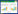文章信息
- 郑杰夫, 殷林波, 宋成博, 傅雅静, 姜拥军, 张子宁
- ZHENG Jiefu, YIN Linbo, SONG Chengbo, FU Yajing, JIANG Yongjun, ZHANG Zining
- T细胞因子1对人类免疫缺陷病毒感染中Th1/Th2细胞平衡的作用
- T cell factor 1 regulates Th1/Th2 cell balance during human immunodeficiency virus infection
- 中国医科大学学报, 2020, 49(2): 97-101
- Journal of China Medical University, 2020, 49(2): 97-101
-
文章历史
- 收稿日期:2019-02-14
- 网络出版时间:2019-12-23 13:18
免疫系统失衡是人类免疫缺陷病毒(human immunodeficiency virus,HIV)感染致病及疾病发生发展的重要原因[1]。作为适应性免疫的重要组成部分,CD4+ T细胞可通过分泌多种细胞因子辅助细胞及体液免疫应答,被称为辅助性T细胞。依据分泌细胞因子的不同,CD4+ T细胞分为Ⅰ型辅助性T细胞(type Ⅰ helper T cell,Th1)及Ⅱ型辅助性T细胞(type Ⅱ helper T cell,Th2)2个亚群。Th1细胞主要分泌白细胞介素(interleukin,IL)-2、γ-干扰素(interferon-γ,IFN-γ)、肿瘤坏死因子等,参与调节细胞免疫;Th2细胞分泌IL-4、IL-6、IL-10等,主要调节体液免疫[2]。以往研究[3]显示,HIV感染中Ⅰ型细胞因子IFN-γ水平出现下降趋势,而Ⅱ型细胞因子IL-4逐渐上升,Thl/Th2细胞平衡向Th2细胞方向漂移。由于Th1细胞有助于增强细胞毒性T细胞应答,而细胞毒性T细胞应答是最重要的抗HIV免疫应答,因此HIV感染中Th1/Th2细胞失衡与AIDS患者病情加重有关[4],而长期不进展者则维持正常的Th1/Th2细胞平衡[5]。迄今为止,HIV感染中Th1/Th2细胞失衡的机制尚未彻底阐明,明确其机制对于HIV临床进程的干预具有重要意义。
T细胞因子1(T cell factor 1,TCF1)是Wnt信号通路的转录因子,结合β-catenin,激活下游信号[6]。TCF1表达在免疫系统、神经系统疾病及肿瘤发病中具有重要作用[7-9]。YU等[10]在对小鼠T淋巴细胞进行研究时发现,TCF1可诱导Th2细胞早期表达,对Th1细胞起到负调控作用。但是,TCF1能否调控HIV感染中Th1/Th2细胞平衡尚无报道。鉴于Th1/Th2细胞漂移在HIV感染致病中的重要作用,本研究探讨了TCF1对HIV感染者Th1/Th2细胞平衡的作用,希望为HIV感染临床结局的免疫调控提供信息。
1 材料与方法 1.1 研究对象11例HIV感染者均来自辽宁,男性,平均年龄为(40.73±15.60)岁(范围:18~64岁),CD4+ T细胞计数绝对值(258.91±63.77)/μL,于抗逆转录病毒治疗前收集血液标本。5例健康对照者为随机选取的HIV抗体阴性的健康成人,男性,平均年龄(31.40±8.43)岁(范围:22~46岁)。研究对象均签署知情同意书。
1.2 siRNA转染使用Ficoll®-Paque PLUS(美国GE Healthcare公司)通过密度离心分离HIV感染者CD4+、CD8+ T细胞的外周血单个核细胞(peripheral blood mononuclear cell,PBMC),将5×105个细胞重悬至160 μL R10培养基(10%胎牛血清,1%青霉素/链霉素,RPMI 1640),制成细胞悬液,接种于96孔板中。每孔加入转染试剂Lipofectamine RNAiMAX Transfection Reagent(美国Thermo Fisher Scientific公司)1.2 μL、TCF1-siRNA(北京华大基因公司)0.2 μL、RPMI 1640(美国GE Healthcare公司)38.6 μL,在37 ℃、5%CO2条件下培养24 h。转染TCF1-siRNA为si-TCF1组,未转染TCF1-siRNA的对照组为TCF1组,Control组为si-TCF1组和TCF1组的画门策略。
1.3 TCF1 mRNA检测采用RNeasy Micro试剂盒(德国QIAGEN公司)提取HIV感染者CD4+、CD8+ T细胞的RNA,采用PrimeScriptTM RT reagent试剂盒(日本TaKaRa公司)实时定量PCR方法将RNA逆转录为cDNA。cDNA合成条件:37 ℃ 15 min,85 ℃ 15 s,16 ℃终止。利用GAPDH作为内参,配置cDNA实时定量体系,接种于96孔板中。实时定量体系:每孔TCF1/GAPDH-R 0.8 μL、TCF1/GAPDH-F 0.8 μL、无核酸水6.5 μL、cDNA 2 μL、SYBR Premix Ex TaqTM Ⅱ(日本TaKaRa公司)10 μL。实时定量PCR(瑞士Roche公司)检测后对Ct值进行分析。实时定量PCR反应条件:95 ℃ 30 s,95 ℃ 5 s,55 ℃ 30 s(扩增40个循环),50 ℃ 30 s。TCF1引物序列:上游引物,5’-CCTTGATGCTAGGTTCTGGTGTACC-3’;下游引物,5’-CACTCTGCAATGACCTTGGCTCTCA-3’。GAPDH引物序列:上游引物,5’-ATGGGGAAGGTGAAGGTCG-3’;下游引物,5’-GGGGTCATTGATGGCAACAATA-3’。
1.4 细胞培养与流式细胞术为了检测HIV感染者及健康对照者Th1和Th2细胞因子分泌水平,将HIV感染者及健康对照者新鲜全血经溶血素溶血后每个流式管加入3 μL Cell Activation Cocktail(美国BioLegend公司),在37 ℃、5%CO2条件下培养4 h,经PBS洗涤离心后进行表面染色与细胞破膜实验,并通过流式细胞仪进行检测。PBMCs转染24 h后,每孔加入1 μL anti-αCD3/CD28 Dynabeads(美国Thermo Fisher Scientific公司)继续培养24 h,在继续培养结束前6 h每孔加入1 μL Golgistop(美国BD公司)。将细胞重悬,使用流式荧光表面抗体Percp-anti-CD3、FITC-anti-CD4、PE(phycoerythrin)-Cy7-anti-CD8进行染色,并置于4 ℃下避光染色30 min。加入LiveDead(美国Life Technologies公司)1 μL,4 ℃避光染色30 min。用FACS缓冲液洗涤2次,加入Cytofix/Cytoperm试剂(美国BD公司)350 μL,4 ℃避光染色40 min。将细胞重悬使用allophycocyanin(APC)-anti-IL-4、BV421-anti-IFN-γ,并置于4 ℃下避光染色30 min。用FACS缓冲液重新悬浮细胞,通过流式细胞仪FaccantoⅡ(美国BD公司)检测后,利用FlowJo软件对数据进行分析。
1.5 统计学分析采用SPSS19.0软件进行数据处理。采用Mann- Whitney U检验进行组间比较,采用Spearman进行等级相关性分析。P < 0.05为差异有统计学意义。采用GraphPad Prism 7.0绘图软件进行绘图。
2 结果 2.1 HIV感染者及健康对照者Th1和Th2细胞的细胞因子胞内表达为了测定HIV感染者及健康对照者Th1和Th2细胞因子分泌水平,对CD4+ T细胞中Ⅰ型细胞因子IFN-γ和Ⅱ型细胞因子IL-4进行分析。结果发现,HIV感染者CD4+ T细胞IFN-γ表达显著低于健康对照者[分别为(3.62±0.87)%和(4.77±0.50)%,P = 0.041,图 1A];IL-4表达显著高于健康对照者[分别为(8.77±1.37)%和(1.33±0.51)%,P < 0.001,图 1B]。提示HIV感染导致细胞因子从Th1型向Th2型漂移。
|
| A, IFN-γ; B, IL-4. 图 1 HIV感染者及健康对照者Th1和Th2细胞的细胞因子胞内表达 Fig.1 Intracellular expression of cytokines in Th1 and Th2 cells from HIV-infected patients versus healthy controls |
2.2 HIV感染者TCF1调节CD4+ T细胞的细胞因子胞内表达
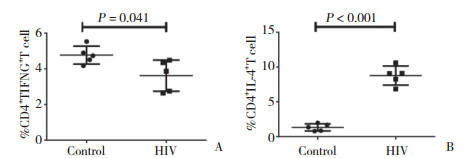
HIV感染者敲除TCF1后,CD4+ T细胞胞内表达的Ⅰ型细胞因子IFN-γ增加,差异有统计学意义[si-TCF1组(5.03±1.54)%,TCF1组(3.23±1.19)%,P = 0.048,图 2A];CD4+ T细胞胞内表达的Ⅱ型细胞因子IL-4减少,差异有统计学意义[si-TCF1组(4.48±1.66)%,TCF1组(7.58±0.94)%,P = 0.004,图 2B]。提示在敲除了HIV感染者TCF1后,使HIV感染者体内Th2细胞向Th1细胞方向漂移。
|
| A, representative images (left panels) and quantitation (right panel) of IFN-γ expressing CD4+ T cells co-cultured with TCF1-knockdown versus control PBMCs by flow cytometry; B, representative images (left panels) and quantitation (right panel) of IL-4 expressing CD4+ T cells co-cultured with TCF1-knockdown versus control PBMCs by flow cytometry. 图 2 TCF1对HIV感染者CD4+ T细胞中IFN-γ和IL-4的调节作用 Fig.2 Regulation of IFN-γ and IL-4 by TCF1 in CD4+ T cells from HIV-infected patients |
2.3 HIV感染者TCF1调节CD8+ T细胞的细胞因子胞内表达
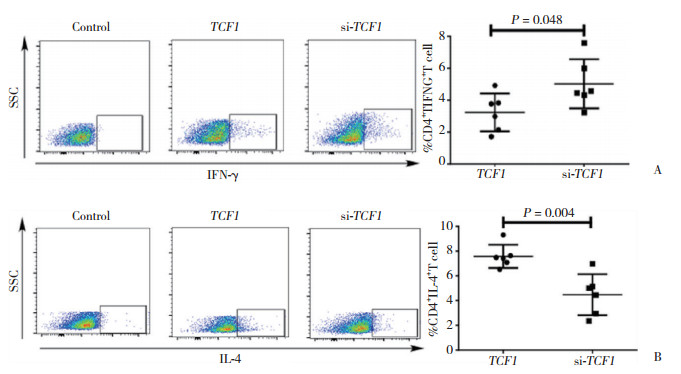
TCF1被敲除后,HIV感染者CD8+ T细胞胞内表达的Ⅰ型细胞因子IFN-γ增加,差异有统计学意义[si-TCF1组(3.58±0.58)%,TCF1组(1.93±0.43)%,P < 0.001,图 3A];CD8+ T细胞胞内表达的Ⅱ型细胞因子IL-4减少,差异有统计学意义[si-TCF1组(2.40±0.53)%,TCF1组(3.88±1.01)%,P = 0.010,图 3B]。
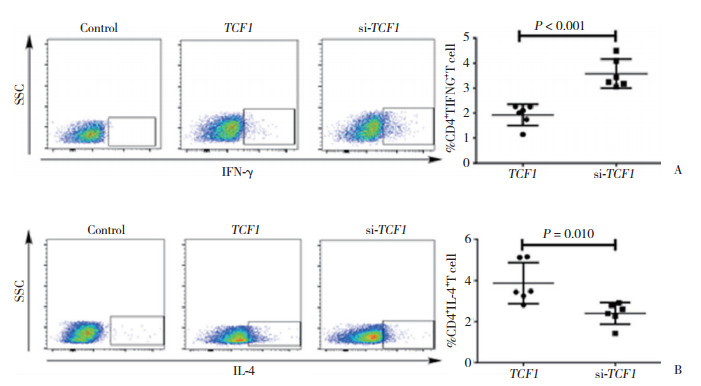
|
| A, representative images (left panels) and quantitation (right panel) of IFN-γ expressing CD8+ T cells co-cultured with TCF1-knockdown versus control PBMCs by flow cytometry; B, representative images (left panels) and quantitation (right panel) of IL-4 expressing CD8+ T cells co-cultured with TCF1-knockdown versus control PBMCs by flow cytometry. 图 3 TCF1对HIV感染者CD8+ T细胞中IFN-γ和IL-4的调节作用 Fig.3 Regulation of IFN-γ and IL-4 by TCF1 in CD8+ T cells from HIV-infected patients |
3 讨论
在人体正常生理条件下,机体Th1/Th2细胞处于动态平衡状态,在病理状态下,这种平衡状态被打破,出现Th1/Th2细胞漂移。有研究[11]发现,器官特异性自身免疫病以及胞内病原体如结核等,Th细胞亚群向Th1漂移。而系统性自身免疫病、过敏、哮喘及肿瘤等,则出现由Th1向Th2的漂移。以往研究显示,HIV感染中,HIV基因中类过敏原结构可诱导Ⅱ型细胞因子IL-4产生增加,通过上调CXCR4等促进合胞体形成病毒的复制,从而加速疾病进展;而Ⅱ型细胞因子增加,进一步抑制CD4+ T细胞向Th1的分化,降低了其对细胞毒性T淋巴细胞的辅助作用,抑制了抗病毒细胞免疫应答,从而加速疾病进展[12]。本研究还证实,HIV感染后,与健康对照者相比,Ⅰ型细胞因子IFN-γ表达下降,Ⅱ型细胞因子IL-4表达升高,出现Th1向Th2的漂移,如何控制HIV感染中Th细胞亚群的失衡对于疾病进展的控制至关重要。
TCF1对T淋巴细胞的发育具有重要的调控作用,可促进记忆性T细胞的产生[13]。有学者发现,在TCF1的调控下活化的小鼠CD4+ T细胞分化为Th1或Th2细胞,诱导Th2细胞早期表达,对Th1细胞起到负调控的作用[14],但在HIV感染中TCF1是否调控Th1/Th2的平衡尚无报道。本研究发现,敲除TCF1后,HIV感染者Th1细胞中的IFN-γ表达增加,Th2细胞中的IL-4表达下降,使得从Th2型向Th1型漂移,提示TCF1有助于纠正HIV感染者Th1/Th2的失衡。此外,研究[15-16]显示,在一些低CD4+ T细胞的HIV感染者中,外周血及皮肤均检出以分泌IL-4等Ⅱ型细胞因子为主的CD8+ T细胞,提示CD8+ T细胞分泌Ⅱ型细胞因子与疾病进展相关。而CD8+ T细胞分泌Ⅰ型细胞因子IFN-γ下降,亦是其功能下降的重要标志。本研究进一步发现,敲除TCF1后,HIV感染者CD8+ T细胞中Ⅰ型细胞因子IFN-γ表达增加,Ⅱ型细胞因子IL-4表达下降,提示敲除TCF1亦有助于恢复HIV感染中CD8+ T细胞的平衡及功能。
综上所述,本研究首次发现敲除TCF1有助于重建HIV感染中T细胞的免疫平衡及功能,为明确HIV感染的致病机制及免疫干预手段提供重要信息。
| [1] |
ZHENG Y, ZHOU H, HE Y, et al. The immune pathogenesis of immune reconstitution inflammatory syndrome associated with highly active antiretroviral therapy in AIDS[J]. AIDS Res Hum Retroviruses, 2014, 30(12): 1197-202. DOI:10.1089/AID.2014.0106 |
| [2] |
ROMAGNANI S. T-cell subsets(Th1 versus Th2)[J]. Ann Allergy Asthma Immunol, 2000, 85(1): 9-18. DOI:10.1016/S1081-1206(10)62426-X |
| [3] |
CLERICI M, SHEARER GM. A TH1: > TH2 switch is a critical step in the etiology of HIV infection[J]. Immunol Today, 1993, 14(3): 107-111. DOI:10.1016/0167-5699(93)90208-3 |
| [4] |
KLEIN SA, DOBMEYER JM, DOBMEYER TS, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry[J]. AIDS, 1997, 11(9): 1111-1118. DOI:10.1097/00002030-199709000-00005 |
| [5] |
PINA AF, MATOS VTG, BONIN CM, et al. Non-polarized cytokine profile of a long-term non-progressor HIV infected patient[J]. Braz J Infect Dis, 2018, 22(2): 142-145. DOI:10.1016/j.bjid.2018.01.003 |
| [6] |
苏尚, 吴畏. Wnt/β-catenin信号通路对靶基因转录的调控[J]. 中国科学:生命科学, 2014, 44(10): 1029-1042. DOI:10.1360/052014-138 |
| [7] |
JANOVSKÁ P, BRYJA V. Wnt signalling pathways in chronic lymphocytic leukaemia and B-cell lymphomas[J]. Br J Pharmacol, 2017, 174(24): 4701-4715. DOI:10.1111/bph.13949 |
| [8] |
YU W, MA YM, SHANKAR S, et al. SATB2/β-catenin/TCF-LEF pathway induces cellular transformation by generating cancer stem cells in colorectal cancer[J]. Sci Rep, 2017, 7(1): 10939. DOI:10.1038/s41598-017-05458-y |
| [9] |
BEM J, BROŻKO N, CHAKRABORTY C, et al. Wnt/β-catenin signaling in brain development and mental disorders:keeping TCF7L2 in mind[J]. FEBS Lett, 2019, 593(13): 1654-1674. DOI:10.1002/1873-3468.13502 |
| [10] |
YU Q, SHARMA A, OH SY, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma[J]. Nat Immunol, 2009, 10(9): 992-999. DOI:10.1038/ni.1762 |
| [11] |
HENDEN AS, HILL GR. Cytokines in graft-versus-host disease[J]. J Immunol, 2015, 194(10): 4604-4612. DOI:10.4049/jimmunol.1500117 |
| [12] |
BECKER Y. The changes in the T helper 1(Th1)and T helper 2(Th2)cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers:a review and hypothesis[J]. Virus Genes, 2004, 28(1): 5-18. DOI:10.1023/B:VIRU.0000012260.32578.72 |
| [13] |
CHEN YP, YU D. TCF-1 at the Tfh and Th1 divergence[J]. Trends Immunol, 2015, 36(12): 758-760. DOI:10.1016/j.it.2015.11.001 |
| [14] |
SCHILHAM MW, WILSON A, MOERER P, et al. Critical involvement of Tcf-1 in expansion of thymocytes[J]. J Immunol, 1998, 161(8): 3984-3991. |
| [15] |
MAGGI E, MAZZETTI M, RAVINA A, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells[J]. Science, 1994, 265(5169): 244-248. DOI:10.1126/science.8023142 |
| [16] |
ROMAGNANI S, PRETE G, MANETTI R, et al. Role of TH1/TH2 cytokines in HIV infection[J]. Immunol Rev, 1994, 140(1): 73-92. DOI:10.1111/j.1600-065x.1994.tb00865.x |
 2020, Vol. 49
2020, Vol. 49