文章信息
- 张燕平, 赵平
- ZHANG Yanping, ZHAO Ping
- 褪黑素对老年女性患者术后早期认知功能障碍的影响
- Effect of Melatonin on Early Postoperative Cognitive Dysfunction after Geriatric Gynecological Surgery
- 中国医科大学学报, 2019, 48(5): 437-441
- Journal of China Medical University, 2019, 48(5): 437-441
-
文章历史
- 收稿日期:2018-03-02
- 网络出版时间:2019-05-13 10:34
术后认知功能障碍(postoperative cognitive dysfunction,POCD)是手术麻醉后常见的并发症,常发生于老年患者心脏手术或非心脏大手术后,主要临床表现为焦虑烦躁、精神错乱、人格改变、记忆学习功能受损和社交能力下降。其表现依严重程度有所不同,轻度仅表现为兴奋与幻觉,轻度记忆损伤,对指令反应功能障碍,重度者可出现严重的记忆损伤、痴呆、丧失判断和语言概括能力以及人格的改变[1-3]。MOLLER等[3]的多中心临床试验表明 > 60岁行非心脏手术患者术后1周POCD发生率为25.8%,术后3个月POCD发生率为9.9%。
褪黑素(melatonin,MT),又称松果体素,主要由哺乳类动物脑松果体合成与分泌,是一种具有广泛生物活性的吲哚类神经内分泌激素,在人体内广泛分布,主要功能是调节人体的生物节律,保持白天清醒和夜间睡眠的“睡眠-觉醒周期”。研究[4]表明,口服外源性MT能够缩短入眠时间、增加总睡眠时间和提高睡眠质量。MT具有调节和影响多个器官功能的作用,能有效调节免疫系统的功能,还可发挥抗应激的作用。MT还可通过与大脑或脊髓背侧的MT1和MT2受体结合,发挥调节疼痛的作用[5]。
研究[6]表明MT可改善认知功能损害。WU等[7]发现POCD的发生与6-巯基硫酸褪黑素含量的变化显著相关。BORAZAN等[8]发现术前口服MT可降低疼痛评分,减少曲马多的用量,增强睡眠质量、镇静评分和主观术后镇痛效果。HANSEN等[9]发现MT增加乳腺癌根治术患者睡眠效率和总睡眠时间,但不影响认知功能。FAN等[10]发现围术期口服外源性MT可能对早期POCD有积极影响。因此,本研究拟探讨MT是否能够减少老年患者全麻妇科手术POCD发生,增加术后总睡眠时间,并改善术后疼痛。
1 材料与方法 1.1 一般资料采用随机、对照、双盲设计,选择我院2017年9月至2018年1月行择期全身麻醉妇科手术的老年患者90例。纳入标准:年龄≥60岁;ASAⅠ~Ⅲ级;拟在全身麻醉下行择期妇科手术,且手术时间 > 2 h。排除标准:简易精神状态量表(mini-mental state examination,MMSE)评分低于相应文化程度的最低评分(文盲 < 17分,小学 < 20分,中学及以上 < 24分);术前生化检查提示肾功能障碍(血肌酐 > 177 μmol/L)或活动性肝病;中枢神经系统或心理疾病;服用镇静剂、抗抑郁药,酗酒;严重的视力、听力障碍或因其他原因与访视者无法交流;术后不愿接受自控镇痛。本研究已通过中国医科大学附属盛京医院伦理委员会伦理审核。纳入患者均签署知情同意书。
根据随机数字表法将纳入患者按1︰1分为MT组(M组)和安慰剂组(P组)。M组于术前1 d晚8点与术前0.5 h口服MT 6 mg,P组给予安慰剂。记录患者年龄、身高、体质量、受教育程度等一般情况,并于术前1 d晚7:00对所有患者进行MMSE量表评分并详细记录。术后第1、3、7天晚7:00进行MMSE量表评分,评估患者术后早期认知功能;术后第1、2天晚7:00评估患者睡眠质量和疼痛状况。
1.2 麻醉方法患者入室后常规监测无创血压、心电图、血氧饱和度、脑电双频指数(bispectral index,BIS),用麻醉深度监测仪指导麻醉药物用量。开放外周静脉通路,并行桡动脉穿刺监测有创血压。
麻醉诱导:长托宁0.5 mg,枸橼酸舒芬太尼0.2 μg/kg,依托咪酯0.2 mg/kg,苯磺顺阿曲库铵0.2 mg/kg;麻醉维持:采用静吸复合麻醉,七氟烷浓度1.0%~2.0%,N2O︰O2=1︰1,流量2 L/min,盐酸瑞芬太尼6~10 µg·kg-1·h-1。术中BIS维持在40~60之间,呼气末二氧化碳分压(end-tidal carbon dioxide partial pressure,PETCO2)维持在35~45 mmHg。根据BIS、PETCO2和血气分析结果调节麻醉深度和呼吸参数。手术结束前30 min停止给予苯磺顺阿曲库铵,手术结束前5 min停止吸入麻醉药。术中血压低于基础值20%时,根据情况减浅麻醉或给予麻黄素,血压高于基础值20%时,根据情况加深麻醉或给予乌拉地尔或其他降压处理,心率 < 50次/min时给予阿托品0.3 mg静推。术中应尽量减少血管活性药的应用。所有患者均应用术后镇痛泵(枸橼酸舒芬太尼2 µg/kg,酮咯酸氨丁三醇3 mg/kg,盐酸雷莫司琼0.6 mg),并给予负荷剂量。记录患者麻醉时间、手术时间、出血量、尿量、补液量等情况。
1.3 统计学分析采用SPSS 22.0统计软件进行统计分析。计量资料以x±s表示,对单变量计量资料采用两独立样本t检验,计数资料采用χ2检验。P < 0.05为差异有统计学意义。
2 结果2组患者一般资料、手术时间及出血量等均无统计学差异(P > 0.05),见表 1。
| Characteristics | P group (n = 45) | M group (n = 45) | P |
| Age (year) | 67.1±5.5 | 67.2±5.4 | 0.88 |
| Height (cm) | 161.3±4.8 | 162.4±5.7 | 0.30 |
| Body weight (kg) | 67.6±6.2 | 66.7±5.6 | 0.47 |
| Degree of education (0/1/2/3) 1) | 6/15/20/4 | 5/13/23/4 | > 0.05 |
| ASA (Ⅰ/Ⅱ/Ⅲ) | 6/30/9 | 7/31/7 | > 0.05 |
| Operation type (A/B/C/D/E/F/G) 2) | 2/7/4/11/0/12/9 | 2/4/6/11/3/10/9 | > 0.05 |
| Anesthesia time (min) | 199.1±44.7 | 210.7±47.8 | 0.24 |
| Operation time (min) | 166.7±40.6 | 173.3±42.0 | 0.45 |
| Urine volume (mL) | 156.7±62.0 | 158.0±67.4 | 0.92 |
| Estimated blood loss (mL) | 77.3±67.8 | 84.4±86.9 | 0.67 |
| Fluid volume (mL) | 1 873.3±488.7 | 1 891.1±441.0 | 0.86 |
| Side effect | |||
| Headache | 5 | 4 | > 0.05 |
| Dizziness | 3 | 4 | > 0.05 |
| Nausea | 11 | 10 | > 0.05 |
| 1) 0= illiteracy,1=primary,2=junior high,3=high;2) A= hysterectomy,B= hysterectomy+ovariectomy,C= hysterectomy+vaginal repair,D= bladder neck suspension+ rectal prolapse suspension+ urethral suspension extension,E= subtotal hysterectomy+ abdominal lymph node dissection,F= extensive hysterectomy+ abdominal lymph node dissection,G= ovarian cancer radical surgery. | |||
2组术前1 d MMSE评分差异无统计学意义(P > 0.05);与术前1 d比较,术后不同时间点2组MMSE评分明显降低(P < 0.05);与P组比较,M组术后不同时间点MMSE评分明显增加(P < 0.05)。见图 1。
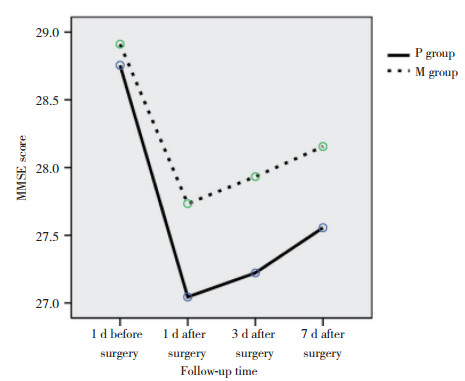
|
| 图 1 2组患者手术前后MMSE评分 Fig.1 MMSE score of two groups before and after surgery |
与P组比较,M组术后第1天、2天总睡眠时间明显增加(P < 0.05),见表 2。
| Time after operation (d) | P group | M group | P |
| 1 | 5.02±0.99 | 5.98±0.92 | < 0.001 |
| 2 | 6.76±1.11 | 7.29±0.80 | 0.01 |
与P组比较,M组术后第1天、2天VAS疼痛评分差异无统计学意义(P > 0.05),见表 3。
| Time after operation (d) | P group | M group | P |
| 1 | 3.84±1.54 | 3.69±1.38 | 0.62 |
| 2 | 2.04±1.15 | 1.71±0.97 | 0.14 |
3 讨论
POCD是一种多因素疾病,发病机制尚不清楚,目前已知年龄是最主要的危险因素[2-3, 11],炎症应激反应可能是最重要的发病机制[12-14]。患者睡眠质量低、昼夜觉醒周期紊乱及围术期疼痛可能也是导致POCD发生的重要因素[14-16]。POCD的发病高峰一般出现在术后1~3 d [17],并可持续至术后数月[3]。有文献[3]报道非心脏手术后3个月POCD发生率仍达9.9%,严重影响了患者术后的生活质量,不可避免地导致患者的尊严和独立性丧失,也消耗了家庭成员的精力,并加重了家庭经济负担[18]。
中枢神经系统神经炎症引发并加速了神经系统退行性病变,短期内的表现如POCD和谵妄,长期不良后果如阿尔茨海默病等[12, 19]。免疫系统是介导急性期神经炎症的主要器官,从细胞、分子水平介导神经炎症的级联反应[12, 20-21]。MT可从多方面调节免疫系统。研究发现,对新生大鼠进行松果体摘除术可使其胸腺退化、免疫功能下降;将健康小鼠的松果体移植到老年体弱的小鼠脑中,发现其萎缩的胸腺再次发育。MT除松果体外也可在人体的淋巴细胞内合成,说明其与免疫作用有重要的相关性。MT生物学效应的分子机制主要涉及2个方面:与高亲和力的G蛋白耦联受体在细胞膜水平结合;和(或)与鞭细胞相互作用,调节信号传导途径和氧化还原过程[22]。这可能是MT减轻神经系统炎症、改善POCD的关键步骤。周围环境、药物影响、术后疼痛、麻醉均会影响正常睡眠觉醒周期,MT可对维持正常的睡眠-觉醒周期发挥关键作用[23]。睡眠-觉醒周期的紊乱会导致认知功能受损[24]。睡眠紊乱会导致术后痛觉过敏,严重疼痛同样会扰乱睡眠周期,两者互为因果,加重POCD的发生。MT能够减轻术后组织损伤的疼痛[25],这可能是由于MT可降低疼痛阈值[10],也可能是因为MT影响β-内啡肽活性,从而间接调控疼痛的阈值[26]。由此可见,MT可通过多种机制改善认知功能,减轻认知损害。
本研究纳入患者均使用自控镇痛泵,2组患者术后第1天、第2天的VAS疼痛评分无统计学差异。舒芬太尼镇痛泵能够显著降低术后疼痛,可能掩盖了MT在调控疼痛方面的作用。后续研究中将选择无镇痛泵的短小择期手术,以进一步观察MT在术后镇痛方面的作用。
目前尚无统一的POCD评价方法与诊断标准。基本检测方法是通过神经心理学测验和问卷调查评估患者手术前后智力、人格等方面的改变,MMSE量表是应用较为广泛的量表之一。但由于MMSE量表的学习效应,随着重复次数的增多可能降低认知损害的检出率[2]。本研究中,术后7 d POCD发生率低于国外文献的报道可能与此有关。
综上所述,本研究发现,MT可减少老年患者妇科手术POCD的发生,延长术后总睡眠时间,但不能改善术后疼痛。
| [1] |
EVERED L, SILBERT B, SCOTT DA, et al. Cerebrospinal fluid biomarker for Alzheimer disease predicts postoperative cognitive dysfunction[J]. Anesthesiology, 2016, 124(2): 353-361. DOI:10.1097/ALN0000000000000953 |
| [2] |
STEINMETZ J, CHRISTENSEN KB, LUND T, et al. Long-term consequences of postoperative cognitive dysfunction[J]. Anesthesiology, 2009, 110(3): 548-555. DOI:10.1097/ALN.0b013e318195b569 |
| [3] |
MOLLER JT, CLUITMANS P, RASMUSSEN LS, et al. Long-term postoperative cognitive dysfunction in the elderly:ISPOCD1 study[J]. Lancet, 1998, 351(9106): 857-861. DOI:10.1016/S0140-6736(97)07382-0 |
| [4] |
HUANG HW, ZHENG BL, JIANG L, et al. Effect of oral melatonin and wearing earplugs and eye masks on nocturnal sleep in healthy subjects in a simulated intensive care unit environment:which might be a more promising strategy for ICU sleep deprivation?[J]. Crit Care, 2015, 19: 124. DOI:10.1186/s13054-015-0842-8 |
| [5] |
ZUROWSKI D NL, MACHOWSKA A. Exogenous melatonin abolishes mechanical allodynia but notthermal hyperalgesia in neuropathic pain. The role of the opioid system and benzodiazepine-gabaergic mechanism[J]. J Physiol Pharmacol, 2012, 63(6): 641-647. DOI:10.1152/advan.00073.2012 |
| [6] |
FORBES D, JANSEN S L, DUNCAN V, et al. Melatonin for cognitive impairment (Protocol)[J]. Cochrane Database Syst Rev, 2009, 25(1): CD003802. DOI:10.1002/14651858.CD003802.pub3 |
| [7] |
WU Y, WANG JW, WU AS, et al. Do fluctuations in endogenous melatonin levels predict the occurrence of postoperative cognitive dysfunction (POCD)?[J]. Int J Neurosci, 2014, 124(11): 787-791. DOI:10.3109/00207454.2014.882919 |
| [8] |
BORAZAN H, TUNCER S, YALCIN N, et al. Effects of preoperative oral melatonin medication on postoperative analgesia, sleep quality, and sedation in patients undergoing elective prostatectomy:a randomized clinical trial[J]. J Anesth, 2010, 24(2): 155-160. DOI:10.1007/s00540-010-0891-8 |
| [9] |
HANSEN MV, MADSEN MT, ANDERSEN LT, et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery:a randomized, double-blind, placebo-controlled trial[J]. Int J Breast Cancer, 2014, 2014: 1-9. DOI:10.1155/2014/416531 |
| [10] |
FAN YX, YUAN L, JI MH, et al. The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty:a randomized controlled trial[J]. J Clin Anesth, 2017, 39: 77-81. DOI:10.1016/j.jclinane.2017.03.023 |
| [11] |
MONK TG, WELDON BC, GARVAN CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery[J]. Anesthesiology, 2008, 108(1): 18-30. DOI:10.1097/01.anes.0000296071.19434.1e |
| [12] |
LYMAN M, LLOYD DG, JI XM, et al. Neuroinflammation:the role and consequences[J]. Neurosci Res, 2014, 79: 1-12. DOI:10.1016/j.neures.2013.10.004 |
| [13] |
HARTEN AEV, SCHEEREN TWL, ABSALOM AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia[J]. Anaesthesia, 2012, 67(3): 280-293. DOI:10.1111/j.1365-2044.2011.07008.x |
| [14] |
KRENK L, RASMUSSEN LS, KEHLET H. New insights into the pathophysiology of postoperative cognitive dysfunction[J]. Acta Anaesthesiol Scand, 2010, 54(8): 951-956. DOI:10.1111/j.1399-6576.2010.02268.x |
| [15] |
FENG L, WU HW, SONG GQ, et al. Chronical sleep interruption-induced cognitive decline assessed by a metabolomics method[J]. Behav Brain Res, 2016, 302: 60-68. DOI:10.1016/j.bbr.2015.12.039 |
| [16] |
WALKER MP. Cognitive consequences of sleep and sleep loss[J]. Sleep Med, 2008, 9(Suppl1): S29-S34. DOI:10.1016/S1389-9457(08)70014-5 |
| [17] |
章放香, 宁俊平, 邱冰, 等. 不同麻醉老年患者术后认知功能障碍发生的比较[J]. 中华麻醉学杂志, 2013, 33(2): 188-190. DOI:10.3760/cma.j.issn.0254-1416.2013.02.014 |
| [18] |
VACAS S, DEGOS V, MAZE M. Fragmented sleep enhances postoperative neuroinflammation but not cognitive dysfunction[J]. Anesth Analg, 2017, 124(1): 270-276. DOI:10.1213/ANE.0000000000001675 |
| [19] |
WITLOX J, EURELINGS LSM, JONGHE JFMD, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia a meta-analysis[J]. JAMA, 2010, 304(4): 443-451. DOI:10.1001/jama.2010.1013 |
| [20] |
WONGPRAYOON P, GOVITRAPONG P. Melatonin attenuates methamphetamine-induced neuroinflammation through the melatonin receptor in the SH-SY5Y cell line[J]. Neurotoxicology, 2015, 50: 122-130. DOI:10.1016/j.neuro.2015.08.008 |
| [21] |
TERRANDO N, MONACO C, MA DQ, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline[J]. Proc Natl Acad Sci USA, 2010, 107(47): 20518-20522. DOI:10.1073/pnas.1014557107 |
| [22] |
HARDELAND R, MADRID JA, TAN DX, et al. Melatonin, the circadian multioscillator system and health:the need for detailed analyses of peripheral melatonin signaling[J]. J Pineal Res, 2012, 52(2): 139-166. DOI:10.1111/j.1600-079x.2011.00934.x |
| [23] |
ALGHAMDI BS. The neuroprotective role of melatonin in neurological disorders[J]. J NeurosciRes, 2018, 96(7): 1136-1149. DOI:10.1002/jnr.24220 |
| [24] |
WRIGHT KP, HULL JT, HUGHES RJ, et al. Sleep and wakefulness out of phase with internal biological time impairs learning in humans[J]. J Cogn Neurosci, 2006, 18(4): 508-521. DOI:10.1162/jocn.2006.18.4.508 |
| [25] |
DANILOV A, KURGANOVA J. Melatonin in chronic pain syndromes[J]. Pain Ther, 2016, 5(1): 1-17. DOI:10.1007/s40122-016-0049-y |
| [26] |
SHAVALI S, HO B, GOVITRAPONG P, et al. Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of beta-endorphin an endogenous opioid[J]. Brain Res Bullet, 2005, 64(6): 471-479. DOI:10.1016/j.brainresbull.2004.09.008 |
 2019, Vol. 48
2019, Vol. 48




