文章信息
- 樊晨星, 王秀艳, 赵千, 赖红, 李昆
- FAN Chenxing, WANG Xiuyan, ZHAO Qian, LAI Hong, LI Kun
- 丹参酮ⅡA诱导人胎盘间充质干细胞向心肌细胞分化、提高预分化细胞归巢能力的研究
- Study of tanshinoneⅡA induction of human placental mesenchymal stem cell differentiation into cardiomyocytes and the enhanced homing of pre-differentiated cells
- 中国医科大学学报, 2020, 49(4): 294-300
- Journal of China Medical University, 2020, 49(4): 294-300
-
文章历史
- 收稿日期:2019-05-21
- 网络出版时间:2020-04-16 9:50
2. 锦州医科大学附属第三医院检验科, 辽宁 锦州 121001;
3. 中国医科大学基础医学院解剖学教研室, 沈阳 110122
2. Department of Laboratory Medicine, the Third Affiliated Hospital of Jinzhou Medical University, Jinzhou 121001, China;
3. Department of Anatomy, College of Basic Medical Science, China Medical University, Shenyang 110122, China
利用干细胞的分化潜能再生心肌细胞已成为治疗缺血性心脏病的一种新策略。由于心肌分化调控的复杂性,无法保证移植入体内的干细胞会向心肌细胞分化的同时不伴有基因突变的发生,因此,移植心肌前体细胞较移植干细胞更为安全有效[1]。目前,人体来源的干细胞定向分化为心肌细胞主要有2种方法,一是通过与异种动物心肌细胞共培养的方法,模拟心肌微环境,促进干细胞向心肌细胞分化[2];一是通过小分子化合物,如5-氮胞苷等,诱导干细胞向心肌细胞分化[3]。但前者容易将外源性有害基因带入人体,后者由于剧毒性使其使用剂量受限,导致诱导效率低下。因此,亟需寻找一种更为安全有效的心肌分化诱导剂。丹参酮ⅡA是我国传统中药丹参的有效成分,因其具有心血管保护作用,广泛应用于缺血性心脏病的治疗[4]。前期研究[5-7]发现,丹参酮ⅡA能够诱导人胎盘间充质干细胞(human placenta-derived mesenchymal stem cells,hPDMSCs)向心肌细胞分化,效果优于共培养法和5-氮胞苷诱导法。本研究拟评估丹参酮ⅡA对hPDMSCs向心肌细胞分化及对预分化细胞归巢能力的影响,并探讨其相关机制。
1 材料与方法 1.1 材料丹参酮ⅡA粉末(20 mg,纯度≥98%)购自上海江莱生物科技有限公司;β-catenin激动剂WAY-262611(1 mg粉末,纯度≥99.09%)购自上海陶素生化科技有限公司;胎牛血清、DMEM低糖培养基、DMEM高糖培养基和青链霉素购自美国Gibco公司;抗心肌转录调节因子4(GATA-binding protein 4,GATA-4)、抗心钠素(atrial natriuretic peptide,ANP)、抗α-横纹肌肌动蛋白(α-sarcomeric actin,α-SCA)、抗心肌肌钙蛋白Ⅰ(cardiac troponin I,cTnI)、抗后促进因子(anaphase promoting factor,APC)、抗糖原合成酶激酶3β(glycogen synthase kinase-3β,Gsk-3β)、抗β-catenin、抗CXC趋化因子受体4(CXC chemokine receptor 4,CXCR-4)、抗CC趋化因子受体2(CC chemokine receptor 2,CCR-2)购自美国Abcam公司;抗CD13、抗CD73、抗CD90、抗CD166、抗人白细胞抗原DR(human leukocyte antigen DR,HLA-DR)、抗CD14、抗CD29、抗CD31、抗CD44、抗CD45、抗CD105、抗人白细胞抗原ABC(human leukocyte antigen ABC,HLA-ABC)抗体均购自美国BD Biosciences公司;SDS-PAGE凝胶试剂盒、ECL超敏化学发光显影液等购自北京碧云天科技有限公司。
1.2 方法 1.2.1 hPDMSCs的分离鉴定采用组织块分离法培养hPDMSCs[5]。取第3代细胞,待其长满培养皿底部后,用胰酶消化处理,PBS洗涤2次,计数1×106细胞置于样品管中,分别加入FITC标记的鼠抗人的HLA-ABC、CD29、CD31、CD44、CD45、CD14、CD105抗体和PE标记的鼠抗人HLA-DR、CD13、CD73、CD90、CD166抗体,4 ℃避光孵育20 min,同时设置空白对照组,流式细胞仪检测细胞上述抗原的表达情况。
1.2.2 细胞培养前期研究[7]显示,0.1 mg/L丹参酮ⅡA对hPDMSCs没有细胞毒性,且诱导hPDMSC向心肌细胞分化的效果优于丹参,故仍然选择0.1 mg/L丹参酮ⅡA用于本研究。将hPDMSCs分别接种于96孔板和10 cm培养皿中(1×103/cm2),用含10%胎牛血清和1%青链霉素的DMEM培养;次日,分别加入0.1 mg/L丹参酮ⅡA或0.1 mg/L丹参酮ⅡA+0.1 μmol/L β-catenin激动剂WAY-262611,同时设置不加药的对照组,每4 d换液1次,3-(4,5-二甲基吡啶-2-基)-5-(3-羧基甲氧基苯基)-2-(4-磺苯基)-2H-四唑[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,MTS]法检测细胞的增殖情况,20 d终止实验,收集细胞进行Western blotting检测。
1.2.3 划痕实验将hPDMSCs接种于6孔板中(1.5×103/cm2),用含10%胎牛血清和1%青链霉素的DMEM培养;次日,分别加入0.1 mg/L丹参酮ⅡA或0.1 mg/L丹参酮ⅡA+0.1 μmol/L β-catenin激动剂WAY-262611,同时设置不加药的对照组,每4 d换液1次。培养2周后,用200 μL的枪头做十字划痕,吸去完全培养基,更换为含0.5%胎牛血清的DMEM培养[8],分别用0.1 mg/L丹参酮ⅡA、0.1 mg/L丹参酮ⅡA+0.1 μmol/L β-catenin激动剂WAY-262611干预24 h后,于显微镜下拍照,观察相同部位细胞向损伤区迁移的情况。
1.2.4 Western blotting收集细胞于EP管中,加入裂解液,-20 ℃过夜;涡旋裂解,4 ℃、12 000 r/min离心25 min,收集总蛋白并定量;加入等量蛋白并进行SDS-PAGE电泳,转膜,封闭,加入抗GAPDH、抗GATA-4、抗ANP、抗α-SCA、抗cTnI、抗APC、抗Gsk-3β、抗β-catenin、抗CXCR-4、抗CCR-2抗体,4 ℃孵育过夜;洗膜后加入HRP标记的二抗,室温孵育1 h;超敏化学发光显影液显色,自动电泳凝胶成像仪采集图像,检测灰度值,以目的蛋白与GAPDH条带灰度值的比值表示目的蛋白表达水平。
1.3 统计学分析采用SPSS 16.0统计软件进行统计学分析。蛋白表达水平均符合正态分布,采用x±s表示,采用t检验比较组间差异,P < 0.05为差异有统计学意义。
2 结果 2.1 hPDMSCs的提取与鉴定分离培养3~5 d时,组织块周围有少量呈短梭形、形态饱满的细胞爬出;随着细胞不断向外扩增,逐渐变成长梭形并形成细胞集落;第3周时,细胞达80%~90%融合;经传代贴壁后,细胞重新变成长梭形并形成细胞集落(图 1A)。流式细胞仪结果显示,来源人胎盘组织的第3代细胞,表达CD13、CD29、CD44、CD73、CD90、CD105、CD166、HLA-ABC,不表达CD14、CD31、CD45、HLA-DR(图 1B),呈现间充质干细胞的表面标记特性。
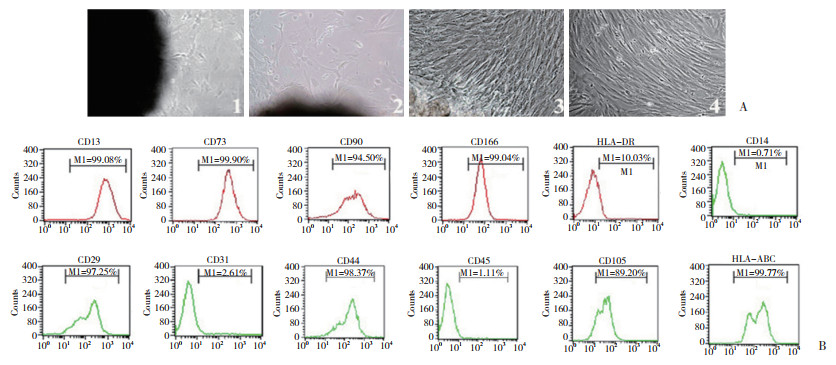
|
| A, morphological observation of the isolated cells; B, flow cytometry identification results. In panel A:1, primary culture day 3;2, primary culture day 10;3, primary culture day 21;4, passage 2. 图 1 细胞分离培养的形态观察及流式细胞术鉴定结果 ×100 Fig.1 Morphological observation and flow cytometry identification results of the isolated cells ×100 |
2.2 丹参酮ⅡA干预对hPDMSCs的增殖及形态的影响
如图 2所示,培养12 d内,对照组细胞增殖速度较快,随后由于细胞密度较大而出现接触性抑制,增殖减慢,整个过程中细胞始终保持成纤维细胞样形态。与对照组相比,丹参酮ⅡA干预组细胞长势缓慢,部分细胞逐渐增大并形成棒状外观,且细胞间出现很多连接细胞的分支。
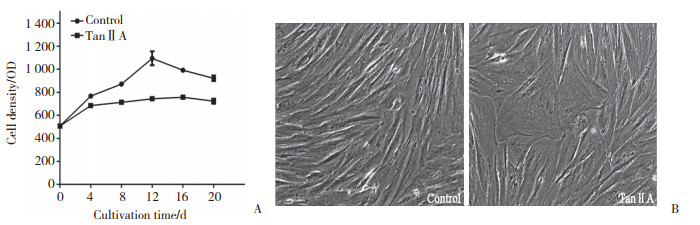
|
| A, effect of tanshinoneⅡA on the proliferation of hPDMSCs; B, effect of tanshinoneⅡA on cell morphology×100. TanⅡA, tanshinoneⅡA. 图 2 丹参酮ⅡA干预前后hPDMSCs增殖形态的变化 Fig.2 Changes in proliferation and morphology of hPDMSCs before and after tanshinone ⅡA intervention |
2.3 丹参酮ⅡA干预对心肌特异性蛋白GATA-4、ANP、α-SCA、cTnI表达及预分化细胞向损伤区迁移能力的影响
如图 3所示,与对照组比较,丹参酮ⅡA组细胞心肌特异性蛋白GATA-4、ANP、α-SCA、cTnI的表达明显增多(P < 0.05),且向损伤区迁移的能力明显增强(P < 0.05)。
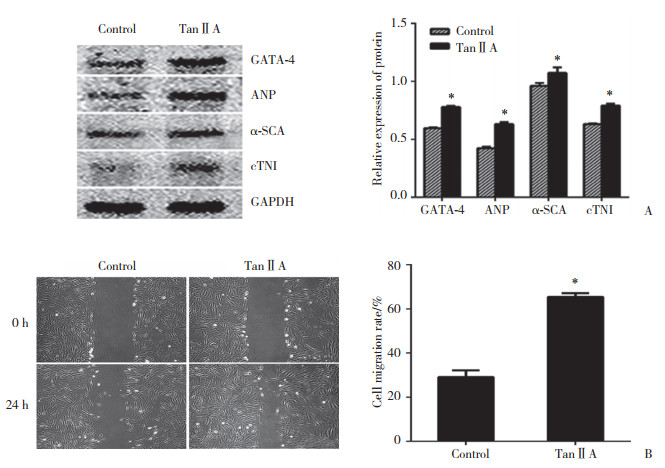
|
| A, effect of tanshinoneⅡA on myocardial specific protein expression; B, effect of tanshinoneⅡA on cell motility. *compared with the control group, P < 0.05. TanⅡA, tanshinoneⅡA. 图 3 丹参酮ⅡA干预前后心肌特异性蛋白的表达变化及预分化细胞向损伤区迁移的情况 Fig.3 Changes of myocardial specific protein expression and migration of pre-differentiated cells to the injured area before and after tanshinone ⅡA intervention |
2.4 丹参酮ⅡA干预对Wnt/β-catenin通路中相关蛋白APC、Gsk-3β、β-catenin及与归巢相关的趋化因子CXCR-4、CCR-2表达的影响
如图 4所示,与对照组比较,丹参酮ⅡA组干预细胞APC、Gsk-3β、CXCR-4、CCR-2的表达明显增多(P < 0.05),β-catenin的表达明显减少(P < 0.05)。
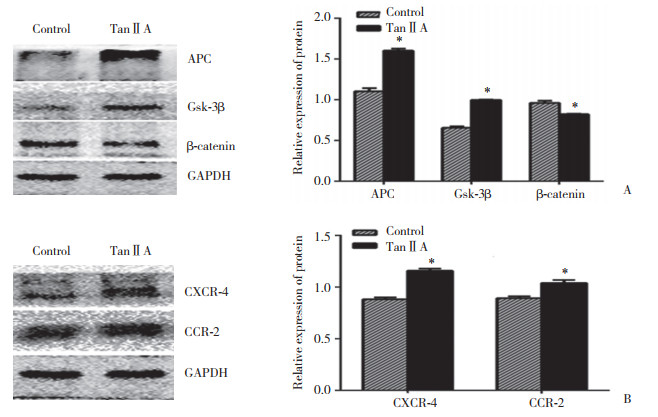
|
| A, effect of tanshinoneⅡA on the expression of related proteins in Wnt/β-catenin pathway; B, effect of tanshinoneⅡA on the expression of CXCR-4 and CCR-2. *compared with the control group, P < 0.05. TanⅡA, tanshinoneⅡA. 图 4 丹参酮ⅡA干预前后Wnt/β-catenin通路中相关蛋白及与归巢相关的趋化因子的表达变化情况 Fig.4 Expressions of the related proteins in Wnt/β-catenin pathway and the homing-related chemokines before and after intervention with tanshinone ⅡA |
2.5 β-catenin激动剂WAY-262611对丹参酮ⅡA诱导hPDMSCs向心肌分化、提高预分化细胞向损伤区归巢能力的影响
经β-catenin激动剂WAY-262611干预后,丹参酮ⅡA组干预细胞β-catenin表达明显增多(P < 0.05)(图 5A),说明β-catenin激动剂WAY-262611干预有效;虽然丹参酮ⅡA能够抑制细胞增殖,促进细胞心肌样形态改变,但经β-catenin激动剂WAY-262611干预后,细胞的增殖速度明显增快,且呈长梭形集落状生长(图 5B、5C);与丹参酮ⅡA干预组相比,丹参酮ⅡA+β-catenin激动剂WAY-262611干预组细胞心肌特异性蛋白GATA-4、ANP、α-SCA、cTnI的表达明显减少(P < 0.05)(图 5D),且细胞向损伤区迁移的能力明显下降(图 5E),与细胞归巢相关的趋化因子CXCR-4、CCR-2的表达也明显减少(P < 0.05)(图 5F)。
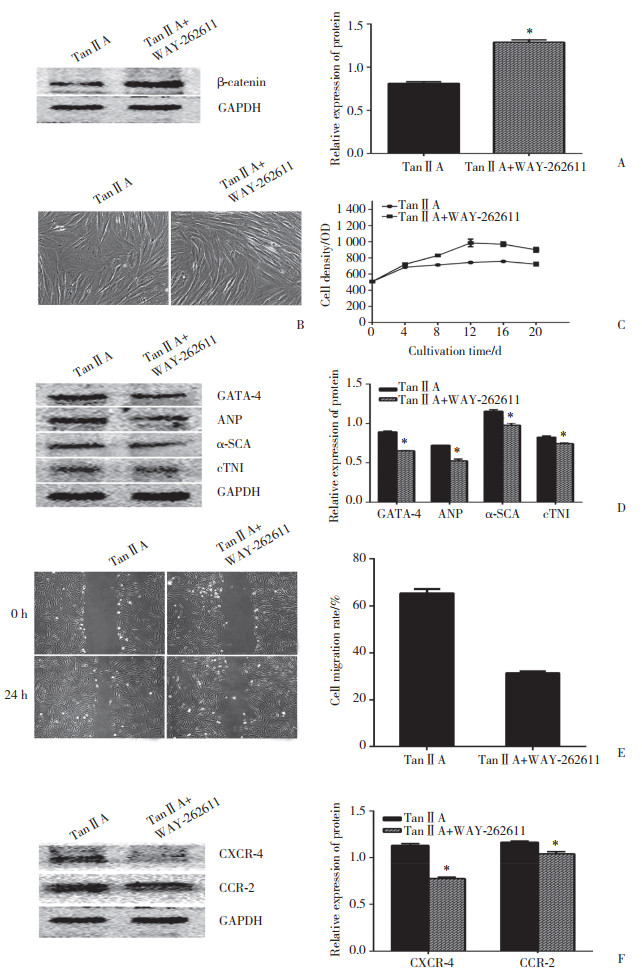
|
| A, Western blotting results of β-catenin; B and C, effect of WAY-262611 on the morphology and proliferation of cells treated by tanshinoneⅡA, ×100;D, effect of WAY-262611 on the expression of GATA-4, ANP, α-SCA and cTNI in cells treated by tanshinoneⅡA; E, effect of WAY-262611 on the motility of cells treated by tanshinoneⅡA, ×100;F, effect of WAY-262611 on the expression of CXCR-4 and CCR-2 in cells treated by tanshinoneⅡA. *compared with tanshinone ⅡA alone, P < 0.05. TanⅡA, tanshinoneⅡA. 图 5 β-catenin激动剂WAY-262611干预前后β-catenin蛋白、细胞增殖形态、心肌特异性蛋白、预分化细胞向损伤区迁移及与归巢相关的趋化因子表达变化的情况 Fig.5 Changes in β-catenin expression, cell proliferation, cell morphology, cardiac-specific proteins, migration of pre-differentiated cells to the injured area, and the expression of homing-related chemokines before and after intervention with the β - catenin agonist WAY-262611 |
3 讨论
胎盘中存在大量易于提取分离的间充质干细胞,这些细胞能表达某些胚胎干细胞的表面标志物,提示hPDMSCs具有很强的自我更新和分化能力;而且,hPDMSCs不仅免疫原性较低,还具有免疫调节特性[9]。因此,hPDMSCs在同种同体及同种异体移植治疗中具有广阔的应用前景。
缺血性心脏病所导致的心肌细胞不可逆的死亡是心功能不全的主要原因。尽管溶栓、介入和冠状动脉旁路搭桥等血运重建技术能够改善冠状动脉粥样硬化性心脏病的缺血状态,但却不能弥补损失的心肌细胞。利用干细胞增殖分化潜能,再生心肌细胞,修复受损心肌组织,恢复心脏功能,已成为治疗缺血性心脏病的一种新策略。但由于心肌分化调控的复杂性,还不能保证移植入体内的干细胞会向心肌细胞分化的同时不伴有基因突变的发生,因此,移植心肌前体细胞较移植干细胞更为安全有效[1]。在众多诱导干细胞向心肌前体细胞分化的方式中,共培养法和5-氮胞苷诱导法最为常用。但是,这2种方法都存在不足,尚不能广泛应用于临床上的心肌再生。另外,在干细胞通过再生心肌细胞修复受损心肌组织的过程中,除被移植的干细胞能否分化为心肌细胞外,另一个制约心肌再生和修复的因素是分化细胞向损伤区归巢(即迁移运动)的能力。
心脏由中胚层发育而来,其形成和发育过程十分复杂,涉及众多信号通路的参与,其中,Wnt/β-catenin通路发挥着不可替代的作用。研究[10-11]显示,在中胚层形成以后,抑制Wnt/β-catenin通路可以完全并有效地指定中胚层细胞向心肌细胞分化。此外,Wnt/β-catenin通路也参与细胞迁移运动能力的调控。细胞的迁移运动能力涉及趋化因子、生长因子、黏附分子等的参与[12-14]。本研究结果显示,经丹参酮ⅡA干预后,hPDMSCs的增殖速度明显减慢,心肌特异性蛋白GATA-4、ANP、α-SCA、cTnI的表达明显增加;在这一过程中,Wnt/β-catenin通路中的APC、Gsk-3β的表达明显增加,β-catenin明显减少,预分化细胞向损伤区迁移运动的能力明显增强,和与归巢相关的趋化因子CXCR-4、CCR-2的表达增加一致。因此,推测丹参酮ⅡA通过调控Wnt/β-catenin通路诱导hPDMSCs向心肌细胞分化,提高预分化细胞归巢能力。为了进一步验证这一推测,本研究应用β-catenin激动剂WAY-262611干预了丹参酮ⅡA诱导分化体系,结果显示,β-catenin激动剂WAY-262611干预后,细胞生长速度明显增快,与单独丹参酮ⅡA干预相比,丹参酮ⅡA+β-catenin激动剂WAY-262611干预后,心肌特异性蛋白GATA-4、ANP、α-SCA、cTnI的表达明显减少,细胞迁移明显受到抑制,与归巢相关的趋化因子CXCR-4、CCR-2的表达也明显减少。
综上所述,本研究发现,丹参酮ⅡA能诱导hPDMSCs向心肌细胞分化,提高预分化细胞的归巢能力,其机制与丹参酮ⅡA调控Wnt/β-catenin通路有关。
| [1] |
JOHNSON TA, SINGLA DK. Therapeutic application of adult stem cells in the heart[J]. Methods Mol Biol, 2017, 1553: 249-264. DOI:10.1007/978-1-4939-6756-8_20 |
| [2] |
BESSER RR, ISHAHAK M, MAYO V, et al. Engineered microenvironments for maturation of stem cell derived cardiac myocytes[J]. Theranostics, 2018, 8(1): 124-140. DOI:10.7150/thno.19441 |
| [3] |
MUNDRE RS, KOKA P, DHANARAJ P, et al. Synergistic role of 5-azacytidine and ascorbic acid in directing cardiosphere derived cells to cardiomyocytes in vitro by downregulating Wnt signaling pathway via phosphorylation of β-catenin[J]. PLoS One, 2017, 12(11): e0188805. DOI:10.1371/journal.pone.0188805 |
| [4] |
LI ZM, XU SW, LIU PQ. Salvia miltiorrhizaBurge (Danshen):a golden herbal medicine in cardiovascular therapeutics[J]. Acta Pharmacol Sin, 2018, 39(5): 802-824. DOI:10.1038/aps.2017.193 |
| [5] |
LI K, LI SZ, ZHANG YL, et al. The effects of Dan-Shen root on cardiomyogenic differentiation of human placenta-derived mesenchymal stem cells[J]. Biochem Biophys Res Commun, 2011, 415(1): 147-151. DOI:10.1016/j.bbrc.2011.10.035 |
| [6] |
LI K, SONG JQ, ZHAO Q, et al. Effective component of Salvia miltiorrhiza in promoting cardiomyogenic differentiation of human placenta-derived mesenchymal stem cells[J]. Int J Mol Med, 2018, 41(2): 962-968. DOI:10.3892/ijmm.2017.3293 |
| [7] |
王秀艳, 樊晨星, 赵千, 等. 丹参酮ⅡA对人胎盘间充质干细胞向心肌细胞分化的诱导作用及其机制[J]. 吉林大学学报(医学版), 2019, 45(1): 33-38, 218. DOI:10.13481/j.1671-587x.20190107 |
| [8] |
LI J, ZHENG H, WANG JF, et al. Expression of Kruppel-like factor KLF4 in mouse hair follicle stem cells contributes to cutaneous wound healing[J]. PLoS One, 2012, 7(6): e39663. DOI:10.1371/journal.pone.0039663 |
| [9] |
刘太行, 王应雄, 丁裕斌. 胎盘来源干细胞研究进展[J]. 中国产前诊断杂志(电子版), 2018, 10(1): 1-5. DOI:10.13470/j.cnki.cjpd.2018.01.001 |
| [10] |
CHOE MS, YEO HC, BAE CM, et al. Trolox-induced cardiac differentiation is mediated by the inhibition of Wnt/β-catenin signaling in human embryonic stem cells[J]. Cell Biol Int, 2019, 43(12): 1505-1515. DOI:10.1002/cbin.11200 |
| [11] |
PARIKH A, WU JC, BLANTON RM, et al. Signaling pathways and gene regulatory networks in cardiomyocyte differentiation[J]. Tissue Eng Part B Rev, 2015, 21(4): 377-392. DOI:10.1089/ten.TEB.2014.0662 |
| [12] |
LI X, HE XT, YIN Y, et al. Administration of signalling molecules dictates stem cell homing for in situ regeneration[J]. J Cell Mol Med, 2017, 21(12): 3162-3177. DOI:10.1111/jcmm.13286 |
| [13] |
HOCKING AM. The role of chemokines in mesenchymal stem cell homing to wounds[J]. Adv Wound Care (New Rochelle), 2015, 4(11): 623-630. DOI:10.1089/wound.2014.0579 |
| [14] |
MOTEGI SI, ISHIKAWA O. Mesenchymal stem cells:The roles and functions in cutaneous wound healing and tumor growth[J]. J Dermatol Sci, 2017, 86(2): 83-89. DOI:10.1016/j.jdermsci.2016.11.005 |
 2020, Vol. 49
2020, Vol. 49




