胞质分裂过程而促进细胞增殖,从而促进卵巢癌的发生。ACAP1是ARF6的特异性GAP [35],2007年MA等[7]在细胞水平验证了ACAP1过表达可以降低ARF6-GTP的水平,而过表达GULP1可部分抑制ACAP1的这种作用。本研究通过GeneMANIA网站构建ACAP1蛋白互作图,结果提示,ACAP1与GULP1存在信号通路及物理互作关系,提示ACAP1可能通过GULP1-ACAP1-ARF6通路促进卵巢癌的发生。
本研究还发现,ACAP1在卵巢癌转移灶中的表达水平高于卵巢癌原发灶,提示ACAP1可能参与卵巢癌的腹腔转移进程。有研究[9-10]通过细胞实验证明敲除ACAP1可抑制细胞迁移,ACAP1被Akt磷酸化后可影响β1整合素内循环,从而促进细胞迁移,提示ACAP1可能通过促进细胞迁移而促进卵巢癌的发展。肿瘤细胞的免疫浸润及肿瘤微环境与肿瘤的发生发展密切相关[36-37]。2020年ZHANG等[20]应用多个生物信息学网站分析ACAP1与卵巢癌肿瘤免疫浸润的关系,结果发现,ACAP1与TILs正相关,ACAP1的表达与多种免疫抑制因子、免疫激活因子、趋化因子相关;通过GSEA数据库分析发现ACAP1与免疫检查点相关;通过Kaplan-Meier plotter网站分析发现,ACAP1高表达与卵巢癌患者预后不良有关。该研究认为,ACAP1与卵巢癌细胞的免疫浸润及肿瘤微环境相关。还有研究[19]发现,ACAP1可能是决定膀胱尿路上皮癌免疫浸润水平的候选基因。以上均表明ACAP1可能与肿瘤细胞的免疫浸润及肿瘤微环境相关,ACAP1可能通过相似的机制促进卵巢癌的腹腔转移。
本研究中,对88例卵巢癌患者进行术后随访,以探讨ACAP1在卵巢癌原发灶中的表达与患者预后的关系。结果显示,ACAP1在卵巢癌原发灶中的高表达与不良预后相关,但ACAP1的表达与患者的年龄、肿瘤分期、CA125水平、淋巴结转移情况、大网膜转移情况及腹膜转移情况无明确相关性。提示ACAP1有可能是影响卵巢癌预后的独立危险因素,但多因素分析结果未能证实此结论,这可能与研究样本数量偏小有关,今后有必要进一步扩大样本量进行分析。
综上所述,本研究首次发现ACAP1蛋白在卵巢浆液性囊腺癌组织中呈高表达,在卵巢癌腹腔转移灶中的表达高于原发灶,原发灶中ACAP1高表达与患者的不良预后相关,提示ACAP1可能参与卵巢癌的发生发展进程,并可能成为卵巢癌诊治的潜在靶点。生物信息学分析结果提示,ACAP1可能通过GULP1-ACAP1-ARF6通路促进卵巢癌的发生。
| [1] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018:globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: A Cancer J Clin, 2018, 68(6): 394-424. DOI:10.3322/caac.21492 |
| [2] |
CHEN WQ, ZHENG RS, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA: A Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [3] |
CORTEZ AJ, TUDREJ P, KUJAWA KA, et al. Advances in ovarian cancer therapy[J]. Cancer Chemother Pharmacol, 2018, 81(1): 17-38. DOI:10.1007/s00280-017-3501-8 |
| [4] |
D'SOUZA-SCHOREY C, CHAVRIER P. ARF proteins: roles in membrane traffic and beyond[J]. Nat Rev Mol Cell Biol, 2006, 7(5): 347-358. DOI:10.1038/nrm1910 |
| [5] |
DONALDSON JG, HONDA A. Localization and function of Arf family GTPases[J]. Biochem Soc Trans, 2005, 33(4): 639-642. DOI:10.1042/bst0330639 |
| [6] |
PASQUALATO S, MÉNÉTREY J, FRANCO M, et al. The structural GDP/GTP cycle of human Arf6[J]. EMBO Rep, 2001, 2(3): 234-238. DOI:10.1093/embo-reports/kve043 |
| [7] |
MA Z, NIE ZZ, LUO RB, et al. Regulation of Arf6 and ACAP1 signaling by the PTB-domain-containing adaptor protein GULP[J]. Curr Biol, 2007, 17(8): 722-727. DOI:10.1016/j.cub.2007.03.014 |
| [8] |
DONALDSON JG, JACKSON CL. Regulators and effectors of the ARF GTPases[J]. Curr Opin Cell Biol, 2000, 12(4): 475-482. DOI:10.1016/S0955-0674(00)00119-8 |
| [9] |
LI J, BALLIF BA, POWELKA AM, et al. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin β1 to control cell migration[J]. Dev Cell, 2005, 9(5): 663-673. DOI:10.1016/j.devcel.2005.09.012 |
| [10] |
CHEN PW, LUO RB, JIAN XY, et al. The Arf6 GTPase-activating proteins ARAP2 and ACAP1 define distinct endosomal compartments that regulate integrin α5β1 traffic[J]. J Biol Chem, 2014, 289(44): 30237-30248. DOI:10.1074/jbc.M114.596155 |
| [11] |
MELLMAN I. Quo vadis: polarized membrane recycling in motility and phagocytosis[J]. J Cell Biol, 2000, 149(3): 529-530. DOI:10.1083/jcb.149.3.529 |
| [12] |
JEAN G. The endocytic pathway: a mosaic of domains[J]. Nat Rev Mol Cell Biol, 2001, 2(10): 721-730. DOI:10.1038/35096054 |
| [13] |
MAXFIELD FR, MCGRAW TE. Endocytic recycling[J]. Nat Rev Mol Cell Biol, 2004, 5(2): 121-132. DOI:10.1038/nrm1315 |
| [14] |
DAI J, LI J, BOS E, et al. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals[J]. Dev Cell, 2004, 7(5): 771-776. DOI:10.1016/j.devcel.2004.10.002 |
| [15] |
RUECKERT C, HAUCKE V. The oncogenic TBC domain protein USP6/TRE17 regulates cell migration and cytokinesis[J]. Biol Cell, 2012, 104(1): 22-33. DOI:10.1111/boc.201100108 |
| [16] |
HOFFMAN JD, GRAFF RE, EMAMI NC, et al. Cis-eQTL-based trans-ethnic meta-analysis reveals novel genes associated with breast cancer risk[J]. PLoS Genet, 2017, 13(3): e1006690. DOI:10.1371/journal.pgen.1006690 |
| [17] |
MILLER CR, PERRY A. Glioblastoma[J]. Arch Pathol Lab Med, 2007, 131(3): 397-406. DOI:10.5858/2007-131-397-g |
| [18] |
ZHANG BG, GU F, SHE CH, et al. Reduction of Akt2 inhibits migration and invasion of glioma cells[J]. Int J Cancer, 2009, 125(3): 585-595. DOI:10.1002/ijc.24314 |
| [19] |
PAN S, ZHAN YH, CHEN XN, et al. Bladder cancer exhibiting high immune infiltration shows the lowest response rate to immune checkpoint inhibitors[J]. Front Oncol, 2019, 9: 1101. DOI:10.3389/fonc.2019.01101 |
| [20] |
ZHANG JW, ZHANG QY, ZHANG J, et al. Expression of ACAP1 is associated with tumor immune infiltration and clinical outcome of ovarian cancer[J]. DNA Cell Biol, 2020, 39(9): 1545-1557. DOI:10.1089/dna.2020.5596 |
| [21] |
YAMAMOTO-FURUSHO JK, BARNICH N, XAVIER R, et al. Centaurin β1 down-regulates nucleotide-binding oligomerization domains 1- and 2-dependent NF-κB activation[J]. J Biol Chem, 2006, 281(47): 36060-36070. DOI:10.1074/jbc.m602383200 |
| [22] |
SUN YN, RONG XF, LU WW, et al. Translational study of Alzheimer's disease (AD) biomarkers from brain tissues in AβPP/PS1 mice and serum of AD patients[J]. J Alzheimer's Dis, 2015, 45(1): 269-282. DOI:10.3233/jad-142805 |
| [23] |
CHAGAS COSTA F, FERREIRA DA CUNHA A, FATTORI A, et al. Gene expression profiles of erythroid precursors characterise several mechanisms of the action of hydroxycarbamide in sickle cell anaemia[J]. Br J Haematol, 2007, 136(2): 333-342. DOI:10.1111/j.1365-2141.2006.06424.x |
| [24] |
RHODES DR, KALYANA-SUNDARAM S, MAHAVISNO V, et al. Oncomine 3.0:genes, pathways, and networks in a collection of 18, 000 cancer gene expression profiles[J]. Neoplasia, 2007, 9(2): 166-180. DOI:10.1593/neo.07112 |
| [25] |
GAO J, AKSOY BA, DOGRUSOZ U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal[J]. Sci Signal, 2013, 6(269): pl1. DOI:10.1126/scisignal.2004088 |
| [26] |
WARDE-FARLEY D, DONALDSON SL, COMES O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function[J]. Nucleic Acids Res, 2010, 38(suppl_2): W214-W220. DOI:10.1093/nar/gkq537 |
| [27] |
ADIB TR, HENDERSON S, PERRETT C, et al. Predicting biomarkers for ovarian cancer using gene-expression microarrays[J]. Br J Cancer, 2004, 90(3): 686-692. DOI:10.1038/sj.bjc.6601603 |
| [28] |
WELSH JB, ZARRINKAR PP, SAPINOSO LM, et al. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer[J]. PNAS, 2001, 98(3): 1176-1181. DOI:10.1073/pnas.98.3.1176 |
| [29] |
LU KH, PATTERSON AP, WANG L, et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis[J]. Clin Cancer Res, 2004, 10(10): 3291-3300. DOI:10.1158/1078-0432.ccr-03-0409 |
| [30] |
BONOME T, LEVINE DA, SHIH J, et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer[J]. Cancer Res, 2008, 68(13): 5478-5486. DOI:10.1158/0008-5472.can-07-6595 |
| [31] |
YOSHIHARA K, TAJIMA A, KOMATA D, et al. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicatesZEB2in tumor progression and prognosis[J]. Cancer Sci, 2009, 100(8): 1421-1428. DOI:10.1111/j.1349-7006.2009.01204.x |
| [32] |
HENDRIX ND, WU R, KUICK R, et al. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of wnt signaling in ovarian endometrioid adenocarcinomas[J]. Cancer Res, 2006, 66(3): 1354-1362. DOI:10.1158/0008-5472.can-05-3694 |
| [33] |
WEBB PM, JORDAN SJ. Epidemiology of epithelial ovarian cancer[J]. Best Pract Res Clin Obstet Gynaecol, 2017, 41: 3-14. DOI:10.1016/j.bpobgyn.2016.08.006 |
| [34] |
FORSTNER R. Early detection of ovarian cancer[J]. Eur Radiol, 2020, 30(10): 5370-5373. DOI:10.1007/s00330-020-06937-z |
| [35] |
JACKSON TR, BROWN FD, NIE ZZ, et al. Acaps are Arf6 gtpase-activating proteins that function in the cell periphery[J]. J Cell Biol, 2000, 151(3): 627-638. DOI:10.1083/jcb.151.3.627 |
| [36] |
CURIGLIANO G. Gyneco-oncological genomics and emerging biomarkers for cancer treatment with immune-checkpoint inhibitors[J]. Semin Cancer Biol, 2018, 52: 253-258. DOI:10.1016/j.semcancer.2018.05.004 |
| [37] |
CHOY CT, WONG CH, CHAN SL. Embedding of genes using cancer gene expression data: biological relevance and potential application on biomarker discovery[J]. Front Genet, 2019, 9: 682. DOI:10.3389/fgene.2018.00682 |
 2021, Vol. 50
2021, Vol. 50




