文章信息
- 李爽, 张丽艳, 李雪建, 周义, 金戈
- LI Shuang, ZHANG Liyan, LI Xuejian, ZHOU Yi, JIN Ge
- β淀粉样蛋白诱导的阿尔茨海默病小鼠模型中BDNF通过上调TRPC3发挥神经保护作用
- Brain-derived neurotrophic factor plays a neuroprotective role by up-regulating TRPC3 expression in a mouse model of amyloid β-protein-induced Alzheimer disease
- 中国医科大学学报, 2020, 49(12): 1086-1090
- Journal of China Medical University, 2020, 49(12): 1086-1090
-
文章历史
- 收稿日期:2019-12-30
- 网络出版时间:2020-12-03 12:17
2. 沈阳医学院基础医学院药理学教研室, 沈阳 110034
2. Department of Pharmacology, College of Basic Medical Sciences, Shenyang Medical College, Shenyang 110034, China
阿尔茨海默病(Alzheimer disease,AD)是一种以记忆力减退、认知功能障碍以及神经元受损为特征的神经退行性疾病,病理表现为老年斑、神经纤维缠结形成和突触功能障碍等[1]。AD发病机制复杂,在众多假说里,β淀粉样蛋白(amyloid β-protein,Aβ)异常沉积占主导地位[2-3]。最近有研究表明,在多种疾病模型中,脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)通过参与神经元成熟和突触重塑发挥神经营养作用[4-5]。在APP/PS1双转基因型AD小鼠中,大脑皮层和海马区BDNF的表达量低于野生型小鼠,并伴随着神经元凋亡和空间学习记忆能力减弱[6]。在本课题组的前期研究[7]中,Aβ诱导的AD大鼠模型BDNF表达下降,外源性给予BDNF后认知功能改善。这些数据表明,BDNF在AD模型中发挥神经营养作用,但其作用机制尚不清楚。经典瞬时受体电位通道(canonical transient receptor potential channel,TRPC)家族成员TRPC3在平滑肌、大脑和小脑组织高度表达。有研究[8]报道,在小鼠胚胎干细胞的分化过程中,TRPC3对神经元的存活、多能性和分化方面有重要作用。在癫痫大鼠海马神经元原代培养中,发现BDNF和TRPC3的表达增加,减轻了癫痫引起的细胞损伤和癫痫发作[9]。在心肌梗死大鼠模型中,BDNF/酪氨酸激酶受体B (tyrosine kinase receptor B,TrkB)通过调控TRPC3/6通道,减轻心肌缺血损伤,抑制心肌细胞凋亡[10]。这些数据表明,BDNF可能通过TRPC3通路发挥神经营养作用。本课题组的前期研究[7]表明,在Aβ诱导的AD大鼠模型中,外源性给予BDNF后TRPC3表达增加且认知功能改善,但BDNF与TRPC3的相互关系尚需进一步确定。本研究通过建立AD小鼠模型,内源性激活BDNF,上调、下调TRPC3的表达,观察小鼠的行为学和神经突触功能,明确BDNF是否通过TRPC3改善AD小鼠的认知功能。
1 材料与方法 1.1 材料 1.1.1 实验动物分组和处理80只5周龄雄性费城癌症研究所小鼠(购自辽宁长生生物技术有限公司),体质量20~24 g,适应性喂养5 d,随机分为对照组、AD组、AD+TrkB受体激动剂7,8-二羟基黄酮(7,8-dihydroxyflavone,7,8-DHF)组(AD+7,8-DHF组)、AD+TRPC3激动剂二酰基甘油类似物(1-oleoyl-2-acetyl-sn-glycerol,OAG)组(AD+OAG组)和AD+7,8-DHF+TRPC3抑制剂吡唑化合物(pyrazole compound,Pyr3)组(AD+7,8-DHF+Pyr3组),每组16只。第6天对照组小鼠给予灭菌生理盐水侧脑室注射,其他4组小鼠给予Aβ1-42侧脑室注射,建立动物模型。待小鼠恢复体力后,AD+7,8-DHF组、AD+OAG组和AD+7,8-DHF+Pyr3组分别给予7,8-DHF (5 mg/kg)、OAG (0.6 mg/kg)、7,8-DHF (5 mg/kg)和Pyr3 (0.1 mg/kg)腹腔注射[11-13],每日给药1次,连续给药21 d后分离小鼠的海马组织和全脑。TrkB受体激动剂7,8-DHF用于内源性激活BDNF信号传导途径[14],TRPC3激动剂OAG和TRPC3抑制剂Pyr3分别用于上调和下调TRPC3的表达。
1.1.2 试剂Aβ1-42和钙/钙调素依赖性蛋白激酶Ⅱ-α (calcium/calmodulin-dependent protein kinase Ⅱ-α,CaMKⅡ-α)兔单克隆抗体(英国Abcam公司);7,8-DHF (大连美伦生物);OAG和Pyr3 (美国Cayman公司);BCA蛋白浓度测定试剂盒和SDS-PAGE凝胶配制试剂盒(上海碧云天公司);TRPC3兔多克隆抗体(台湾Arigo公司);synapsin-Ⅰ兔单克隆抗体(美国Cell公司);β-actin小鼠单克隆抗体和辣根酶标记山羊抗兔IgG (中国Boster生物公司)。
1.2 方法 1.2.1 AD模型制备将Aβ1-42溶于DMSO中,终浓度控制在0.3% (用无菌生理盐水稀释),然后在37 ℃温箱中孵育120 h。根据小鼠脑立体定位图谱(第2版)确定侧脑室注射位点为前囟后0.5 mm,中线向右旁开1.1 mm。用微量注射器缓慢注射Aβ1-42 3 μL,注射速度为0.6 μL/min,留针5 min。
1.2.2 Morris水迷宫实验Morris水迷宫是评估小鼠空间学习和记忆的经典测试[15]。水迷宫实验(中国上海吉良软件技术有限公司)从造模后第16天开始,持续6 d。圆形水池中装满黑色墨水,平台固定在水池的第三象限,水位在平台上1 cm处,水温保持在(25±1) ℃。水迷宫实验包括定位航行实验(前5 d)和空间探索实验(第6天),分别测量小鼠的学习和记忆能力。前5 d,将小鼠面对水池壁于不同的象限放入水中,寻找隐藏的平台,每日3次。第6天,将平台移走,观察小鼠在1 min内的动作轨迹。
1.2.3 ELISA使用ELISA试剂盒(台湾Arigo公司),利用酶标仪测量海马中Aβ1-42的浓度(n = 6)。Aβ1-42浓度(ng/mL) =样品浓度×稀释倍数。
1.2.4 Western blotting海马组织中加入含磷酸酶抑制剂的RIPA裂解液吹打混匀后进行超声处理,处理完后冰上静置0.5 h,高速离心10 min。取少量上清液,采用BCA法测定蛋白浓度,剩余上清液加蛋白缓冲液进行100 ℃沸水浴变性。取30 μg变性后的蛋白溶液上样于5%SDS-聚丙烯凝胶中,室温下电泳2 h,4 ℃下用转膜仪(中国天能公司)将蛋白转移到硝酸纤维素膜1 h。将膜浸泡在含有5%脱脂牛奶的TBST中,摇床上室温封闭1.5 h,TBST漂洗3次,每次15 min。之后分别与TRPC3小鼠多克隆抗体(1:500)、synapsin-Ⅰ (1:2 000)、CaMKⅡ-α (1:10 000)和β-actin (1:1 500) 4 ℃孵育过夜。第2天,TBST漂洗3次,4 ℃摇床上与辣根过氧化酶标记的山羊抗小鼠IgG (1:5 000)孵育2 h,TBST漂洗3次。最后,用化学发光成像系统(中国天能公司)进行ECL显影并用Image J软件进行条带灰度的定量分析。
1.3 统计学分析采用Graphpad Prism 7.0软件进行统计学处理,数据以x±s表示,组间比较采用单因素方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 激活BDNF和TRPC3对AD小鼠空间学习记忆能力的改善作用水迷宫实验结果显示,与对照组小鼠相比,AD组小鼠第5天逃避潜伏期延长(P < 0.001),第6天目标象限停留时间减少(P < 0.001),第6天穿越平台次数减少(P < 0.05),结果表明,AD组小鼠的空间学习记忆能力下降。与AD组小鼠相比,AD+7,8-DHF组和AD+OAG组小鼠第5天逃避潜伏期缩短(P < 0.01),第6天目标象限停留时间增加(P < 0.01),第6天穿越平台次数增加(P < 0.05),结果表明,内源性激活BDNF和上调TRPC3后,AD小鼠空间学习记忆能力得到明显改善。见表 1。
| Group | n | Escape latency (s) | Target quadrant occupancy (s) | Number of platform crossing | ||||
| 1 d | 2 d | 3 d | 4 d | 5 d | ||||
| Control | 6 | 48.27±9.02 | 34.89±9.56 | 33.38±7.48 | 25.21±4.59 | 11.19±2.06 | 26.50±2.70 | 3.67±0.82 |
| AD | 6 | 59.83±0.41 | 53.49±5.21 | 46.41±7.81 | 40.09±8.171) | 36.53±6.571) | 12.47±1.272) | 1.33±1.031) |
| AD+OAG | 6 | 49.68±10.97 | 41.30±4.05 | 34.49±4.18 | 25.94±2.163) | 15.11±1.424) | 21.51±1.364) | 3.17±0.983) |
| AD+7,8-DHF | 6 | 55.81±5.00 | 45.29±9.33 | 35.71±3.90 | 26.66±3.753) | 15.08±2.474) | 20.92±1.524) | 3.33±1.213) |
| AD+7,8-DHF+Pyr3 | 6 | 56.67±5.25 | 49.44±8.76 | 49.76±5.28 | 44.43±1.556) | 33.71±8.287) | 11.09±0.637) | 1.16±0.675) |
| Compared with control group,1) P < 0.01,2) P < 0.001;compared with AD group,3) P < 0.05,4) P < 0.001;compared with AD + 7,8-DHF group,5) P < 0.05,6) P < 0.01,7) P < 0.001. AD,Alzheimer disease;7,8-DHF,7,8-dihydroxyflavone;OAG,1-oleoyl-2-acetyl-sn-glycerol;Pyr3,pyrazole compound. | ||||||||
2.2 激活BDNF和TRPC3能够减轻Aβ1-42异常沉积
采用ELISA检测各组海马Aβ1-42浓度,对照组、AD组小鼠、AD+OAG组、AD+7,8-DHF组和AD+7,8-DHF+Pyr3组小鼠Aβ1-42浓度分别为(271.1±34.03)、(837.9±66.09)、(523.6±79.29)、(458.7±59.37)、(954.8±23.19) pg/mL。与对照组相比,AD组小鼠Aβ1-42浓度增高(P < 0.001);与AD组小鼠相比,AD+7,8-DHF组和AD+OAG组小鼠Aβ1-42浓度降低(均P < 0.01)。结果表明,激活BDNF和TRPC3减轻了Aβ1-42的异常沉积。
2.3 激活BDNF和TRPC3促进突触相关蛋白synapsin-Ⅰ和CaMKⅡ-α的表达Western blotting结果显示,与对照组小鼠相比,AD组小鼠TRPC3、突触相关蛋白(synapsin-Ⅰ、CaMKⅡ-α)表达均降低(分别为P < 0.01,P < 0.001,P < 0.001),表明AD组小鼠神经突触功能受损。与AD组小鼠相比,AD+7,8-DHF组和AD+OAG组小鼠TRPC3、突触相关蛋白(synapsin-Ⅰ、CaMKⅡ-α)表达均增加(分别为P < 0.01,P < 0.001,P < 0.01),表明激活BDNF和TRPC3明显改善神经突触功能。见图 1、表 2。
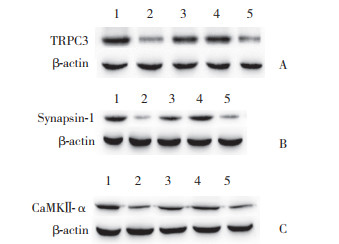
|
| A, TRPC3 protein expression; B, synapsin-Ⅰ protein expression; C, CaMKⅡ-α protein expression. 1, control group; 2, AD group; 3, AD+7, 8-DHF group; 4, AD+OAG group; 5, AD+7, 8-DHF+Pyr3 group. 图 1 Western blotting检测各组小鼠海马区TRPC3、synapsin-Ⅰ和CaMKⅡ-α蛋白表达 Fig.1 Expression of TRPC3, synapsin-Ⅰ, and CaMKⅡ-α proteins in the hippocampus of mice in each group detected using Western blotting |
| Protein | Control group (n = 5) | AD group (n = 5) | AD+OAG group (n = 5) | AD+7,8-DHF group (n = 5) | AD+7,8-DHF+Pyr3 group (n = 5) |
| TRPC3 | 1.05±0.16 | 0.51±0.161) | 1.06±0.283) | 0.96±0.173) | 0.56±0.125) |
| Synapsin-Ⅰ | 0.78±0.09 | 0.12±0.022) | 0.58±0.064) | 0.47±0.054) | 0.30±0.076) |
| CaMKⅡ-α | 0.76±0.08 | 0.27±0.032) | 0.67±0.033) | 0.57±0.063) | 0.38±0.105) |
| Compared with control group,1) P < 0.01,2) P < 0.001;compared with AD group,3) P < 0.01,4) P < 0.001;compared with AD + 7,8-DHF group,5) P < 0.05,6) P < 0.01. | |||||
2.4 BDNF通过上调TRPC3减轻Aβ沉积进而改善AD小鼠的认知和神经突触功能
水迷宫实验结果显示,与AD+7,8-DHF组小鼠相比,AD+7,8-DHF+Pyr3组小鼠第5天逃避潜伏期延长(P < 0.001),第6天目标象限停留时间减少(P < 0.01),第6天穿越平台次数减少(P < 0.05),结果表明,抑制TRPC3表达降低了BDNF对AD小鼠认知功能的改善作用。见表 1。
ELISA结果显示,与AD+7,8-DHF组小鼠相比,AD+7,8-DHF+Pyr3组小鼠Aβ1-42浓度显著降低(P < 0.001),结果表明,抑制TRPC3表达降低了BDNF对AD小鼠中Aβ1-42沉积的清除作用。
Western blotting结果显示,与AD+7,8-DHF组小鼠相比,AD+7,8-DHF+Pyr3组小鼠TRPC3、synapsin-Ⅰ和CaMKⅡ-α蛋白表达均降低(分别为P < 0.05,P < 0.01,P < 0.05),结果表明,抑制TRPC3表达降低了BDNF对AD小鼠神经突触功能改善作用。见表 2。
以上结果表明,BDNF通过上调TRPC3的表达减轻了Aβ异常沉积,进而改善AD小鼠的认知和神经突触功能。
3 讨论已有研究[16-17]报道,BDNF在AD模型中表达下降,上调BDNF后AD模型认知和神经突触功能得到改善,但TRPC3对AD的认知和神经突触功能的作用未见报道。本课题组的前期研究[7]发现,AD大鼠中BDNF和TRPC3蛋白表达降低,侧脑室注射BDNF后TRPC3表达增加,认知功能得到改善。本研究中,AD小鼠TRPC3表达降低,给予7,8-DHF (内源性激活BDNF)后TRPC3表达增加,与之前的研究结果一致。给予OAG (激活TRPC3)后TRPC3表达增加,AD小鼠认知和神经突触功能改善,说明TRPC3具有改善AD小鼠认知和神经突触功能的作用。另外,上调BDNF后AD小鼠的认知和神经突触功能得到改善,但抑制TRPC3通道后再上调BDNF,AD小鼠认知和神经突触功能并未得到改善,说明BDNF通过上调TRPC3的表达来发挥神经保护作用。
Aβ是由β淀粉样蛋白前体蛋白水解而成,其在脑内异常沉积形成老年斑。老年斑是AD的主要病理变化,也是神经元死亡的关键因素[18]。本研究中,上调BDNF和TRPC3均使AD小鼠Aβ沉积减轻,而使用TRPC3抑制剂上调BDNF并没有减轻Aβ沉积,说明BDNF可能通过TRPC3清除Aβ沉积,从而达到神经保护作用。但TRPC3通过何种途径清除Aβ沉积,具体机制并不清楚。目前,Aβ主要通过血脑屏障和脑血管周隙-淋巴2种途径进行清除[19-20]。但有研究[21]报道,缺氧模型中TRPC通过钙离子内流导致血脑屏障受损。因此,TRPC3是否影响血脑屏障以及是否通过血脑屏障清除Aβ沉积,尚需进一步研究证明。
已有研究报道,在急性心肌梗死大鼠模型中BDNF/TrkB通过TRPC3通道抑制缺血引起的心肌细胞凋亡[10]。嗅鞘细胞的迁移对神经再生和嗅觉发育至关重要。WANG等[22]发现BDNF通过TRPC3促进嗅鞘细胞的迁移。然而,ARAVAMUDAN等[23]认为,TRPC3/6正向调控BDNF的释放,在哮喘患者气道平滑肌细胞中,以RNA干扰技术检测到TRPC3/6通过调控BDNF的分泌来调节气道平滑肌的变化。这些证据说明,BDNF和TRPC3具有协同作用,但具体机制有待进一步探讨。
综上所述,本研究表明,BDNF/TrkB通过调节AD小鼠海马中TRPC3的表达,减轻Aβ的沉积,从而发挥其对AD小鼠认知功能和突触功能障碍的改善作用。BDNF和TRPC3激动剂可能成为AD治疗的候选药物。
| [1] |
HONIG LS, VELLAS B, WOODWARD M, et al. Trial of solanezumab for mild dementia due to Alzheimer's disease[J]. N Engl J Med, 2018, 378(4): 321-330. DOI:10.1056/nejmoa1705971 |
| [2] |
MUSIEK ES, HOLTZMAN DM. Three dimensions of the amyloid hypothesis:time, space and 'wingmen'[J]. Nat Neurosci, 2015, 18(6): 800-806. DOI:10.1038/nn.4018 |
| [3] |
SELKOE DJ, HARDY J. The amyloid hypothesis of Alzheimer's disease at 25 years[J]. EMBO Mol Med, 2016, 8(6): 595-608. DOI:10.15252/emmm.201606210 |
| [4] |
YOSHⅡ A, CONSTANTINE-PATON M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease[J]. Devel Neurobio, 2010, 70(5): 304-322. DOI:10.1002/dneu.20765 |
| [5] |
MOHAMMADI A, AMOOEIAN VG, RASHIDI E. Dysfunction in brain-derived neurotrophic factor signaling pathway and susceptibility to schizophrenia, Parkinson's and Alzheimer's diseases[J]. Curr Gene Ther, 2018, 18(1): 45-63. DOI:10.2174/1566523218666180302163029 |
| [6] |
郝继伟, 李莺歌, 王来, 等. BDNF在APP/PS1转基因小鼠皮层和海马内的表达及其对学习记忆能力的影响[J]. 中国病理生理杂志, 2019, 35(5): 858-864. DOI:10.3969/j.issn.1000-4718.2019.05.014 |
| [7] |
张利华, 张丽艳, 张小康, 等. β-淀粉样蛋白诱导的阿尔茨海默病大鼠模型中BDNF与TRPC3的关系[J]. 中国医科大学学报, 2018, 47(3): 217-221. DOI:10.12007/j.issn.0258-4646.2018.03.006 |
| [8] |
HAO HB, WEBB SE, YUE J, et al. TRPC3 is required for the survival, pluripotency and neural differentiation of mouse embryonic stem cells (mESCs)[J]. Sci China Life Sci, 2018, 61(3): 253-265. DOI:10.1007/s11427-017-9222-9 |
| [9] |
YANG ZW, WU F, ZHANG SL. Effects of ganoderic acids on epileptiform discharge hippocampal neurons:insights from alterations of BDNF, TRPC3 and apoptosis[J]. Die Pharmazie, 2016, 71(6): 340-344. |
| [10] |
HANG P, ZHAO J, CAI B, et al. Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents[J]. Int J Biol Sci, 2015, 11(5): 536-545. DOI:10.7150/ijbs.10754 |
| [11] |
AGRAWAL R, NOBLE E, TYAGI E, et al. Flavonoid derivative 7, 8-DHF attenuates TBI pathology via TrkB activation[J]. Biochim Biophys Acta, 2015, 1852(5): 862-872. DOI:10.1016/j.bbadis.2015.01.018 |
| [12] |
KIYONAKA S, KATO K, NISHIDA M, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound[J]. Proc Natl Acad Sci U S A, 2009, 106(13): 5400-5405. DOI:10.1073/pnas.0808793106 |
| [13] |
KITAJIMA N, WATANABE K, MORIMOTO S, et al. TRPC3-mediated Ca2+ influx contributes to Rac1-mediated production of reactive oxygen species in MLP-deficient mouse hearts[J]. Biochem Biophys Res Commun, 2011, 409(1): 108-113. DOI:10.1016/j.bbrc.2011.04.124 |
| [14] |
LIU X, OBIANYO O, CHAN CB, et al. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7, 8-dihydroxyflavone in the binding and activation of the TrkB receptor[J]. J Biol Chem, 2014, 289(40): 27571-27584. DOI:10.1074/jbc.M114.562561 |
| [15] |
BARNHART CD, YANG D, LEIN PJ. Using the Morris water maze to assess spatial learning and memory in weanling mice[J]. PLoS One, 2015, 10(4): e0124521. DOI:10.1371/journal.pone.0124521 |
| [16] |
ZHANG L, FANG Y, LIAN Y, et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer's disease induced by Aβ1-42[J]. PLoS One, 2015, 10(4): e0122415. DOI:10.1371/journal.pone.0122415 |
| [17] |
JIAO SS, SHEN LL, ZHU C, et al. Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer's disease[J]. Transl Psychiatry, 2016, 6(10): e907. DOI:10.1038/tp.2016.186 |
| [18] |
WEI Z, CHEN XC, SONG Y, et al. Amyloid β protein aggravates neuronal senescence and cognitive deficits in 5XFAD mouse model of Alzheimer's disease[J]. Chin Med J (Engl), 2016, 129(15): 1835-1844. DOI:10.4103/0366-6999.186646 |
| [19] |
HICKS K, O'NEIL RG, DUBINSKY WS, et al. TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress[J]. Am J Physiol Cell Physiol, 2010, 298(6): C1583-C1593. DOI:10.1152/ajpcell.00458.2009 |
| [20] |
ZUROFF L, DALEY D, BLACK KL, et al. Clearance of cerebral Aβ in Alzheimer's disease:reassessing the role of microglia and monocytes[J]. Cell Mol Life Sci, 2017, 74(12): 2167-2201. DOI:10.1007/s00018-017-2463-7 |
| [21] |
ŠIMIĆ G, ŠPANIĆ E, LANGER HORVAT L, et al. Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer's disease[J]. Prog Mol Biol Transl Sci, 2019, 168: 99-145. DOI:10.1016/bs.pmbts.2019.06.003 |
| [22] |
WANG Y, TENG HL, GAO Y, et al. Brain-derived neurotrophic factor promotes the migration of olfactory ensheathing cells through TRPC channels[J]. Glia, 2016, 64(12): 2154-2165. DOI:10.1002/glia.23049 |
| [23] |
ARAVAMUDAN B, THOMPSON MA, PABELICK CM, et al. Mechanisms of BDNF regulation in asthmatic airway smooth muscle[J]. Am J Physiol Lung Cell Mol Physiol, 2016, 311(2): L270-L279. DOI:10.1152/ajplung.00414.2015 |
 2020, Vol. 49
2020, Vol. 49