文章信息
- 徐杰, 张一杨, 马玉, 贾云鹏, 杜莹, 冯福民
- XU Jie, ZHANG Yiyang, MA Yu, JIA Yunpeng, DU Ying, FENG Fumin
- SIRT1及炎性细胞因子在不同抗结核药物致肝损伤中的作用
- Role of SIRT1 and Inflammatory Factors in Liver Injury Induced by Different Anti-Tuberculosis Drugs
- 中国医科大学学报, 2019, 48(11): 983-989
- Journal of China Medical University, 2019, 48(11): 983-989
-
文章历史
- 收稿日期:2018-09-05
- 网络出版时间:2019-11-20 13:00
结核病是全球第九大致死疾病,患者主要死因为单一病原体感染造成[1]。世界卫生组织推荐的标准抗结核治疗方案是将异烟肼(isoniazid,INH)、利福平(rifampin,RFP)、吡嗪酰胺(pyrazinamide,PZA)作为不可替代的一线抗结核药物,但用药后可导致患者产生明显的抗结核药物性肝损伤(anti-tuberclosis drug-induced liver injury,ADLI)[2]。目前,ADLI的发生机制尚不明确,随着对其研究的不断深入,发现在肝细胞损伤后,打破了组蛋白乙酰化酶(histone acetyltransferase,HAT)和组蛋白去乙酰化酶(histone deacetylase,HDAC)之间动态平衡,造成炎性细胞因子等异常表达[3]。已有研究[4-8]显示HDAC沉默信息调节因子-1(silent mating type information regulation 2 homolog-1,SIRT1)与核转录因子-κB(nuclear factor-κB,NF-κB)在肝脏炎症等损伤中起重要作用,且NF-κB是SIRT1的靶分子,能够调控下游炎性细胞因子IL-6、TNF-α的表达。故推测HDAC SIRT1可能通过作用于NF-κB信号通路调控ADLI过程中炎症反应的发生。
本研究在INH、RFP、PZA致人肝细胞损伤模型中观察SIRT1的表达变化,并分别给予特异性SIRT1激动剂、抑制剂干预,观察其对炎性细胞因子的影响,进而探讨SIRT1在不同的抗结核药物致肝损伤过程中的作用,为ADLI治疗提供依据。
1 材料与方法 1.1 试剂与仪器人正常肝细胞株HL-7702购自上海中科院细胞所;异烟肼(批号YSMXE-OM)、二甲基亚砜(dimethyl sulfoxide,DMSO;批号W3OKK-MI)购自日本TCI公司;青霉素与链霉素(批号1634678)购自德国BI公司;0.25%胰蛋白酶(批号25200-072)购自美国GIBCO公司;SIRT1激动剂SRT1720(批号S112906)和抑制剂EX527(批号S154103)均购自美国Selleck公司;PrimeScriptTM RT Master Mix试剂盒(批号AK4601)、SYBR® Premix Ex TaqTMⅡPCR试剂盒(批号AK4602)购自大连宝生物工程有限公司;TRI pure Reagent总RNA抽提试剂盒(批号0020161010)购自北京百泰克生物技术有限公司;ALT、AST试剂盒(批号20170301)购自南京建成生物工程研究所;SIRT1、NF-κB p65、TNF-α ELISA检测试剂盒(批号201612)购自北京冬歌伟业生物技术有限公司;IL-6 ELISA检测试剂盒(批号210660124)购自杭州联科生物技术有限公司。Heraeus高速冷冻离心机(德国Thermo Scientific公司)、CA94089型连续光谱酶标仪(美国Molecular Devices公司)、C1000PCR扩增仪(美国BIO-RAD公司)、实时荧光定量PCR仪(美国Applied Biosystems公司)。
1.2 方法 1.2.1 细胞培养将HL-7702细胞接种于含90%RPMI 1640、10%胎牛血清、1%双抗培养液中,收集细胞悬液,于37 ℃、5%CO2的细胞培养箱中进行培养。对于未长满的细胞进行隔天换液;对于生长密度达到培养瓶底部面积80%以上的细胞进行传代处理。选取对数生长期细胞进行同细胞传代,调整细胞浓度为1×105/mL,接种到6孔板上,每孔为2 mL,接种预培养24 h后给予相应处理,于48 h后收集细胞及培养液进行检测。
1.2.2 细胞分组及干预确定最佳药物浓度(800 µg/mL INH、200 µg/mL RFP、400 µg/mL PZA)及SIRT1激动剂、抑制剂浓度(1 µmol/L SIRT1激动剂SRT1720、1 µmol/L SIRT1抑制剂EX527)后,将细胞分为INH组、RFP组、PZA组3组。各组再分为以下亚组,空白对照组、药物组、药物+SIRT1激动剂(SRT1720)组、激动剂对照组、药物+SIRT1抑制剂(EX527)组和抑制剂对照组。
1.2.3 细胞上清液中谷丙转氨酶(alanine transaminase,ALT)、谷草转氨酶(aspartate transaminase,AST)含量检测收集细胞培养的上清液,严格按照ALT、AST检测试剂盒说明书进行操作,测定ALT、AST含量。
1.2.4 肝细胞中SIRT1、NF-κB p65 mRNA及其靶基因IL-6、TNF-α mRNA表达检测采用实时PCR方法测定,严格按照试剂盒说明书进行操作。收集各组处理后的细胞用Trizol提取RNA,mRNA表达水平检测以GAPDH为内参,采用2-ΔΔCT计算mRNA表达水平;酶标仪检测各组RNA样本吸光度,OD260/OD280在1.8~2.0范围内提示RNA纯度较好。取RNA在逆转录酶的作用下合成cDNA。用SYBR Green嵌合荧光法进行实时PCR扩增。反转录条件为37 ℃ 15 min,85 ℃5 s,4 ℃ 1 min。定量PCR反应条件为95 ℃预变性30 s,95 ℃变性5 s,60 ℃退火30 s,72 ℃延伸60 s,共40个循环,引物由上海生物工程公司负责设计与合成,引物序列见表 1。
| Gene | Primer sequence 5’-3’ |
| GAPDH | F,GAAGGTCGGAGTCAACGGATT;R,CCTGGAAGATGGTGATGGGAT |
| SIRT1 | F,CATAGACACGCTGGAACAGG;R,TTGAGGGAAGACCCAATAACA |
| NF-κB p65 | F,GCGAGAGGAGCACAGATACC;R,GCACAGCATTCAGGTCGTAG |
| IL-6 | F,CACACAGACAGCCACTCACC;R,GCTCTGGCTTGTTCCTCACT |
| TNF-α | F,CAGCCTCTTCTCCTTCCTGA;R,TGAGGTACAGGCCCTCTGAT |
1.2.5 肝细胞中SIRT1、NF-κB p65、IL-6、TNF-α蛋白含量检测
采用Western blotting检测肝细胞中SIRT1、NF-κB p65蛋白表达水平;采用酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)检测肝细胞中IL-6、TNF-α蛋白表达水平,严格参照试剂盒说明书进行操作。
1.3 统计学分析采用SPSS 23.0统计软件进行数据分析。各组间差异比较采用单因素方差分析,两两比较采用SNK-q检验,P < 0.05为差异有统计学意义。
2 结果 2.1 各组肝细胞形态学变化HE染色后于光学显微镜下观察各组细胞形态,结果显示,空白对照组、SIRT1激动剂对照组和SIRT1抑制剂对照组的肝细胞密集,形态正常,提示对照组细胞正常,肝细胞对激动剂和抑制剂无不良反应。而各药物组肝细胞数量明显减少,形态异常。联用SIRT1激动剂后可减轻肝细胞损伤情况,而联用SIRT1抑制剂则加重了细胞损伤。见图 1。
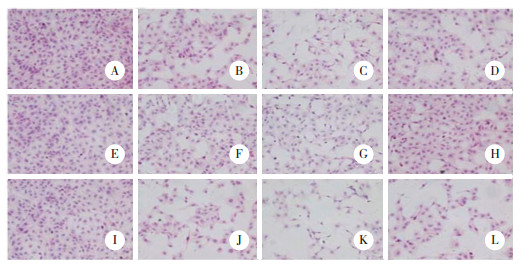
|
| A, control group; B, INH group; C, RFP group; D, PZA group; E, SRT1720 group; F, INH+ SRT1720 group; G, RFP+ SRT1720 group; H, PZA+ SRT1720 group; I, EX527 group; J, INH+ EX527 group; K, RFP+ EX527 group; L, PZA+ EX527 group. 图 1 各组肝细胞形态学变化 HE×100 Fig.1 Morphological changes of HL-7702 in the different treatment groups HE×100 |
2.2 各组细胞上清液中ALT和AST含量比较
与其空白对照组比较,INH组、RFP组、PZA组均引起上清液ALT、AST含量增加,差异具有统计学意义(均P < 0.05),提示造模成功。SIRT1激动剂对照组和抑制剂对照组则与空白对照组比较差异均无统计学意义(P > 0.05),提示激动剂、抑制剂对人肝细胞无影响。INH、RFP、PZA分别联用SIRT1激动剂,使ALT、AST含量降低;而联用SIRT1抑制剂使ALT、AST含量升高(均P < 0.05),提示加入SIRT1激动剂可缓解抗结核药物导致的肝细胞损伤,加入SIRT1抑制剂则加重了损伤发生。见表 2~4。
| Group | ALT | AST |
| Control | 4.95±0.25 | 7.58±0.52 |
| INH | 10.45±0.621) | 12.89±0.541) |
| INH+ SRT1720 | 8.02±0.382) | 10.74±0.542) |
| SRT1720 | 3.03±0.24 | 7.90±0.27 |
| INH+ EX527 | 13.61±0.632) | 15.96±1.082) |
| EX527 | 5.01±0.17 | 8.07±0.45 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs INH group. | ||
| Group | ALT | AST |
| Control | 4.97±0.57 | 7.23±0.46 |
| RFP | 10.75±0.751) | 13.04±0.381) |
| RFP+ SRT1720 | 7.44±0.342) | 9.49±0.452) |
| SRT1720 | 5.06±0.31 | 7.61±0.25 |
| RFP+ EX527 | 14.72±0.812) | 16.13±0.642) |
| EX527 | 5.16±0.65 | 7.61±0.44 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs RFP group. | ||
| Group | ALT | AST |
| Control | 4.98±0.41 | 7.30±0.25 |
| PZA | 9.63±0.451) | 13.05±0.541) |
| PZA+ SRT1720 | 7.60±0.192) | 10.36±0.312) |
| SRT1720 | 5.13±0.14 | 7.38±0.15 |
| PZA+ EX527 | 12.50±0.392) | 16.57±0.542) |
| EX527 | 5.39±0.49 | 7.23±0.30 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs PZA group. | ||
2.3 各组细胞相关指标mRNA及蛋白表达变化
与其空白对照组比较,INH、RFP、PZA刺激均造成HL-7702细胞的SIRT1 mRNA和蛋白表达降低,差异具有统计学意义(均P < 0.05),提示SIRT1表达参与抗结核药物致肝细胞损伤过程。其中INH组的NF-κB p65、IL-6及TNF-α mRNA及蛋白表达与空白对照组比较差异具有统计学意义(均P < 0.05);而与空白对照组比较,RFP组与PZA组各指标差异无统计学意义(均P > 0.05),提示INH组细胞出现了炎症损伤,而RFP组和PZA组细胞的炎症损伤并不明显。SIRT1激动剂对照组和抑制剂对照组与空白对照组相关指标比较差异无统计学意义(均P > 0.05),提示SIRT1激动剂及抑制剂对细胞无影响。
与INH组比较,INH+ SRT1720组NF-κB p65、IL-6及TNF-α mRNA及蛋白表达均减少(均P < 0.05),提示SIRT1的激活可以减轻细胞炎症损伤。而INH+ EX527组与INH组比较NF-κB p65、IL-6及TNF-α mRNA及蛋白表达均增加(均P < 0.05),说明SIRT1的抑制可加重细胞炎症损伤。见表 5~10,图 2~4。
| Group | SIRT1 | NF-κB | IL-6 | TNF-α |
| Control | 0.97±0.18 | 1.01±0.15 | 1.02±0.22 | 1.07±0.48 |
| INH | 0.67±0.081) | 2.33±0.241) | 1.99±0.291) | 2.22±0.511) |
| INH+ SRT1720 | 0.87±0.142) | 1.62±0.092) | 1.43±0.072) | 1.50±0.232) |
| SRT1720 | 1.24±0.21 | 0.97±0.12 | 0.94±0.15 | 1.05±0.17 |
| INH+ EX527 | 0.45±0.122) | 3.01±0.262) | 2.73±0.202) | 3.17±0.462) |
| EX527 | 0.87±0.23 | 1.03±0.05 | 1.03±0.31 | 1.04±0.29 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs INH group. | ||||
| Group | SIRT1 | NF-κB | IL-6 | TNF-α |
| Control | 1.00±0.04 | 0.92±0.23 | 1.01±0.16 | 1.01±0.15 |
| RFP | 0.73±0.051) | 1.33±0.09 | 1.29±0.22 | 1.36±0.46 |
| RFP + SRT1720 | 0.86±0.042) | 1.20±0.20 | 1.14±0.04 | 1.20±0.20 |
| SRT1720 | 1.05±0.07 | 1.04±0.05 | 1.17±0.24 | 1.03±0.56 |
| RFP + EX527 | 0.56±0.052) | 1.42±0.22 | 1.35±0.10 | 1.45±0.10 |
| EX527 | 0.93±0.10 | 0.98±0.06 | 1.08±0.18 | 1.04±0.30 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs RFP group. | ||||
| Group | SIRT1 | NF-κB | IL-6 | TNF-α |
| Control | 1.01±0.12 | 1.04±0.32 | 1.01±0.13 | 1.01±0.11 |
| PZA | 0.77±0.011) | 1.28±0.07 | 1.24±0.43 | 1.29±0.36 |
| PZA + SRT1720 | 0.87±0.052) | 1.20±0.27 | 1.14±0.26 | 1.16±0.06 |
| SRT1720 | 1.01±0.06 | 1.01±0.22 | 1.01±0.03 | 1.10±0.07 |
| PZA + EX527 | 0.60±0.082) | 1.43±0.12 | 1.35±0.07 | 1.42±0.06 |
| EX527 | 0.97±0.05 | 1.06±0.27 | 1.08±0.02 | 1.01±0.05 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs PZA group. | ||||
| Group | SIRT1 | NF-κB | IL-6 | TNF-α |
| Control | 0.71±0.02 | 0.79±0.00 | 47.38±1.50 | 80.51±1.04 |
| INH | 0.43±0.011) | 1.11±0.011) | 56.31±1.001) | 91.98±1.031) |
| INH+ SRT1720 | 0.53±0.052) | 0.98±0.022) | 52.43±0.852) | 87.31±0.782) |
| SRT1720 | 0.70±0.01 | 0.79±0.01 | 47.03±1.84 | 79.65±1.83 |
| INH+ EX527 | 0.31±0.042) | 1.23±0.032) | 61.00±1.652) | 96.57±0.852) |
| EX527 | 0.71±0.01 | 0.79±0.01 | 47.04±1.31 | 80.24±1.30 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs INH group. | ||||
| Group | SIRT1 | NF-κB | IL-6 | TNF-α |
| Control | 0.71±0.01 | 0.78±0.01 | 51.07±1.71 | 73.52±0.42 |
| RFP | 0.45±0.021) | 0.79±0.01 | 53.10±4.72 | 75.30±4.99 |
| RFP + SRT1720 | 0.56±0.042) | 0.78±0.00 | 52.18±3.19 | 74.65±5.66 |
| SRT1720 | 0.70±0.01 | 0.78±0.00 | 51.02±4.60 | 73.75±2.01 |
| RFP + EX527 | 0.31±0.052) | 0.78±0.01 | 54.21±2.81 | 76.25±5.54 |
| EX527 | 0.70±0.00 | 0.78±0.01 | 51.44±4.42 | 73.47±3.39 |
| 1)P < 0.05 vs control group;2)P < 0.05 vs RFP group. | ||||
| Group | SIRT1 | NF-κB | IL-6 | TNF-α |
| Control | 0.75±0.00 | 0.78±0.13 | 48.58±7.10 | 71.70±5.39 |
| PZA | 0.46±0.031) | 0.79±0.01 | 51.85±7.18 | 74.34±5.68 |
| PZA + SRT1720 | 0.57±0.012) | 0.78±0.01 | 50.01±8.21 | 72.45±2.51 |
| SRT1720 | 0.72±0.02 | 0.78±0.00 | 49.73±4.13 | 71.41±1.76 |
| PZA + EX527 | 0.35±0.052) | 0.78±0.00 | 53.15±8.16 | 75.98±4.57 |
| EX527 | 0.71±0.03 | 0.78±0.00 | 49.17±3.86 | 71.34±5.00 |
| 1) P < 0.05 vs control group;2)P < 0.05 vs PZA group. | ||||
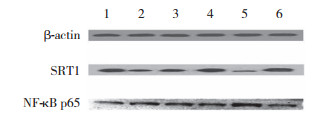
|
| 1, control group; 2, INH group; 3, INH+ SRT1720 group; 4, SRT1720 group; 5, INH+ EX527 group; 6, EX527 group. 图 2 INH各亚组细胞中SIRT1、NF-κB蛋白表达比较 Fig.2 Comparison of SIRT1 and NF-κB protein expressim in cells of each subgroups treated with isoniazid |
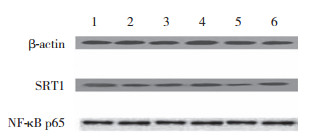
|
| 1, Control group; 2, RFP group; 3, RFP + SRT1720 group; 4, SRT1720 group; 5, RFP + EX527 group; 6, EX527 group. 图 3 RFP各亚组细胞中SIRT1、NF-κB蛋白表达比较 Fig.3 Comparison of SIRT1 and NF-κB protein expression in cells of each subgroups treated with rifampin |
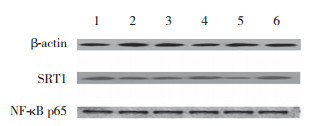
|
| 1, control group; 2, PZA group; 3, PZA + SRT1720 group; 4, SRT1720 group; 5, PZA + EX527 group; 6, EX527 group. 图 4 PZA各亚组细胞中SIRT1、NF-κB蛋白表达比较 Fig.4 Comparison of SIRT1 and NF-κB protein expression in the cells of each subgroups treated with pyrazinamide |
3 讨论
一线抗结核药物INH、RFP和PZA最严重且最受临床关注的不良反应就是ADLI,为此深入探讨ADLI的发生机制就显得尤为重要。SIRT1是一类烟酰胺腺嘌呤二核苷酸(NAD+)依赖性的Ⅲ型HDAC,且肝脏是SIRT1主要表达的器官之一[9],已被证实它与细胞凋亡、抗氧化应激以及抑制炎症等细胞的多种功能活动有关[10-13]。例如小鼠骨髓细胞特异性破坏SIRT1时,提示腹腔巨噬细胞缺乏SIRT1可诱导NF-κB过度乙酰化,增加肝脏NF-κB转录激活,导致肝脏炎症[14]。这些都提示SIRT1表达改变与肝损伤发生密切相关。本研究通过建立ADLI细胞模型,检测SIRT1的表达水平发现,INH组、RFP组、PZA组中SIRT1表达水平与空白对照组比较均降低,证明SIRT1表达下调在ADLI发生机制中可能起着重要作用。
抗结核药物代谢过程中出现炎症反应越来越受到人们关注,这很可能为ADLI防治提供新的参考依据。目前,大多数观点认为SIRT1可调控炎症反应相关信号通路,但具体机制尚不清楚。NF-κB是SIRT1的靶分子,它能够调控下游炎性细胞因子IL-6、TNF-α的表达。有研究[15]报道,SIRT1激活可抑制NF-κB信号转导,促进炎症消退。NLRP3炎症小体通过NF-κB信号传导参与了日本血吸虫肝纤维化进程,进而影响下游炎性细胞因子的表达,提示NF-κB与肝脏疾病密切相关,并在炎症发生中起着极其重要的作用[16]。本研究结果显示,与空白对照组比较,INH、RFP、PZA 3组均可引起肝细胞损伤中SIRT1表达减少,但是只有INH组的NF-κB p65、IL-6及TNF-α的表达水平升高。为了观察SIRT1表达变化对各组肝细胞炎症损伤的影响,加入SIRT1激动剂后发现,只有INH组诱导的NF-κB p65呈现低表达,进而减少了IL-6、TNF-α等炎性细胞因子的产生;加入SIRT1抑制剂后,只有INH组诱导的炎性细胞因子NF-κB p65、IL-6、TNF-α的表达随之升高。这表明INH组的细胞出现了炎症损伤,而RFP与PZA组虽然导致SIRT1表达改变,但是其炎症损伤并不明显。故推测INH导致的人肝细胞炎症损伤的机制可能是SIRT1通过作用于NF-κB p65信号通路进而影响IL-6、TNF-α等炎性细胞因子表达产生的。
AST和ALT是反映肝损害的敏感指标,可用于评估肝功能或肝损伤。本研究中ALT、AST的升高表示INH、RFP与PZA 3组细胞在抗结核药物作用下出现了一定的肝损伤,加入SIRT1激动剂、抑制剂后观察ALT、AST水平发现,人肝细胞损伤情况也随之发生了改变。但观察SIRT1表达对炎性细胞因子影响时却发现,只有INH组出现了炎症损伤,RFP、PZA组炎症损伤不明显。因此推测这很有可能与过氧化物酶增殖物激活受体(peroxisome proliferator activated receptor,PPAR)信号通路有关。早有研究报道PPAR信号通路是SIRT1的调控靶点,SIRT1可以通过PPAR抑制脂肪细胞分化[17],并且PPAR激动剂能够影响人体内肝脏脂质稳态和能量平衡,使用几种激动剂重复激活PPAR可以导致人肝素细胞减少,脂肪变性[18]。而RFP治疗可显著升高血浆ALT、AST水平,其组织病理学典型表现为肝脂肪变性[19]。还有研究[20]发现,PZA诱导肝脏脂质代谢紊乱,PPAR表达下调,与肝脏损伤程度呈显著负相关。故推测SIRT1影响RFP、PZA组ALT、AST变化造成肝脏损伤是通过调控PPAR信号通路来实现的。
综上所述,本研究证实SIRT1参与INH致人肝损伤的机制是通过作用于NF-κB p65信号通路进而影响炎性细胞因子的表达来实现的,并且SIRT1可能通过调控PPAR信号通路参与到RFP、PZA致人肝细胞损伤的过程中。本研究没有考虑细胞系的多样性,只运用人正常肝细胞系HL-7702进行了实验,因此仍需在其他人正常肝细胞系中验证相关机制,以便为ADLI的防治提供新依据。
| [1] |
MOLTON JS, SINGH S, CHEN LJ, et al. International tuberculosis research collaborations within Asia[J]. BMC Res Notes, 2017, 10(1): 462. DOI:10.1186/s13104-017-2769-4 |
| [2] |
World Health Organization. Global tuberculosis report 2017[M]. Geneva: World Health Organization, 2017.
|
| [3] |
李金凤, 杜莹, 李标, 等. 异烟肼致肝细胞损伤过程中组蛋白乙酰化对内质网应激的调控作用[J]. 山东医药, 2018, 58(8): 34-37. DOI:10.3969/j.issn.1002-266X.2018.08.009 |
| [4] |
WANG Q, YAN C, XIN MM, et al. Sirtuin 1(Sirt1) overexpression in BaF3 cells contributes to cell proliferation promotion, apoptosis resistance and pro-inflammatory cytokine production[J]. Med Sci Monit, 2017, 23: 1477-1482. DOI:10.12659/msm.900754 |
| [5] |
FAN Z, JING HR, YAO JH, et al. The protective effects of curcumin on experimental acute liver lesion induced by intestinal ischemia-reperfusion through inhibiting the pathway of NF-κB in a rat model[J]. Oxidative Med Cell Longev, 2014, 2014: 1-8. DOI:10.1155/2014/191624 |
| [6] |
CUI XL, CHEN Q, DONG Z, et al. Inactivation of Sirt1 in mouse livers protects against endotoxemic liver injury by acetylating and activating NF-κB[J]. Cell Death Dis, 2016, 7(10): e2403. DOI:10.1038/cddis.2016.270 |
| [7] |
DENG ZT, JIN JW, WANG ZH, et al. The metal nanoparticle-induced inflammatory response is regulated by SIRT1 through NF-κB deacetylation in aseptic loosening[J]. Int J Nanomedicine, 2017, 12: 3617-3636. DOI:10.2147/IJN.S124661 |
| [8] |
史哲.一线抗结核药物诱导小鼠肝损伤对肝细胞NF-κB表达的影响[D].唐山: 华北理工大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10081-1015808232.htm
|
| [9] |
DING RB, BAO JL, DENG CX. Emerging roles of SIRT1 in fatty liver diseases[J]. Int J Biol Sci, 2017, 13(7): 852-867. DOI:10.7150/ijbs.19370 |
| [10] |
WELLMAN AS, METUKURI MR, KAZGAN N, et al. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota[J]. Gastroenterology, 2017, 153(3): 772-786. DOI:10.1053/j.gastro.2017.05.022 |
| [11] |
YOSHIMURA K, MATSUU A, SASAKI K, et al. Detection of Sirtuin-1 protein expression in peripheral blood leukocytes in dogs[J]. J Vet Med Sci, 2018, 80(7): 1068-1076. DOI:10.1292/jvms.17-0499 |
| [12] |
NAKAMURA K, ZHANG M, KAGEYAMA S, et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury[J]. J Hepatol, 2017, 67(6): 1232-1242. DOI:10.1016/j.jhep.2017.08.010 |
| [13] |
KANG XM, YANG W, WANG RQ, et al. Sirtuin-1(SIRT1) stimulates growth-plate chondrogenesis by attenuating the PERK-eIF-2α-CHOP pathway in the unfolded protein response[J]. J Biol Chem, 2018, 293(22): 8614-8625. DOI:10.1074/jbc.M117.809822 |
| [14] |
DING RB, BAO JL, DENG CX. Emerging roles of SIRT1 in fatty liver diseases[J]. Int J Biol Sci, 2017, 13(7): 852-867. DOI:10.7150/ijbs.19370 |
| [15] |
WANG JY, KAINRAD N, SHEN H, et al. Hepatic knockdown of splicing regulator Slu7 ameliorates inflammation and attenuates liver injury in ethanol-fed mice[J]. Am J Pathol, 2018, 188(8): 1807-1819. DOI:10.1016/j.ajpath.2018.05.004 |
| [16] |
ZHANG WJ, FANG ZM, LIU WQ. NLRP3 inflammasome activation from Kupffer cells is involved in liver fibrosis of Schistosoma japonicum-infected mice via NF-κB[J]. Parasit Vectors, 2019, 12(1): 29. DOI:10.1186/s13071-018-3223-8 |
| [17] |
MAYORAL R, OSBORN O, MCNELIS J, et al. Adipocyte SIRT1 knockout promotes PPARγ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity[J]. Mol Metab, 2015, 4(5): 378-391. DOI:10.1016/j.molmet.2015.02.007 |
| [18] |
BRUNMEIR R, XU F. Functional regulation of PPARs through post-translational modifications[J]. Int J Mol Sci, 2018, 19(6): 1738. DOI:10.3390/ijms19061738 |
| [19] |
黄家慧.利福平诱导小鼠脂肪肝及部分作用机理[D].合肥: 安徽医科大学, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10366-1016149848.htm
|
| [20] |
ZHANG Y, GUO HL, HASSAN HM, et al. Pyrazinamide induced hepatic injury in rats through inhibiting the PPARα pathway[J]. J Appl Toxicol, 2016, 36(12): 1579-1590. DOI:10.1002/jat.3319 |
 2019, Vol. 48
2019, Vol. 48




