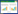文章信息
- 李阳, 姜凌峰, 廉飞玥, 樊保良, 邵雨双, 秦宇
- LI Yang, JIANG Lingfeng, LIAN Feiyue, FAN Baoliang, SHAO Yushuang, QIN Yu
- miR-125b在小鼠白内障模型与人晶状体上皮细胞凋亡模型中的调控作用
- Investigations on the regulatory role of microRNA-125b in a mouse cataract model and human lens epithelial cell apoptosis model
- 中国医科大学学报, 2020, 49(9): 769-775
- Journal of China Medical University, 2020, 49(9): 769-775
-
文章历史
- 收稿日期:2019-12-20
- 网络出版时间:2020-09-11 8:57
白内障作为全球首位致盲性眼病, 严重影响患者的视觉质量与生活质量[1]。虽然手术是白内障目前最为有效的治疗方法, 但存在手术禁忌证、并发症、后发性白内障等诸多问题。因此, 非手术靶向治疗白内障的研究具有重要的社会效益和经济效益。
在种类众多的非编码RNA中, 微小RNA (microRNA, miRNA)是一类长20~25个核苷酸的单链非编码RNA, 通过直接与靶基因mRNA 3’非编码区结合的经典途径以及靶向调控其他非编码RNA的前体RNA等非经典途径, 进行细胞内调控[2-3]。研究[4]表明, miR-125b与细胞凋亡密切相关, 而晶状体上皮细胞凋亡是非先天性白内障形成的共同细胞学基础[5]。本研究组前期利用生物信息学软件预测到促凋亡蛋白BCL-2拮抗剂/杀手1 (BCL-2 antagonist killer 1, BAK1)可能与miR-125b存在直接结合位点。BAK1属于促凋亡家族BCL-2, 定位于线粒体的外膜上, 是通过线粒体通路传递凋亡信号的必要组分[6-7]。因此, 本研究拟观察miR-125b在紫外线诱导的小鼠白内障模型与人晶状体上皮细胞凋亡模型中的表达, 及其对预测靶基因BAK1与人晶状体上皮细胞凋亡的调控作用。
1 材料与方法 1.1 材料人晶状体上皮细胞系SRA01/04由中国医科大学附属第四医院眼科晶状体实验室提供。PrimerScriptTM RT 5 Enzyme Mix、SYBR premix Ex Taq Ⅱ (日本Takara公司);BAK1 (D4E4) Rabbit mAb (美国CST公司);山羊抗兔二抗(美国Proteintech公司);miR-125b mimic、miR-125b mimic control;miR-125b inhibitor、miR-125b inhibitor control (广州锐博生物科技有限公司);RNAiso (美国Invitrogen公司);TUNEL细胞凋亡检测试剂盒(江苏凯基生物技术有限公司);LipofectamineTM RNAiMAX (美国Invitrogen公司)。ABI 7500 real-time pCR系统(美国Applied Biosystems公司);荧光显微镜(日本Olympus公司);XX-15B型紫外线灯、紫外线仪(美国Spectroline Westbury公司)。miR-125b与RNU6B的RT引物、特异性上游引物和通用下游引物由Ribobio公司合成;BAK1和β-actin的特异性上下游引物由生工生物工程(上海)股份有限公司合成。
1.2 方法 1.2.1 人晶状体上皮细胞凋亡模型的构建将SRA01/04细胞用DEME培养基(含10%胎牛血清, 100 U/mL青霉素, 100 μg /mL链霉素)于37℃、5%CO2、100%湿度条件下, 培养至对数生长期, 弃培养液, 加入1 mL PBS。打开培养皿盖, 置于紫外灯下照射25 min, 紫外灯光谱范围在280~320 nm, 峰值为312 nm, 照射强度为360 μW/cm2, 总剂量1.5 J/m2[8]。照射后将PBS更换为DMEM培养基, 并继续培养4 h。
1.2.2 小鼠白内障模型的构建取10只8周龄SPF级C57BL/6小鼠, 照射前5 min用复方托吡卡胺滴眼液点眼扩瞳, 用紫外线UVB (照射强度360 μW/cm2)直接照射左眼, 5 min/次, 1次/d, 连续照射7 d, 总能量2.1 J/m2[9]。右眼不进行任何干预。颈椎脱位法处死小鼠, 收集晶状体囊膜。本研究经中国医科大学附属第四医院伦理委员会批准, 实验动物的使用和喂养遵循ARVO声明。
1.2.3 细胞转染将SRA01/04培养至对数生长期, 细胞密度达30%~50%。用LipofectamineTM RNAiMAX将50 nmol/L的miR-125b mimic、mimic control和100 nmol/L的miR-125b inhibitor、inhibitor control分别转染至SRA01/04中, 培养48 h后, 用紫外线照射构建人晶状体上皮细胞凋亡模型。
1.2.4 实时qPCR检测miR-125b与BAK1 mRNA的表达用RNAiso提取总RNA, PrimerScriptTM RT 5 Enzyme Mix反转录为cDNA。用SYBR premix Ex TaqⅡ检测miR-125b与BAK1 mRNA的表达水平, RNU6B为miR- 125b内参对照, β-actin为BAK1内参对照。引物序列如下:人β-actin上游引物5’-CTCCATCCTGGCCTCGC TGT-3’, 下游引物5’-GCTGTCACCTTCACCGTTC C-3’;小鼠β-actin上游引物5’-CGGATTTGGTCGTAT TGGG-3’, 下游引物5’-TGCTGGAAGATGGTGATG GGATT-3’;人BAK1上游引物5’-GGGTCTATGTTCC CCAGGAT-3’, 下游引物5’-AGCAGGGGTAGAGTTG AGCA-3’;小鼠Bak1上游引物5’-CCAACATTGCA TGGTGCTAC-3’, 下游引物5’-CAGTGGAGAAAA AGGGTGGA-3’。反应体系为20 μL, 反应条件为95 ℃预变性30 s, 95℃变性5 s, 60℃退火34 s, 共40个循环。数据经3次独立实验后获得, 利用公式RQ=2-ΔΔCt进行计算。
1.2.5 Western blotting检测BAK1蛋白表达水平利用RIPA裂解液提取总蛋白, BCA法检测蛋白浓度。取变性后蛋白30 μg行12% SDS-PAGE凝胶电泳, 湿转法转移至PVDF膜。5%脱脂奶粉室温封闭1 h, 加入一抗(1:1 000稀释), 4 ℃过夜。TBST洗涤3次, 10 min/次。封闭液稀释二抗(1:6 000)后, 常温下孵育2 h。TBST洗涤3次, 10 min/次。ECL发光, GAPDH做内参对照, 采用Image J软件对结果进行灰度分析。
1.2.6 TUNEL法检测细胞凋亡4%多聚甲醛室温固定各组细胞30 min, PBS漂洗3次, 5 min/次;用预冷的100 μL 1%Triton X-100/10 mmol·L-1 PBS处理5 min, PBS漂洗3次;样本周围用吸水纸吸干, 每个样本上滴加50 µL TdT酶反应液, 放入湿盒中, 37 ℃避光反应60 min;PBS漂洗3次后加入Streptavidin-Fluorescein标记液, 放入湿盒, 37 ℃避光反应30 min;DAPI复染2 min, 防淬灭剂封片, 荧光显微镜下观察。结果采用Image J软件进行分析。
1.3 统计学分析采用SPSS 25.0统计软件进行分析。数据以x±s表示, 用独立样本t检验进行比较。P < 0.05为差异有统计学意义。
2 结果 2.1 人晶状体上皮细胞凋亡模型中miR-125b与BAK1的表达(图 1)
|
| A, miR-125b expression in human lens epithelial cell apoptosis model; B, BAK1 mRNA expression in human lens epithelial cell apoptosis model; C, BAK1 protein expression in human lens epithelial cell apoptosis model. *P < 0.05 vs control. 图 1 人晶状体上皮细胞凋亡模型中miR-125b与BAK1的表达 Fig.1 Expression of miR-125b and BAK1 in a human lens epithelial cell apoptosis model |
人晶状体上皮细胞凋亡模型中miR-125b mRNA表达水平显著低于正常人晶状体上皮细胞系(0.452±0.126 5 vs 1.000±0.000 1, t = 7.504, P = 0.017), BAK1 mRNA的表达水平显著高于正常人晶状体上皮细胞系(3.858±0.488 8 vs 1.0±0.001 0, t = 10.13, P = 0.009 6), BAK1蛋白的表达水平显著高于正常人晶状体上皮细胞系(0.68±0.019 vs 0.45±0.017, t = 15.34, P < 0.001), 差异均有统计学意义。
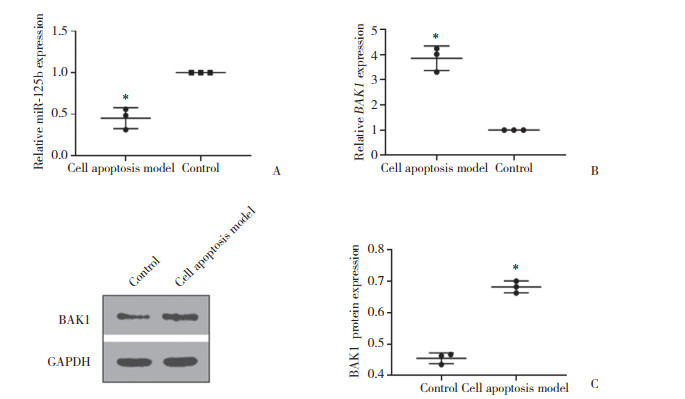
2.2 小鼠白内障模型晶状体囊膜中miR-125b与Bak1的表达(图 2)
|
| A, miR-125b expression in lens capsule of mouse cataract model; B, Bak1 mRNA expression in lens capsule of mouse cataract model; C, Bak1 protein expression in lens capsule of mouse cataract model. **P < 0.01, *P < 0.05 vs control. 图 2 小鼠白内障模型晶状体囊膜中miR-125b与Bak1 mRNA和BAK1蛋白的表达 Fig.2 Expression of miR-125b and Bak1 mRNA and BAK1 protein in lens capsule of mouse cataract model |
小鼠白内障模型晶状体囊膜中, miR-125b mRNA的表达水平显著低于未照射组小鼠(0.301±0.318 3 vs 0.762±0.076 2, t = 9.674, P = 0.000 6), Bak1 mRNA表达水平显著高于未照射组(1.228±0.078 3 vs 1.000±0.001 0, t = 5.042, P = 0.037), Bak1蛋白表达水平显著高于未照射组(1.039±0.053 1 vs 0.512±0.014 7, t = 16.59, P < 0.001), 差异均有统计学意义。
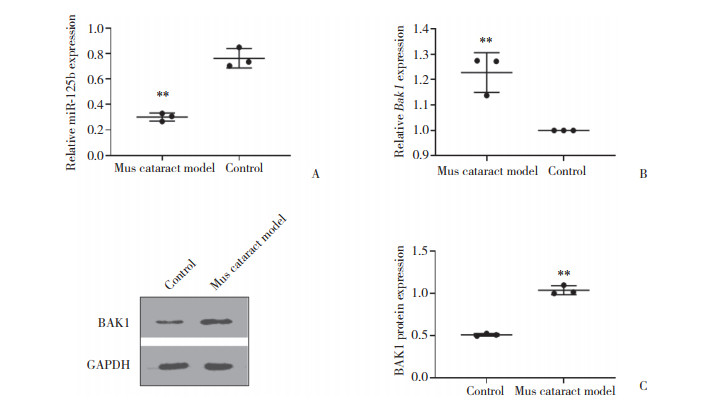
2.3 人晶状体上皮细胞凋亡模型细胞凋亡情况(图 3)
|
| 图 3 人晶状体上皮细胞凋亡模型细胞凋亡检测结果 Fig.3 Apoptosis detection in human lens epithelial cell apoptosis model |
TUNEL结果显示, 人晶状体上皮细胞凋亡模型细胞凋亡率显著高于正常人晶状体上皮细胞[(49.37±5.121) % vs (35.20±3.000) %, t = 4.135, P = 0.001 4], 差异有统计学意义。
2.4 miR-125b对BAK1表达的调控人晶状体上皮细胞凋亡模型中, 转染miR-125b mimic组miR-125b的表达显著高于转染mimic control组, BAK1 mRNA和蛋白的表达水平显著低于转染mimic control组, 差异均有统计学意义;转染miR-125b inhibitor组miR-125b的表达水平显著低于转染inhibitor control组, BAK1 mRNA和蛋白表达水平显著高于转染inhibitor control组, 差异均有统计学意义。见表 1。
| Group | miR-125b mRNA | BAK1 mRNA | BAK1 protein |
| miR-125b mimic | 2 451±376.200 0 | 0.266 5±0.030 0 | 0.074±0.002 0 |
| miR-125b mimic control | 1±0.000 1 | 1.000 0±0.001 | 0.573±0.014 0 |
| t | 11.28 | 42.07 | 60.89 |
| P | 0.007 | < 0.001 | < 0.001 |
| miR-125b inhibitor | 0.000 5±0.000 6 | 3.866±0.237 | 1.142±0.045 |
| miR-125b inhibitor control | 1.000 0±0.000 1 | 1.000±0.001 | 0.069±0.024 |
| t | 20 950 | 20.94 | 15.25 |
| P | < 0.001 | < 0.001 | < 0.001 |
2.5 miR-125b调控人晶状体上皮细胞凋亡
人晶状体上皮细胞凋亡模型中, 转染miR-125b mimic组细胞凋亡较对照组显著减少, 而转染miR-125b inhibitor组细胞凋亡较对照组显著增加。miR-125b mimic组细胞凋亡率显著低于miR-125b mimic control组[(24.97±4.937) % vs (41.53±1.879) %, t = 5.429, P = 0.005 6];miR-125b inhibitor组细胞凋亡率显著高于miR-125b inhibitor control组[71.23±4.700) % vs (53.49±4.064) %, t = 4.006, P = 0.016), 差异均有统计学意义。见图 7。
3 讨论除年龄因素外, 紫外线辐射也是白内障发生的一个重要危险因素。太阳辐射是紫外线的主要来源[10]。按照波长的不同, 紫外线分为紫外线A (波长315~400 nm)、紫外线B (波长280~315 nm)和紫外线C (波长100~280 nm), 部分紫外线B可被角膜吸收, 部分紫外线B和紫外线A可穿过角膜和房水而被晶状体吸收[11]。动物实验证明暴露于紫外线B可导致白内障的发生[12]。体外研究[13]表明, 受到紫外线照射的细胞通过启动细胞内信号转导通路发生细胞凋亡。Liu等[14]发现紫外线能导致晶状体上皮细胞的凋亡。因此, 本研究利用紫外线照射构建了小鼠白内障模型, 模拟白内障发生过程中晶状体上皮细胞及细胞内分子水平的改变。
miRNA是一种小的非编码调节RNA分子, 通过与mRNA的3’非编码区结合, 促进mRNA降解或抑制mRNA的表达, 从而调节基因的表达[15]。miRNA在多种组织中均有表达, 而这些特定miRNA丰度的改变可以调节某些miRNA介导的转录网络, 对包括凋亡在内的病理生理学行为产生影响[16]。研究表明, miRNA与白内障的发生发展有密切联系。KUBO等[17]通过对不同发育阶段的大鼠晶状体以及白内障和正常大鼠晶状体中miRNA进行对比检测发现, let-7c、miR-29a、miR-29c和miR-126表达趋势与年龄增长趋势一致, 白内障对照组中该趋势更为明显, 提示上述miRNA可能在晶状体发育过程中有重要作用。研究[18]发现, 人的透明与浑浊晶状体中let-7b、miR-125b和miR-26a表达显著不同, 提示它们在白内障发生过程中可能起着重要作用。
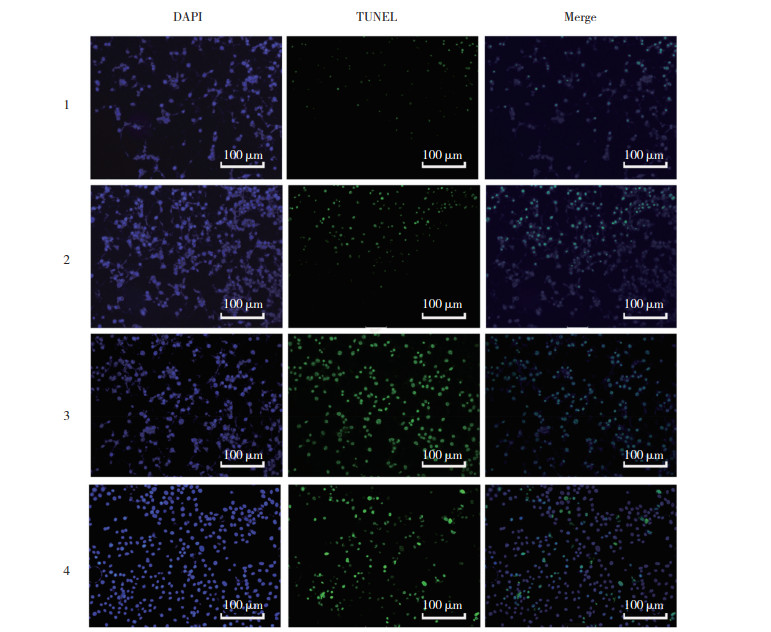
|
| 1, miR-125b mimic group; 2, miR-125b mimic control group; 3, miR-125b inhibitor group; 4, miR-125b inhibitor control group. 图 4 miR-125b对人晶状体上皮细胞系凋亡的调控 Fig.4 Regulation of miR-125b on apoptosis of human lens epithelial cells |
miR-125b是一种广泛存在的miRNA, 与细胞凋亡、增殖、迁移和分化等密切相关[19-20]。ZHU等[21]发现miR-125b上调与脓毒症患者病情加重、炎症和死亡率增加相关。之前关于miR-125b调控机制的研究[22]主要集中于调控野生型p53进而影响细胞的凋亡, 而与BCL-2蛋白家族之间作用机制的研究较少。BCL-2蛋白家族与内部线粒体凋亡途径相关, 包括促凋亡基因BAK1、BAX、BAD等以及抗凋亡基因BCL-2、BCLXL等[23]。本研究组前期通过检索在线数据库Target Scan (http://www.targetscan.org/), miRNA(http://www.microrna.org/microrna) (http://www.microrna.org/microrna/home/Do)及picTar (http://pictar.mde-berlin.de/), 预测在BAK1 3’非编码区存在miR-125b结合位点, 而BAK1作为关键的促凋亡基因, 可能是miR-125b调控细胞凋亡的重要作用机制。
本研究利用紫外线照射构建人晶状体上皮细胞凋亡模型和小鼠白内障模型, 以研究miR-125b在白内障的发生发展过程中可能的作用机制。结果表明, miR-125b在人晶状体上皮细胞凋亡模型与小鼠白内障模型中的表达较对照组显著降低, 而BAK1表达量与miR-125b呈负相关趋势, 且当转染miR-125b mimic后, BAK1表达量显著降低, 细胞凋亡减少, 而转染miR-125b inhibitor后, BAK1表达增加, 细胞凋亡也增加。提示miR-125b可能通过与BAK1结合调控细胞的凋亡, 并可能通过该机制影响白内障的发生和发展。本研究首次证明了在晶状体上皮细胞中, miR-125b通过靶向作用BAK1影响细胞凋亡的作用机制。本研究的不足之处在于未能确切证明miR-125b与BAK1的直接结合关系。miRNA的作用机制十分复杂, 除与编码基因相互作用外, 与非编码基因间同样存在调控网络, 这种庞大的功能网络需要进一步研究探讨。
综上所述, miR-125b在紫外线诱导的小鼠白内障模型和人晶状体上皮细胞凋亡模型中呈低表达, miR-125b可能通过靶向负调控BAK1的表达, 抑制人晶状体上皮细胞凋亡, 从而在白内障的发生发展过程中发挥一定的调控作用, 以上结果可能为非手术靶向治疗白内障提供了新的思路。
| [1] |
SONG PG, WANG H, THEODORATOU E, et al. The national and subnational prevalence of cataract and cataract blindness in China:a systematic review and meta-analysis[J]. J Glob Heal, 2018, 8(1): 010804. DOI:10.7189/jogh.08.010804 |
| [2] |
BARTEL DP. MicroRNAs:genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281-297. DOI:10.1016/S0092-8674(04)00045-5 |
| [3] |
DRAGOMIR MP, KNUTSEN E, CALIN GA. SnapShot:unconventional miRNA functions[J]. Cell, 2018, 174(4): 1038-1038.e1. DOI:10.1016/j.cell.2018.07.040 |
| [4] |
ZHAO Y, LI X, ZHU SQ. Rs78378222 polymorphism in the 3'-untranslated region of TP53 contributes to development of age-associated cataracts by modifying microRNA-125b-induced apoptosis of lens epithelial cells[J]. Mol Med Rep, 2016, 14(3): 2305-2310. DOI:10.3892/mmr.2016.5465 |
| [5] |
LI WC, KUSZAK JR, DUNN K, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals[J]. J Cell Biol, 1995, 130(1): 169-181. DOI:10.1083/jcb.130.1.169 |
| [6] |
HAUSEMAN ZJ, HARVEY EP, NEWMAN CE, et al. Homogeneous oligomers of pro-apoptotic BAX reveal structural determinants of mitochondrial membrane permeabilization[J]. Mol Cell, 2020. DOI:10.1016/j.molcel.2020.05.029 |
| [7] |
WEI MC, ZONG WX, CHENG EH, et al. Proapoptotic BAX and BAK:a requisite gateway to mitochondrial dysfunction and death[J]. Science, 2001, 292(5517): 727-730. DOI:10.1126/science.1059108 |
| [8] |
HE J, LONG CD, HUANG ZX, et al. PTEN reduced UVB-mediated apoptosis in retinal pigment epithelium cells[J]. Biomed Res Int, 2017, 2017: 3681707. DOI:10.1155/2017/3681707 |
| [9] |
MESA R, BASSNETT S. UV-B-induced DNA damage and repair in the mouse lens[J]. Invest Ophthalmol Vis Sci, 2013, 54(10): 6789. DOI:10.1167/iovs.13-12644 |
| [10] |
HUA H, YANG TY, HUANG LT, et al. Protective effects of lanosterol synthase up-regulation in UV-B-induced oxidative stress[J]. Front Pharmacol, 2019, 10: 947. DOI:10.3389/fphar.2019.00947 |
| [11] |
LÖFGREN S. Solar ultraviolet radiation cataract[J]. Exp Eye Res, 2017, 156: 112-116. DOI:10.1016/j.exer.2016.05.026 |
| [12] |
GALICHANIN K, LÖFGREN S, BERGMANSON J, et al. Evolution of damage in the lens after in vivo close to threshold exposure to UV-B radiation:cytomorphological study of apoptosis[J]. Exp Eye Res, 2010, 91(3): 369-377. DOI:10.1016/j.exer.2010.06.009 |
| [13] |
SU WC, WANG L, FU XT, et al. Protective effect of a fucose-rich fucoidan isolated from Saccharina japonica against ultraviolet B-induced photodamage in vitro in human keratinocytes and in vivo in zebrafish[J]. Mar Drugs, 2020, 18(6): 316. DOI:10.3390/md18060316 |
| [14] |
LIU K, ZHAO JF, YANG LS, et al. Protective effects of calbindin-D28K on the UVB radiation-induced apoptosis of human lens epithelial cells[J]. Int J Mol Med, 2020, 45(6): 1793-1802. DOI:10.3892/ijmm.2020.4552 |
| [15] |
GU XL. MicroRNA-124 prevents H2O2-induced apoptosis and oxidative stress in human lens epithelial cells via inhibition of the NF-κB signaling pathway[J]. Pharmacology, 2018, 102(3/4): 213-222. DOI:10.1159/000491433 |
| [16] |
MOHR A, MOTT J. Overview of microRNA biology[J]. Semin Liver Dis, 2015, 35(1): 003-011. DOI:10.1055/s-0034-1397344 |
| [17] |
KUBO ER, HASANOVA N, SASAKI H, et al. Dynamic and differential regulation in the microRNA expression in the developing and mature cataractous rat lens[J]. J Cell Mol Med, 2013, 17(9): 1146-1159. DOI:10.1111/jcmm.12094 |
| [18] |
WU CR, YE M, QIN L, et al. Expression of lens-related microRNAs in transparent infant lenses and congenital cataract[J]. Int J Ophthalmol, 2017, 361-365. DOI:10.18240/ijo.2017.03.06 |
| [19] |
ZHENG Z, QU JQ, YI HM, et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-κB signaling pathway[J]. Cell Death Dis, 2017, 8(6): e2855. DOI:10.1038/cddis.2017.211 |
| [20] |
NIE J, JIANG HC, ZHOU YC, et al. MiR-125b regulates the proliferation and metastasis of triple negative breast cancer cells via the Wnt/β-catenin pathway and EMT[J]. Biosci Biotechnol Biochem, 2019, 83(6): 1062-1071. DOI:10.1080/09168451.2019.1584521 |
| [21] |
ZHU XP. MiR-125b but not miR-125a is upregulated and exhibits a trend to correlate with enhanced disease severity, inflammation, and increased mortality in sepsis patients[J]. J Clin Lab Anal, 2020, 34(3): e23094. DOI:10.1002/jcla.23094 |
| [22] |
QIN Y, ZHAO JY, MIN XJ, et al. MicroRNA-125b inhibits lens epithelial cell apoptosis by targeting p53 in age-related cataract[J]. Biochim Biophys Acta, 2014, 1842(12): 2439-2447. DOI:10.1016/j.bbadis.2014.10.002 |
| [23] |
Huska JD, Lamb HM, Hardwick JM. Overview of BCL-2 family proteins and therapeutic potentials[J]. Methods Mol Biol, 2019, 1877: 1-21. DOI:10.1007/978-1-4939-8861-7_1 |
 2020, Vol. 49
2020, Vol. 49