文章信息
- 夏桂辉, 贾昌俊, 徐锋, 赵阳, 戴朝六
- XIA Guihui, JIA Changjun, XU Feng, ZHAO Yang, DAI Chaoliu
- 吲哚菁绿荧光成像技术在肝癌切除术中识别微小癌灶的价值
- The value of indocyanine fluorescence imaging for the identification of microhepatocellular carcinoma in hepatectomy
- 中国医科大学学报, 2020, 49(10): 899-906
- Journal of China Medical University, 2020, 49(10): 899-906
-
文章历史
- 收稿日期:2019-11-14
- 网络出版时间:2020-10-07 16:10
2. 沈阳二四二医院普通外科, 沈阳 110141
2. Department of General surgery, shenyang 242 nd hospital, Shenyang 110141, China
在全球恶性肿瘤患病率及死亡率的统计报告中,肝癌均位居前列[1-2]。2015年中国癌症统计数据报告指出肝癌是我国癌症致死的主因[3]。我国肝癌患者的主要致病因素为病毒性肝炎及肝硬化,多数患者早期无临床症状,就诊晚,预后差,且早期小肝癌不易被常规影像学检查检测到,因此肝癌的早期诊断是肝癌治疗的重点。
《原发性肝癌诊疗规范(2017年版) 》 [4]中提及的肝癌临床影像学检查方法主要有腹部超声、CT、MRI、DSA、PET-CT和发射单光子计算机断层。而现有研究[5-6]报告的关于肝癌诊断的上述影像学检查中,对于直径 < 1 cm的微小病灶检出率较低,易发生遗漏,导致术后短期内即出现肝内转移和复发灶,对预后造成极大的负面影响。
2009年首次报道了应用吲哚菁绿(indocyanine green,ICG)分子荧光成像技术在肝细胞癌切除术中探测到术前常规检查手段难以检测到的微小肝内转移病灶[7-8]。不仅如此,该成像技术还具有精准度高的特点,探测发现的最小肝癌病灶直径约0.15 cm[8-11]。ICG荧光成像技术是利用ICG的荧光成像特点及其在肝脏肿瘤细胞中特定的靶向滞留效应[12-13],使用具有高度特异性的荧光探针,作用于特定的靶细胞,得到具有明暗对比度的影像,从而实现对肝癌病灶的精准定位。
但目前研究表明该项技术有穿透力弱[14]和假阳性率高[15-17] 2点不足。假阳性病灶的检出率及其特征仍需要临床研究来进一步明确。故本研究拟针对这一课题进行探讨。
1 材料与方法 1.1 临床资料 1.1.1 研究对象收集2016年12月至2017年9月于中国医科大学附属盛京医院肝胆脾外科行肝细胞癌肝切除手术患者的临床资料。纳入标准: (1)术前超声造影、增强CT和(或)增强MRI诊断为原发性肝癌,肝癌病灶距离肝脏表面均≤1 cm;(2)术前行ICG 15分钟潴留率(indocyanine green 15 retention rate,ICGR15)测定;(3)术中用ICG分子荧光成像系统探测肝脏肿物显像情况及显像微小病灶(微小病灶)并取材;(4)术后病理证实肝脏肿瘤为肝细胞癌。纳入患者共59例,其中,女11例,男48例。年龄35~75岁,中位年龄为58岁。乙型肝炎患者41例,丙型肝炎患者4例,脂肪肝患者1例。ICGR15值为0.8%~40.9%,中位值为5.1%。ICG注射时间为9.0~341.0 h,中位时间为74.0 h。
1.1.2 主要试剂和仪器注射用ICG购自丹东医创药业有限责任公司(产品编号:20160701),近红外荧光成像系统由北京数字精准医疗科技有限公司生产。
1.2 方法应用“ICG试敏针”进行试敏检测,确认入选对象无过敏反应。于术前1~14 d,以灭菌注射用水将ICG稀释成5 mg/mL,将按照0.5 mg/kg体质量计算的ICG溶液由肘静脉注入,密切观察患者反应,一般在10秒内注完。测定并记录患者ICGR15及ICG术前注射时间(以h为单位)。肝癌切除术中,标本离体后马上用ICG介导的免疫荧光探测仪探查已切除的离体标本,除既定切除肿物以外,有荧光反应的微小病灶予以切除,记为实验组;同时于该离体标本上取出既定切除肿物外无荧光反应的肝组织,记为对照组。
1.3 统计学分析采用SPSS 22.0进行统计学分析,年龄分析采用独立样本t检验,其余因素采用非参数曼惠特尼U检验,分类指标采用χ2检验。P < 0.05为差异有统计学意义。
2 结果 2.1 肝癌病灶肿瘤表面显影和瘤周微小病灶样显影病灶情况本组59例患者肿瘤病理结果均为肝细胞癌,肿瘤直径为0.5~20.0 cm,中位直径为3.1 cm。显影者50例,未显影者9例,ICG荧光成像肿瘤表面显影检出率为84.74% (图 1);其中肿瘤剖面显影46例(高亮显影33例,混杂显影14例,环状显影8例),肿瘤剖面不显影4例(图 2)。其中26例(检出率44.07%)患者在荧光成像探测下新发现肝脏微小病灶65枚,65枚微小病灶全显影,对照组织不显影(图 3)。微小病灶直径为0.2~2.0 cm,中位直径为0.8 cm。
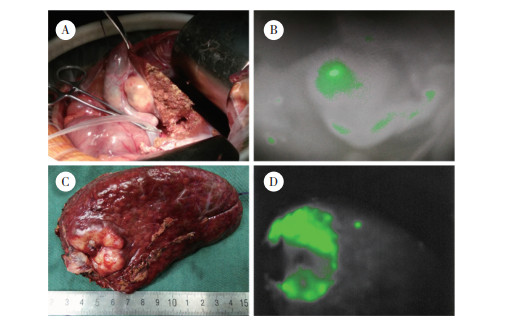
|
| A, cancer of the liver in vivo; B, fluorescence imaging of a hepatocellular carcinoma in vivo; C, in vitro liver cancer; D, fluorescence imaging of an in vitro hepatocellular carcinoma and peripheral microlesions. 图 1 肝癌表面荧光成像和瘤周微小病灶荧光成像 Fig.1 Fluorescence imaging of the surface of a hepatocellular carcinoma and microscopic lesions around the carcinoma |
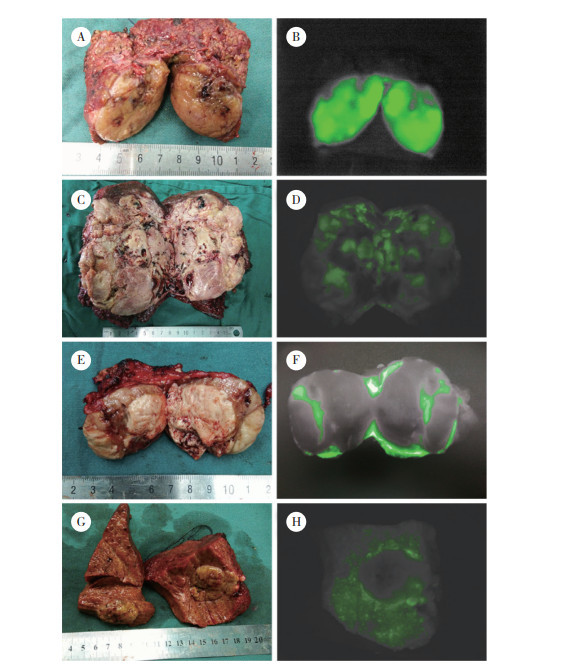
|
| A, tumor profile; B, complete fluorescence imaging of the tumor profile; C, tumor profile; D, mixed fluorescence imaging of the tumor profile; E, tumor profile; F, annular fluorescence imaging of the tumor profile; G, tumor profile; H, no fluorescence imaging of the tumor profile. 图 2 肝癌剖面荧光显影类型 Fig.2 Fluorescence imaging type of the hepatocellular carcinoma profile |
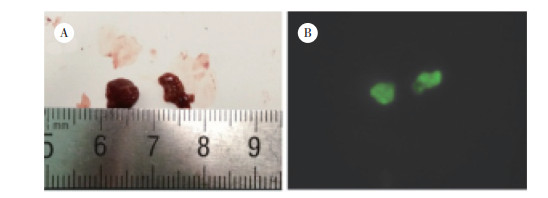
|
| A, naked-eye view of liver microlesions; B, fluorescence imaging of liver microlesions. 图 3 肝脏微小病灶荧光成像 Fig.3 Fluorescence imaging of liver microlesions |
2.2 微小病灶病理结果
65枚显影微小肝脏病灶的病理结果为肝细胞癌4枚,非肝细胞癌61枚(肝细胞增生20枚,肝硬化28枚,肝纤维化13枚),28枚对照组肝组织的病理结果均为非肝细胞癌(肝硬化23枚,肝纤维化5枚)。
2.3 肝癌显影情况差异分析在不同显影组中,仅肿瘤直径这一因素存在统计学差异(P < 0.05),见表 1。肿瘤表面显影组患者的肿瘤直径显著高于不显影患者的肿瘤直径,即肝脏肿瘤在ICG荧光显影探测下显像的概率与肿瘤直径相关;肿瘤直径越大者,越容易在ICG荧光显影探测中显像,见图 4。
| Item | Fluorescence imaging of the liver tumor surface | t/Z/χ2 | P | |
| Yes (n = 50) | No (n = 9) | |||
| Age (year) | 56.54±9.52 | 53.67±7.52 | 0.875 | 0.395 |
| Tumor size (cm) | 4(2.50-6.18) | 1.5(1.10-2.60) | -3.084 | 0.002 |
| ICGR15 | 4.9(2.60-7.43) | 6.5(4.40-29.70) | -1.866 | 0.062 |
| ICG injection time (h) | 77(36.00-110.38) | 53.5(50.00-138.50) | -0.474 | 0.635 |
| Sex [n (%)] | - | 1.000 | ||
| Male | 40(83.3) | 8(16.7) | ||
| Female | 10(90.9) | 1(9.1) | ||
| Hepatic injury [n (%)] | - | 0.671 | ||
| No | 13(92.9) | 1(7.1) | ||
| Yes | 37(82.2) | 8(17.8) | ||
| Liver cirrhosis [n (%)] | - | 0.333 | ||
| No | 10(100.0) | 0(0.0) | ||
| Yes | 40(81.6) | 9(18.4) | ||
| Tumor onset [n (%)] | - | 0.397 | ||
| Secondary | 2(66.7) | 1(33.3) | ||
| Primary | 48(85.7) | 8(14.3) | ||
| Tumor differentiation [n (%)] | 0.395 | 0.693 | ||
| Poor | 9(90.0) | 1(10.0) | ||
| Moderate | 26(83.9) | 5(16.1) | ||
| Well | 15(83.3) | 3(16.7) | ||
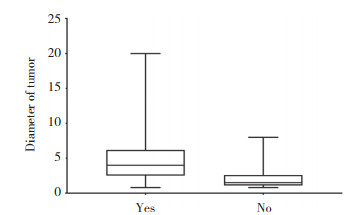
|
| 图 4 不同直径肝癌表面显影情况差异分析 Fig.4 Variance analysis of surface imaging of liver tumors of different diameters |
2.4 肝癌肿瘤剖面显影情况的差异分析
在荧光成像设备下探测,切除后的肝脏肿瘤剖面会呈现4种显影状态:高亮、混合、环状及无显影。如表 2所示,不同分化程度肿瘤的剖面显影率存在统计学差异(P < 0.05)。进一步分析显示,将肿瘤分化作为自变量,剖面显影为因变量,对其采用有序logistic回归分析,结果显示,分化程度与肿瘤剖面显影显著相关(P < 0.05)。分化程度低的肿瘤剖面显影亮度低的概率是分化高肿瘤的8.91倍,即肿瘤分化程度越高,出现高亮显影的概率越高。见表 3。
| Item | Fluorescence imaging types of hepatocellular carcinoma profile | F/Z/χ2 | P | |||
| Bright (n = 33) | Mixed (n = 14) | Annular (n = 8) | None (n = 0) | |||
| Age (year) | 54.36±9.19 | 58.50±9.09 | 57.50±10.46 | 59.25±7.63 | 0.915 | 0.440 |
| Tumor size (cm) | 3.00(1.65-5.00) | 4.55(2.43-8.13) | 5.50(2.75-9.75) | 3.25(2.10-3.88) | 7.171 | 0.067 |
| ICGR15 | 5.10(2.50-7.90) | 5.50(3.08-7.78) | 5.35(1.45-12.03) | 3.55(2.13-11.73) | 0.451 | 0.929 |
| ICG injection time (h) | 56.00(43.75-115.75) | 88.75(55.13-135.00) | 64.00(32.50-81.00) | 55.75(26.50-81.63) | 3.135 | 0.371 |
| Sex | -0.822 | 0.411 | ||||
| Male | 26(54.2) | 11(22.9) | 7(14.6) | 4(8.3) | ||
| Female | 7(63.6) | 3(27.3) | 1(9.1) | 0(0.0) | ||
| Hepatic Injury | -0.258 | 0.797 | ||||
| No | 8(57.1) | 4(28.6) | 1(7.1) | 1(7.1) | ||
| Yes | 25(55.6) | 10(22.2) | 7(15.6) | 3(6.7) | ||
| Hepatic cirrhosis | -1.123 | 0.261 | ||||
| No | 4(40.0) | 3(30.0) | 2(20.0) | 1(10.0) | ||
| Yes | 29(59.2) | 11(22.4) | 6(12.2) | 3(6.1) | ||
| Tumor onset | -0.058 | 0.954 | ||||
| Secondary | 2(66.7) | 0(0.0) | 0(0.0) | 1(33.3) | ||
| Primary | 31(55.4) | 14(25.0) | 8(14.3) | 3(5.4) | ||
| Tumor differentiation | 7.757 | 0.005 | ||||
| Poor | 4(40.0) | 1(10.0) | 3(30.0) | 2(20) | ||
| Moderate | 15(48.4) | 10(32.3) | 4(12.9) | 2(6.5) | ||
| Well | 14(77.8) | 3(16.7) | 1(5.6) | 0(0.0) | ||
| Tumor differentiation | Estimation | SE | Wald | df | P | 95% CI |
| Well | 2.187 | 0.822 | 7.084 | 1 | 0.008 | 0.577 - -3.798 |
| Moderate | 1.279 | 0.665 | 3.701 | 1 | 0.054 | -0.024 - -2.581 |
| Poor | 0 | - | - | 0 | - | - |
2.5 肿瘤旁探测出荧光显影微小病灶的情况分析
各项因素在探出微小病灶数目上均无统计学差异(P > 0.05)。即肝癌患者术中应用ICG荧光成像探测仪是否能探出显影微小病灶,与患者年龄、性别、有无肝损伤史、有无肝硬化、ICGR15、ICG注射时间、肿瘤直径、肿瘤为原发或复发及肿瘤分化程度等因素均不相关。见表 4。
| Item | Nomber of microhepatic lesions | F/Z/χ2 | P | ||
| 0(n = 33) | 1(n = 10) | > 1(n = 16) | |||
| Age (year) | 56.42±9.79 | 53.5±9.69 | 57.06±8.00 | 0.494 | 0.613 |
| Tumor size (cm) | 3.50(2.20-6.00) | 2.75(1.38-4.25) | 4.00(2.63-8.00) | 1.888 | 0.169 |
| ICGR15 | 4.60(3.00-7.90) | 3.40(1.68-11.48) | 6.25(3.65-7.38) | 1.409 | 0.235 |
| ICG injection time (h) | 77.00(47.50-114.25) | 56.25(28.00-116.25) | 66.50(36.13-117.75) | 0.006 | 0.937 |
| Sex | -0.544 | 0.587 | |||
| Male | 28(58.3) | 7(14.6) | 13(27.1) | ||
| Female | 5(45.5) | 3(27.3) | 3(27.3) | ||
| Hepatic injury | -0.020 | 0.984 | |||
| No | 8(57.1) | 2(14.3) | 4(28.6) | ||
| Yes | 25(55.6) | 8(17.8) | 12(26.7) | ||
| Hepatic cirrhosis | -0.113 | 0.910 | |||
| No | 6(60.0) | 1(10.0) | 3(30.0) | ||
| Yes | 27(55.1) | 9(18.4) | 13(26.5) | ||
| Tumor onset | -0.174 | 0.862 | |||
| Secondary | 2(66.7) | 0(0.0) | 1(33.3) | ||
| Primary | 31(55.4) | 10(17.9) | 15(26.8) | ||
| Tumor differentiation | 1.942 | 0.163 | |||
| Poor | 7(70.0) | 2(20.0) | 1(10.0) | ||
| Moderate | 17(54.8) | 6(19.4) | 8(25.8) | ||
| Well | 9(50.0) | 2(11.1) | 7(38.9) | ||
2.6 瘤周微小病灶样显影病灶的病理情况分析
病理结果显示,同一患者可以探测出多个微小病灶样显影病灶,而且同一患者探出的多个微小病灶样显影病灶可以有不同的病理结果。以微小病灶样显影病灶而非患者为研究对象。首先以病理阳性(肝癌)和阴性(肝细胞增生、肝硬化、肝纤维化)分组,进行四格表Fisher确切概率计算,结果显示,2组在病理结果上无统计学差异(P > 0.05),见表 5。本研究中ICG荧光成像技术诊断小肝癌的正确率为34.40 %;特异度为36.14 %;阳性预测值为6.15 %;假阳性率为68.53%;Youden指数为0.361 4。本研究微小肝癌检出率虽然较低,但Youden指数为0.361 4,且探测出有荧光反应的65枚微小病灶中有20枚为肝细胞增生,肝细胞低度异型增生为肝脏癌前病变表现,说明ICG免疫荧光成像技术对检测肝脏微小病灶及识别肝脏癌前病变有临床价值。
| Group | Positive | Negative | Total |
| Test | 4 | 61 | 65 |
| Control | 0 | 28 | 28 |
| Total | 4 | 89 | 93 |
2.7 实验组假阳性情况分析
根据计算结果得知微小病灶假阳性率过高,因此对实验组假阳性微小病灶的可能影响因素进行分析。将实验组微小病灶分为阳性(肝癌)和假阳性(增生、肝硬化、肝纤维化) 2组,结果显示,不同肿瘤剖面荧光类型的假阳性存在统计学差异(P < 0.05)。见表 6。
| Item | False-positive | Z/χ2 | P | |
| No | Yes | |||
| Age (year) | 56.50(46.25-66.00) | 58.00(48.00-63.00) | -0.178 | 0.859 |
| ICGR15 | 5.95(5.18-19.93) | 5.70(3.40-6.50) | -0.727 | 0.467 |
| ICG injection time (h) | 40.75(28.88-73.25) | 56.00(31.50-122.00) | -1.221 | 0.222 |
| Tumor size (cm) | 6.25(3.25-8.88) | 4.00(2.00-8.00) | -1.234 | 0.217 |
| Microlesions | 0.75(0.50-1.75) | 0.80(0.50-1.00) | -0.526 | 0.599 |
| Sex | - | 1.000 | ||
| Male | 3(6.0) | 47(94.0) | ||
| Female | 1(6.7) | 14(93.3) | ||
| Hepatic injury | - | 1.000 | ||
| No | 0(0.0) | 8(100.0) | ||
| Yes | 4(7.0) | 53(93.0) | ||
| Hepatic cirrhosis | - | 0.458 | ||
| No | 1(11.1) | 8(88.9) | ||
| Yes | 3(5.4) | 53(94.6) | ||
| In the same hepatic lobe | - | 0.574 | ||
| No | 2(10.5) | 17(89.5) | ||
| Yes | 2(4.3) | 44(95.7) | ||
| Same fluorescence imaging | - | 0.144 | ||
| No | 0(0.0) | 26(100.0) | ||
| Yes | 4(10.3) | 35(89.7) | ||
| Imaging of carcinoma profile | 11.898 | 0.004 | ||
| Bright | 1(3.0) | 32(97.0) | ||
| Mixed | 1(3.7) | 26(96.3) | ||
| Annular | 2(100.0) | 0(0.0) | ||
| None | 0(0.0) | 3(100.0) | ||
3 讨论
微小肝癌为早期肝癌,肝癌的早期及精准诊断是肝癌治疗的重点和难点。近年来,ICG介导的近红外分子荧光成像技术实现了对肝癌病灶的精准定位[18],在肝癌的诊断以及手术定位方面表现突出[19-20],为肝癌的早期诊断提供了一个新思路。
本研究主要针对肝癌早期的微小病灶,探讨了在现有常规影像学检查基础上对术中离体标本进行ICG免疫荧光成像检测的可行性。结果显示,ICG介导的免疫荧光成像技术在发现微小肝癌方面,比常规影像学检查具有更高的灵敏度和更大的潜能,能发现微小的肝脏肿瘤,利于预防肝癌术后早期复发;同时有助于肿瘤分期及预后判断,可以考虑在肝脏手术中作为传统影像学检查的补充。
ICG荧光成像系统探测深度较浅,最大深度仅1 cm,故可能有遗漏病灶尚未检出。检出微小病灶的病理结果假阳性率较高,现有研究[7, 15-17, 20]报道,该项探测技术针对合并肝硬化的肝癌患者检测假阳性率可达40%~50%,本研究患者中大部分均合并肝硬化,假阳性率达68.53%,高于文献报道。本研究已将可能的影响因素如肝脏损伤史、肝硬化、肿瘤与微小病灶是否位于同一肝叶、主标本肿瘤荧光类型、主标本肿瘤直径、ICGR15及ICG注射时间等进行分析,但可能由于受样本量有限,并未显示有明显相关因素。
本研究结果显示,肝癌手术中实时荧光成像探测出癌周高亮荧光显影微小癌灶的概率与患者年龄、性别、有无肝损伤史、有无肝硬化、ICGR15、ICG注射时间、肿瘤直径、肿瘤为原发或复发及肿瘤分化程度等因素无明显相关性。术中探测出的高亮荧光微小病灶病理阳性率虽低,但在灵敏度方面高于常规影像学检查,且有助于进一步明确肝癌患者的TNM分期,对诊断及治疗均有一定帮助。该项检测的Youden指数为0.361 4(> 0),说明ICG免疫荧光成像技术在检测肝脏微小病灶方面有临床价值。若能有大量样本,应进一步细化各因素,进行分层探讨,可能会得到更严谨的结论。
综上所述,ICG免疫荧光探测有望成为今后肝脏手术中实时检测小肝癌和肝脏癌前病变的常规诊疗技术。本研究结果显示,ICG荧光成像技术对肝脏微小病灶的诊断具有指导意义及临床价值,证实了肝脏肿瘤分化程度可能对ICG荧光成像诊断小肝癌灶的假阳性率有一定影响,但未能探寻出更好的减少假阳性率的具体方法。本研究为离体标本上进行,若能进一步完善,将来亦可能为肝癌的术中实时诊断及治疗提供依据。
| [1] |
SIA D, VILLANUEVA A, FRIEDMAN SL, et al. Liver cancer cell of origin, molecular class, and effects on patient prognosis[J]. Gastroenterology, 2017, 152(4): 745-761. DOI:10.1053/j.gastro.2016.11.048 |
| [2] |
HUANG H, HAN Y, ZHANG C, et al. HNRNPC as a candidate biomarker for chemoresistance in gastric cancer[J]. Tumor Biol, 2016, 37(3): 3527-3534. DOI:10.1007/s13277-015-4144-1 |
| [3] |
CHEN WQ, ZHENG RS, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA:A Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [4] |
中华人民共和国国家卫生和计划生育委员会. 原发性肝癌诊疗规范(2017年版)[J]. 传染病信息, 2017, 30(3): 111-127. DOI:10.3969/j.issn.1007-8134.2017.03.001 |
| [5] |
DAHIYA D, WU TJ, LEE CF, et al. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients:a 20-year experience[J]. Surgery, 2010, 147(5): 676-685. DOI:10.1016/j.surg.2009.10.043 |
| [6] |
LIM C, VIBERT E, AZOULAY D, et al. Indocyanine green fluorescence imaging in the surgical management of liver cancers:current facts and future implications[J]. J Visc Surg, 2014, 151(2): 117-124. DOI:10.1016/j.jviscsurg.2013.11.003 |
| [7] |
ISHIZAWA T, FUKUSHIMA N, SHIBAHARA J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging[J]. Cancer, 2009, 115(11): 2491-2504. DOI:10.1002/cncr.24291 |
| [8] |
YOKOYAMA N, OTANI T, HASHIDATE H, et al. Real-time detection of hepatic micrometastases from pancreatic cancer by intraoperative fluorescence imaging[J]. Cancer, 2012, 118(11): 2813-2819. DOI:10.1002/cncr.26594 |
| [9] |
王金伟, 张雅敏, 刘子荣, 等. 荧光导航肝切除术中应用吲哚菁绿的可行性[J]. 山东医药, 2016, 56(19): 86-88. DOI:10.3969/j.issn.1002-266X.2016.19.032 |
| [10] |
ZHANG YM, SHI R, HOU JC, et al. Liver tumor boundaries identified intraoperatively using real-time indocyanine green fluorescence imaging[J]. J Cancer Res Clin Oncol, 2017, 143(1): 51-58. DOI:10.1007/s00432-016-2267-4 |
| [11] |
ABO T, NANASHIMA A, TOBINAGA S, et al. Usefulness of intraoperative diagnosis of hepatic tumors located at the liver surface and hepatic segmental visualization using indocyanine green-photodynamic eye imaging[J]. Eur J Surg Oncol EJSO, 2015, 41(2): 257-264. DOI:10.1016/j.ejso.2014.09.008 |
| [12] |
HUANG L, VORE M. Multidrug resistance p-glycoprotein 2 is essential for the biliary excretion of indocyanine green[J]. Drug Metab Dispos:Biol Fate Chem, 2001, 29(5): 634-637. |
| [13] |
DE GRAAF W, HÄUSLER S, HEGER M, et al. Transporters involved in the hepatic uptake of 99mTc-mebrofenin and indocyanine green[J]. J Hepatol, 2011, 54(4): 738-745. DOI:10.1016/j.jhep.2010.07.047 |
| [14] |
MIYATA A, ISHIZAWA T, KAMIYA M, et al. Photoacoustic tomography of human hepatic malignancies using intraoperative indocyanine green fluorescence imaging[J]. PLoS One, 2014, 9(11): e112667. DOI:10.1371/journal.pone.0112667 |
| [15] |
ISHIZAWA T, KOKUDO N. Identification of hepatocellular carcinoma[M]. Frontiers of Gastrointestinal Research. Basel: S. KARGER AG, 2013: 10-17. DOI: 10.1159/000348601.
|
| [16] |
GOTOH K, YAMADA T, ISHIKAWA O, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation[J]. J Surg Oncol, 2009, 100(1): 75-79. DOI:10.1002/jso.21272 |
| [17] |
ISHIZUKA M, KUBOTA K, KITA J, et al. Intraoperative observation using a fluorescence imaging instrument during hepatic resection for liver metastasis from colorectal cancer[J]. Hepato-gastroenterology, 2011, 59(113): 90. DOI:10.5754/hge11223 |
| [18] |
WEISSLEDER R. Molecular imaging:exploring the next frontier[J]. Radiology, 1999, 212(3): 609-614. DOI:10.1148/radiology.212.3.r99se18609 |
| [19] |
MIZUGUCHI T, KAWAMOTO M, MEGURO M, et al. Preoperative liver function assessments to estimate the prognosis and safety of liver resections[J]. Surg Today, 2014, 44(1): 1-10. DOI:10.1007/s00595-013-0534-4 |
| [20] |
黄文琪, 许金超, 闵峰, 等. 吲哚菁绿清除试验对肝硬化患者肝脏储备功能的评估价值[J]. 实用肝脏病杂志, 2015, 18(5): 468-471. DOI:10.3969/j.issn.1672-5069.2015.05.006 |
 2020, Vol. 49
2020, Vol. 49




