文章信息
- 林特, 夏志军
- LIN Te, XIA Zhijun
- 盆腔器官脱垂患者子宫骶韧带COX2、PGE2和p16的表达及意义
- Expression and significance of COX2, PGE2, and p16 in the uterosacral ligaments of patients with pelvic organ prolapse
- 中国医科大学学报, 2020, 49(1): 21-26
- Journal of China Medical University, 2020, 49(1): 21-26
-
文章历史
- 收稿日期:2019-01-03
- 网络出版时间:2019-12-12 14:15
盆腔器官脱垂(pelvic organ prolapse,POP)是由各种原因引起的盆底支持组织出现薄弱而导致器官位置及功能异常,是影响全球近50%的50岁以上女性的健康问题[1]。POP的确切发病机制目前尚不明确,但其发生与经阴道分娩次数、年龄、慢性腹压增高、职业及合并疾病等因素有关。研究[2]表明,POP患病率及风险随年龄增加而增高。但也有研究[3]显示,年龄并不能作为POP的独立危险因素。p16是衰老相关的有效生物标志物[4]。前列腺素E2(prostaglandin E2,PGE2)能促进相关组织分泌炎性细胞因子诱导炎症反应发生,但也有助于急性炎症消退与后续组织修复[5]。同时,PGE2是环氧化酶2(cyclooxygenase 2,COX2)/PGE2信号通路的产物,与肌腱、骨、软骨等组织代谢相关[6]。因此,本研究拟观察POP患者子宫骶韧带组织中COX2、PGE2、p16、Ⅰ型胶原蛋白(collagenⅠ,COLⅠ)和Ⅲ型胶原蛋白(collagen Ⅲ,COLⅢ)的表达变化及其与年龄的相关性,探讨组织衰老、损伤与子宫骶韧带纤维组织变化及POP发生发展的关系。
1 材料与方法 1.1 材料 1.1.1 研究对象选择2016年1月至2018年3月于我院行子宫全切术的患者(已获得本院伦理委员会批准,患者已知情同意)共81例。根据是否患有POP,将全部患者分为POP组与对照组。POP诊断标准参照国际尿控协会公布的POP定量分期法(POP-Q),所有POP患者分期均为Ⅲ~Ⅳ度。对照组均为因良性疾病行子宫全切术,且近3个月未服用激素类药物,无雌激素相关疾病,无盆腔炎、妇科恶性肿瘤、结缔组织疾病等。根据年龄再将2组患者分别分为 < 55岁与 > 65岁2个亚组,即 < 55岁POP组(20例),> 65岁POP组(20例),< 55岁对照组(21例),> 65岁对照组(20例)。2组的一般资料具有可比性,见表 1。
| Item | Control group (n = 41) | POP group (n = 40) | P |
| Age | 58.37±14.37 | 58.65±15.32 | >0.05 |
| <55-year-old | 45.81±5.83 | 44.55±6.63 | >0.05 |
| >65-year-old | 71.55±6.45 | 72.75±4.38 | >0.05 |
| Delivery | 1.44±0.95 | 1.65±0.80 | >0.05 |
| <55-year-old | 1.10±0.77 | 1.15±0.76 | >0.05 |
| >65-year-old | 1.00±0.73 | 1.80±0.84 | >0.05 |
| BMI (kg/m2) | 23.99±3.03 | 23.80±3.12 | >0.05 |
| <55-year-old | 23.87±3.03 | 23.88±3.06 | >0.05 |
| >65-year-old | 24.11±3.09 | 23.71±3.25 | >0.05 |
| BMI, body mass index. | |||
1.1.2 主要试剂
COX2抗体(美国Cell Signaling Technology公司);PGE2抗体、COLⅠ抗体[艾博抗Abcam(上海)贸易有限公司];p16INK4A抗体、COLⅢ抗体(美国Proteintech Group有限公司);兔SP试剂盒(兔链霉卵白素-生物素法检测系统,中国上海碧云天生物技术有限公司);DAB显色试剂盒(中国北京索莱宝科技有限公司)。
1.2 方法从手术切除的组织中取接近宫颈的子宫骶韧带组织,大小约1 cm×1 cm×1 cm,经固定脱水、包埋,制成石蜡标本,4 µm厚连续切片。常规脱蜡、抗原修复,按SP免疫组化试剂盒说明书操作,检测各组组织中COX2、PGE2、p16、COLⅠ、COLⅢ的表达情况,DAB显色,苏木素复染返蓝,脱水透明,中性树胶封片。
采用双盲评分法对免疫组化结果进行判定,并由2位观察者阅片。在400倍视野下随机选取5个视野,阳性表达呈棕黄色颗粒状或片状,按阳性细胞着色强度进行评分,无着色为0分,浅黄色为1分,黄色为2分,棕黄色以及棕褐色为3分。每张切片在400倍光学显微镜下取3个高倍视野观察,按阳性细胞数所占百分比评分,评分后取均值,阴性为0分,阳性细胞百分比≤10%为1分,> 10%~50%为2分,> 50%~75%为3分,> 75%为4分。取2项评分的乘积作为总分,≤2分为阴性(-),3~4分为弱阳性(+),5~8为中等阳性(++),9~12分为强阳性(+++)。-/+为低表达,++/+++为高表达。
1.3 统计学分析采用SPSS 23.0统计软件进行χ2检验和Spearman相关性分析,P < 0.05为差异有统计学意义。
2 结果 2.1 子宫骶韧带组织中PGE2的表达免疫组化结果显示,PGE2表达于细胞质,见图 1。POP组PGE2表达水平高于对照组,差异有统计学意义(P < 0.01),但 < 55岁和 > 65岁亚组并无明显差异,见表 2。

|
| A, PGE2 in POP group; B, PGE2 in control group; C, COX2 in POP group; D, COX2 in control group. 图 1 子宫骶韧带组织中PGE2和COX2的表达SP ×400 Fig.1 PGE2 and COX2 expression in uterosacral ligaments of each group SP ×400 |
| Group | -/+ | ++/+++ |
| POP (n = 40) | 6 | 341) |
| <55-year-old | 2 | 182) |
| >65-year-old | 4 | 16 |
| Control (n = 41) | 33 | 8 |
| <55-year-old | 17 | 43) |
| >65-year-old | 16 | 4 |
| 1) χ2=34.781,P < 0.01 vs control group; 2) χ2=0.874,P = 0.661 vs > 65-year-old POP group; 3) χ2=0.076, P = 0.939 vs > 65-year-old control group. | ||
2.2 子宫骶韧带组织中COX2的表达
免疫组化结果显示,COX2表达于细胞质,见图 1。POP组COX2表达水平高于对照组,差异有统计学意义(P < 0.01),但 < 55岁和 > 65岁亚组并无统计学差异,见表 3。
| Group | -/+ | ++/+++ |
| POP(n = 40) | 13 | 271) |
| <55-year-old | 7 | 132) |
| >65-year-old | 6 | 14 |
| Control(n = 41) | 29 | 12 |
| <55-year-old | 14 | 73) |
| >65-year-old | 15 | 5 |
| 1)χ2=11.854,P < 0.01 vs control group;2)χ2=0.114,P = 0.736 vs > 65-year-old POP group;3)χ2=0.344,P = 0.558 vs > 65-year-old control group. | ||
2.3 子宫骶韧带组织中p16的表达
p16蛋白表达于组织细胞核和细胞质中,见图 2。POP组与对照组p16蛋白表达水平无统计学差异,但 > 65岁亚组表达水平较 < 55岁亚组增高,见表 4。
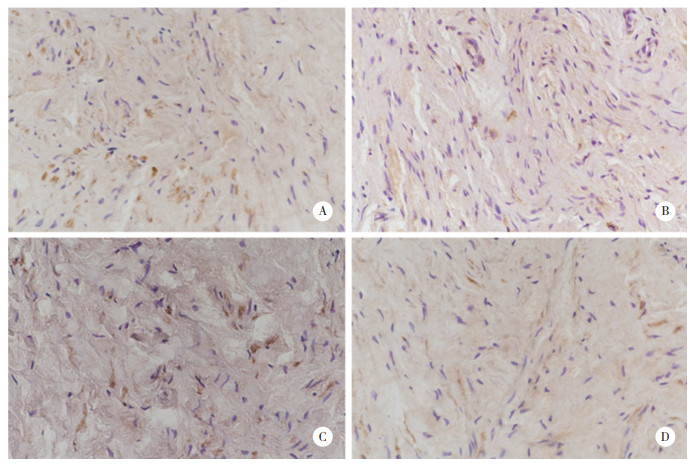
|
| A, > 65-year-old control group; B, < 55-year-old control group; C, > 65-year-old POP group; D, < 55-year-old POP group. 图 2 各组患者子宫骶韧带组织中p16的表达SP×400 Fig.2 p16 expression in uterosacral ligaments from each group SP×400 |
| Group | -/+ | ++/+++ |
| POP(n = 40) | 21 | 191) |
| <55-year-old | 14 | 62) |
| >65-year-old | 7 | 13 |
| Control(n = 41) | 19 | 22 |
| <55-year-old | 12 | 73) |
| >65-year-old | 7 | 15 |
| 1)χ2=0.307,P = 0.659,vs control group;2)χ2=4.912,P = 0.027,vs > 65-year-old POP group;3)χ2=4.027,P = 0.045,vs > 65-year-old control group. | ||
2.4 子宫骶韧带组织中COLⅠ和COLⅢ的表达
COLⅠ和COLⅢ表达于细胞质中,见图 3。POP组二者表达水平均低于对照组,见表 5。
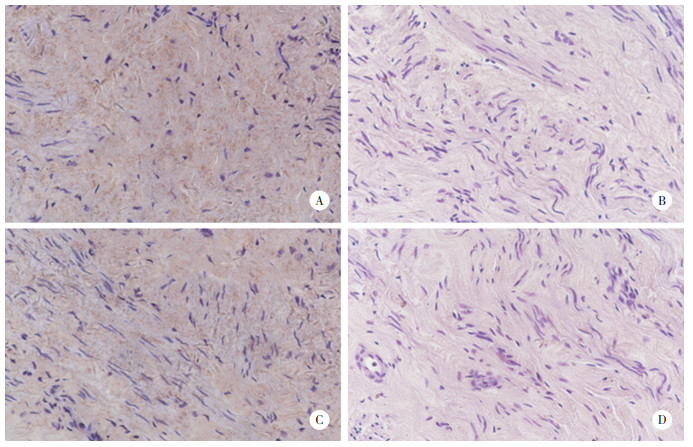
|
| A, COLⅠ in control group; B, COLⅠ in POP group; D, COLⅢ in control group; B, COLⅢ in POP group. 图 3 子宫骶韧带组织中COLⅠ、COLⅢ的表达SP×400 Fig.3 COLⅠ and COLⅢ expression in uterosacral ligaments of each group SP×400 |
| Group | n | COLⅠ | COLⅢ | |||||||
| -/+ | ++/+++ | χ2 | P | -/+ | ++/+++ | χ2 | P | |||
| POP | 40 | 27 | 13 | 5.53 | 0.019 | 26 | 14 | 4.50 | 0.034 | |
| Control | 41 | 17 | 24 | 17 | 24 | |||||
2.5 相关性分析
Spearman相关分析结果显示,COX2与PGE2的表达呈正相关,与COLⅠ、COLⅢ的表达呈负相关,见表 6。
| Protein | PGE2 | r | P | |
| -/+ | ++/+++ | |||
| COX2 | 0.532 | < 0.01 | ||
| -/+ | 29 | 11 | ||
| +/+++ | 8 | 33 | ||
| COLⅠ | -0.406 | < 0.01 | ||
| -/+ | 13 | 31 | ||
| +/+++ | 26 | 11 | ||
| COLⅢ | -0.579 | < 0.01 | ||
| -/+ | 9 | 34 | ||
| +/+++ | 30 | 8 | ||
3 讨论
POP是一种多因素疾病,严重影响女性的生活质量。目前的研究认为POP的发生与盆底组织结构变化有关。Ⅰ型和Ⅲ型胶原纤维对器官有支撑作用,而这种支撑需要细胞外基质各成分含量和比例的平衡,无论何种原因引起的失衡均会降低组织的机械强度,使POP易于发生。本研究结果发现,COLⅠ和COLⅢ的表达在POP组子宫骶韧带中显著减少,提示胶原纤维含量的减少可能与POP发生相关。有研究证实,盆底功能障碍患者盆底支持组织中胶原蛋白含量减少并不是因为合成减少,而是由于胶原蛋白降解增多,这与基质金属蛋白酶家族(matrix metalloproteinase,MMPs)、金属蛋白酶组织抑制剂(tissue inhibitor of metalloproteinase,TIMPs)及两者的比例相关。转换生长因子-β(transforming growth factor beta,TGF-β)对MMPs和TIMPs的调节作用在胶原代谢中起重要作用[7-8]。盆底组织在承受牵拉、挤压等外力作用时,逐渐释放炎性细胞因子,导致胶原代谢紊乱,韧带组织松弛。
COX2/PGE2信号通路对应力刺激敏感,在病理性瘢痕组织中,COX2蛋白表达高于正常组织,其代谢产物会增加炎症反应,影响成纤维细胞的迁移[9]。也有研究[10-11]证实,在皮肤、血管壁、牙周等组织中,PGE2可拮抗胶原纤维的形成。在树突细胞中,PGE2可诱导MMP9的表达[12],PGE2低表达可使MMP/TIMP比值下降,在血管壁重塑和胶原纤维形成中起重要作用[13];PGE2还能在肝星形细胞、乳腺上皮细胞和肺成纤维细胞中拮抗TGF-β信号通路[14]。本研究结果表明,COX2/PGE2在POP患者子宫骶韧带组织中的表达高于对照组,说明POP患者子宫骶韧带组织炎性细胞因子表达增多,且COX2/PGE2表达与年龄无关。同时,相关性分析发现PGE2和COX2的表达呈正相关,与COLⅠ和COLⅢ的表达呈负相关,说明COX2表达增加可使PGE2表达增加,而PGE2却可使胶原纤维蛋白表达减少,提示COX2/PGE2信号通路在胶原代谢中起重要作用。
> 65岁的POP组与对照组p16的表达均高于 < 55岁组,提示其可能作为组织衰老的指标。而p16在POP组与对照组中的表达无统计学差异,提示这2组组织衰老程度无显著差异。年龄增加导致的组织衰老并不能完全解释POP的发生,在2组患者组织衰老程度无明显差异的情况下,POP组患者细胞外基质中与炎症相关的因子COX2/PGE2的含量与组织中COLⅠ、COLⅢ的含量存在相关性,即COX2/PGE2表达增加可使组织中胶原纤维含量减少,组织成分改变,正常组织呈现病理状态,与POP发生发展相关。COX2/PGE2表达增加可能与既往组织损伤相关,也可能与个体自身调节能力相关,即与遗传因素导致的个体差异相关。推测以上可能是临床患者,尤其是中青年女性POP患者主要的发病机制。
综上所述,本研究结果提示COX2/PGE2信号通路与子宫骶韧带组织中胶原纤维代谢密切相关,而且老龄并不是POP发生的主要危险因素,组织中炎症相关因子的变化使盆底支持组织呈现松弛状态可能是POP发生的主要原因。
| [1] |
BUDATHA M, ROSHANRAVAN S, ZHENG Q, et al. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans[J]. J Clin Invest, 2011, 121(5): 2048-2059. DOI:10.1172/JCI45636 |
| [2] |
MASENGA GG, SHAYO BC, RASCH V. Prevalence and risk factors for pelvic organ prolapse in Kilimanjaro, Tanzania:a population based study in Tanzanian rural community[J]. PLoS One, 2018, 13(4): e0195910. DOI:10.1371/journal.pone.0195910 |
| [3] |
ELBISS HM, OSMAN N, HAMMAD FT. Prevalence, risk factors and severity of symptoms of pelvic organ prolapse among Emirati women[J]. BMC Urol, 2015, 15: 66. DOI:10.1186/s12894-015-0062-1 |
| [4] |
LAPAK KM, BURD CE. The molecular balancing act of p16(INK4a) in cancer and aging[J]. Mol Cancer Res, 2014, 12(2): 167-183. DOI:10.1158/1541-7786.MCR-13-0350 |
| [5] |
CHEN EP, SMYTH EM. COX-2 and PGE2-dependent immunomodulation in breast cancer[J]. Prostaglandins Other Lipid Mediat, 2011, 96(1/4): 14-20. DOI:10.1016/j.prostaglandins.2011.08.005 |
| [6] |
SPIESZ EM, THORPE CT, CHAUDHRY S, et al. Tendon extracellular matrix damage, degradation and inflammation in response to in vitro overload exercise[J]. J Orthop Res, 2015, 33(6): 889-897. DOI:10.1002/jor.22879 |
| [7] |
KUSHNER L, MATHRUBUTHAM M, BURNEY T, et al. Excretion of collagen derived peptides is increased in women with stress urinary incontinence[J]. Neurourol Urodyn, 2004, 23(3): 198-203. DOI:10.1002/nau.10174 |
| [8] |
LEEGANT A, ZUCKERWISE LC, DOWNING K, et al. Transforming growth factor β1 and extracellular matrix protease expression in the uterosacral ligaments of patients with and without pelvic organ prolapse[J]. Female Pelvic Med Reconstr Surg, 2015, 21(1): 53-58. DOI:10.1097/SPV.0000000000000130 |
| [9] |
BUSCH F, MOBASHERI A, SHAYAN P, et al. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes[J]. J Biol Chem, 2012, 287(45): 38050-38063. DOI:10.1074/jbc.m112.377028 |
| [10] |
LIAO CH, FEI W, SHEN ZH, et al. Expression and distribution of TNF-α and PGE2 of periodontal tissues in rat periodontitis model[J]. Asian Pac J Trop Med, 2014, 7(5): 412-416. DOI:10.1016/S1995-7645(14)60067-5 |
| [11] |
LI Y, LEI D, SWINDELL WR, et al. Age-associated increase in skin fibroblast-derived prostaglandin E2 contributes to reduced collagen levels in elderly human skin[J]. J Invest Dermatol, 2015, 135(9): 2181-2188. DOI:10.1038/jid.2015.157 |
| [12] |
YEN JH, KOCIEDA VP, JING HE, et al. Prostaglandin E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1(AP-1) activation[J]. J Biol Chem, 2011, 286(45): 38913-38923. DOI:10.1074/jbc.M111.252932 |
| [13] |
GOMEZ I, BENYAHIA C, LOUEDEC L, et al. Decreased PGE2 content reduces MMP-1 activity and consequently increases collagen density in human varicose vein[J]. PLoS One, 2014, 9(2): e88021. DOI:10.1371/journal.pone.0088021 |
| [14] |
REMES LENICOV F, PALETTA AL, GONZALEZ PRINZ M, et al. Prostaglandin E2 antagonizes TGF-β actions during the differentiation of monocytes into dendritic cells[J]. Front Immunol, 2018, 9: 1441. DOI:10.3389/fimmu.2018.01441 |
 2020, Vol. 49
2020, Vol. 49




