文章信息
- 邓纯博, 刘林, 肖正俊, 阿良, 李洪秋
- DENG Chunbo, LIU Lin, XIAO Zhengjun, A Liang, LI Hongqiu
- 骨质疏松小鼠骨组织中miR-21及Smad7的表达及其与骨密度的相关性
- Expression of miR-21 and Smad7 in the Osseous Tissues of Osteoporotic Mice and Its Correlation with Bone Mineral Density
- 中国医科大学学报, 2019, 48(1): 44-47
- Journal of China Medical University, 2019, 48(1): 44-47
-
文章历史
- 收稿日期:2018-02-23
- 网络出版时间:2018-12-29 14:39
Smad7是转化生长因子β /骨形态发生蛋白(transforming growth factor β/bone morphogenetic protein,TGF-β/BMP)信号通路内源性负反馈调节因子[1],Smad7与R-Smad蛋白结合干扰拮抗TGF-β信号,BMP与活化的TGF-βRI和Smad7有协同作用[2]。Smad7也可以通过激活核因子κβ(NF-kappaB inhibitor alpha,NF-κB)或信号传导子和转录活化子1(signal transducer and activator of transcription 1,STAT1)来抑制TGF-β信号[3]。TGF-β/BMP信号在成骨分化及骨形成过程发挥着重要作用[4],前期研究发现,miR-21在骨质疏松患者血浆中表达下降,miR-21可通过调节Smad7促进成骨细胞分化和矿化,因此深入研究miR-21及Smad7的作用机制是有必要的。
通过建立卵巢切除(ovariectomized,OVX)鼠模型发现,miR-21可以促进成骨细胞分化,抑制miR-21可减轻雌激素缺乏引起的骨质疏松症[6-7]。研究[8-10]表明,Smad7蛋白表达改变常导致人类疾病。但是,Smad7在骨质疏松中的具体作用目前没有报道。Smad7能够抑制小鼠成骨细胞增殖、分化和矿化[11],部分敲除Smad7基因可调控TGF-β/BMP信号通路进而影响小鼠体内骨形成及矿化[12]。本研究建立鼠骨质疏松动物模型,检测miR-21/Smad7在骨组织内的表达及其与骨密度的关系,旨在揭示miR-21/Smad7在绝经后骨质疏松中的作用机制。
1 材料与方法 1.1 实验动物选取30只12周龄WTC57BL/6J雌性小鼠,体质量18~22 g,由中国医科大学附属盛京医院动物实验中心提供,分笼饲养,自由摄食饮水。根据不同观察时间点,将30只小鼠随机分成6组:切除卵巢组、切除卵巢1周组、切除卵巢2周组、切除卵巢4周组、切除卵巢6周组和切除卵巢8周组,每组5只。
1.2 主要试剂及仪器戊巴比妥钠(上海生工生物工程公司),TRIzol Reagent Invitrogen(美国Sigma公司),逆转录试剂盒(日本TaKaRa公司),real-time PCR试剂盒(日本TaKaRa公司),抗鼠Smad7抗体(美国Abcom公司),兔抗小鼠二抗(武汉博士德生物工程有限公司),Inveon micro-CT系统(德国Siemens AG公司),组织切片机(德国Leica公司),荧光显微镜(德国Leica公司),二氧化碳恒温孵箱(美国Forma公司),离心机(日本Kubota公司),倒置相差显微镜及照相系统(日本Olympas公司),分光光度仪(德国Eppendorf公司),双能X线骨密度仪(美国Hologic公司)。
1.3 实验方法 1.3.1OVX小鼠骨质疏松模型:所用小鼠经腹腔注射1%戊巴比妥钠(0.1 mL/100 g),待小鼠完全麻醉后,取俯卧位手术。在双侧第3腰椎背侧正中做一约0.5 cm切口,钝性分离,剪开腹膜后用组织镊探入切口,找到卵巢,将其从靠近输卵管端结扎,眼科剪剪除,随后将剩余组织还纳腹腔,缝合切口。将30只小鼠按观察时间点分为:切除卵巢0周组、切除卵巢1周组、切除卵巢2周组、切除卵巢4周组、切除卵巢6周组和切除卵巢8周组。
1.3.2取材:在OVX后不同观察时间点将行骨密度测定的小鼠颈椎脱臼处死,用手术剪从髋关节及膝关节离断分离双侧股骨,去除肌肉和筋膜。每只小鼠的双侧股骨进行骨密度检测,将行骨密度的股骨随机取一侧股骨行免疫组化染色,另一侧股骨行miR-21和Smad-7 mRNA含量检测[5]。
1.4 主要观察指标 1.4.1小鼠骨密度测定:在OVX后不同观察时间点,使用双能X线骨密度仪并应用小动物骨密度测定软件,对小鼠双侧股骨远端行骨密度检测。
1.4.2免疫组织化学染色:将OVX后不同观察时间点小鼠随机选取的一侧股骨于4%中性甲醛浸泡固定;4 ℃过夜,15%中性EDTA-2Na脱钙4周,经梯度脱水、石蜡包埋后作厚5 μm切片。应用Smad7免疫组化试剂盒按说明测定smad7在骨组织内的表达。
1.4.3实时PCR检测:将OVX后不同时间的另一侧小鼠股骨干骺端在液氮中研磨成干粉状,按照TRIZOL说明书进行操作,从股骨远端取样提取RNA,进行RNA质量和浓度检测后逆转录合成cDNA。参考早期研究行股骨骨组织中miR-21和Smad-7的mRNA测定,miRNAs表达含量计算方法为2-ΔΔCt相对定量法。
1.5 统计学分析所有计量资料以x±s表示,应用SPSS 22.0软件对数据进行单因素方差分析,应用Pearson相关系数分析miR-21、Smad-7与骨密度的相关性,P < 0.05为差异有统计学意义。
2 结果 2.1 Smad7在骨质疏松小鼠骨组织中的表达水平通过免疫组化染色检测Smad7在骨组织中的表达水平,随着卵巢切除的时间逐渐增加,Smad7的表达逐渐增强,见图 1。
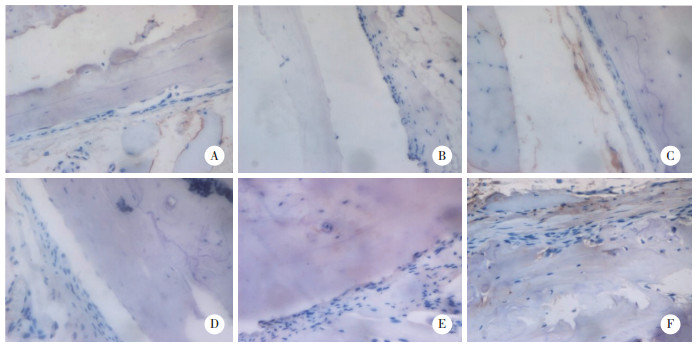
|
| A, 0th week; B, 1st week; C, 2nd week; D, 4th week; E, 6th week; F, 8th week. 图 1 骨质疏松小鼠OVX后不同时间点Smad7的表达情况×200 Fig.1 Expression levels of Smad7 in osteoporotic mice at different time points after OVX × 200 |
2.2 miR-21及smad7在骨质疏松小鼠骨内表达水平
通过实时PCR检测miR-21及smad7在骨组织RNA表达水平,随着卵巢切除的时间逐渐增加,miR-21的表达逐渐降低,组间差异有统计学意义,而随着卵巢切除的时间逐渐增加,smad7的表达逐渐增强,组间有统计学差异,见表 1。
| Item | 0 week | 1 week | 2 weeks | 4 weeks | 6 weeks | 8 weeks |
| miR-21 mRNA | 1.00±0.00 | 0.92±0.02 | 0.72±0.02 | 0.63±0.01 | 0.46±0.03 | 0.26±0.01 |
| Smad7 mRNA | 1.00±0.00 | 1.21±0.07 | 1.73±0.03 | 2.10±0.08 | 2.52±0.05 | 3.25±0.13 |
| BMD(g/cm2) | 0.26±0.02 | 0.22±0.01 | 0.21±0.02 | 0.20±0.01 | 0.17±0.01 | 0.14±0.01 |
2.3 小鼠骨质疏松模型的骨密度变化情况
通过检测计算小鼠股骨的骨密度值发现,随着时间的推移,去势后小鼠的骨密度逐渐下降,第4、6、8周组与0周组有统计学差异(P < 0.05),见表 1。
2.4 miR-21及Smad7与骨质疏松鼠骨骨密度相关性通过检测鼠骨密度值并与miRNA-21、Smad7进行相关分析发现,miR-21与骨密度值呈正相关(r = 0.866,P < 0.01),Smad7与骨密度值呈负相关(r = -0.867,P < 0.01)。
3 讨论微小RNA(microRNAs,miRNAs)在骨形成的构成中发挥重要作用,miR-29家族是调控骨重建代表性的miRNA[13]。miR-214升高的水平与年龄相关的骨折标本中骨形成较低有关,miR-214的抑制对骨的形成有着至关重要的作用[14]。miR-218增强了成骨相关转录因子的骨特异性活性衰减,干扰了正常骨沉积和重塑[15]。观察miR-206的转基因鼠发现,转基因小鼠的骨小梁间隙较大,骨量较低、骨形成率下降,miR-206是成骨分化的抑制因子[16]。miR-21在破骨细胞分化过程中高度表达,这表明它在破骨发生中起重要作用[17]。在破骨细胞诱导miR-21的拮抗雌激素,能够抑制骨吸收,通常会降低雌激素在破骨细胞中miR-21的表达。核因子κB受体活化因子配体(receptor activator of nuclear factor-κB ligand,RANKL)通过诱导miR-21来下调程序性细胞死亡基因4(programmed cell death gene 4,PDCD4)蛋白水平,PDCD4表达减弱反过来会删除c-Fos的压制,允许破骨发生。随后确保初始RANKL信号延续c-Fos的依赖性,激活miR-21将减少PDCD4水平[18]。本研究表明,miR-21在骨质疏松鼠骨组织内表达下降,并与小鼠骨密度呈正相关,这与早期的研究[19]结果一致,miR-21在骨质疏松和骨量减少组内表达上升,且髋部和脊柱的骨密度值呈正相关。miR-21可能在绝经后骨质疏松中发挥着抑制骨形成或促进骨吸收的重要作用。
Smad7是TGF-β/Smad蛋白活动信号通路的重要成员,抑制或调节多种细胞过程,如细胞增殖、分化、凋亡、黏附和迁移[20-21],也参与疾病的病理过程[22-24]。本研究显示,Smad7在骨质疏松鼠骨组织内表达逐渐升高,Smad7可能通过调节成骨与破骨细胞来参与骨重塑,Smad7的本身也可能通过这两个过程调节绝经后骨质疏松,是治疗绝经后骨质疏松的新机制。早期的研究表明,miR-21可通过调节Smad7促进体外成骨细胞分化和矿化,然而,miR-21是否在体内通过调节Smad7在绝经后骨质疏松中发挥作用还没有文献报道。本研究结果预示在绝经后骨质疏松骨组织中miR-21可能通过调控Smad7发挥着抑制骨形成或促进骨吸收的作用。
本研究未进行体内转染或基因敲出,只能说明现象而不能阐明作用机制,miR-21通过调控Smad7在骨质疏松骨形成、骨吸收、骨折愈合以及可能的药物干预的机制方面还需进一步研究。
| [1] |
NICKLAS D, SAIZ L. Characterization of negative feedback network motifs in the TGF-β signaling pathway[J]. PLoS One, 2013, 8(12): e83531. DOI:10.1371/journal.pone.0083531 |
| [2] |
YAN X, LIN Z, CHEN F, et al. human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling[J]. J Biol Chem, 2009, 284(44): 30097-30104. DOI:10.1074/jbc.M109.049304 |
| [3] |
YANG YJ, CHUANG CC, YANG HB, et al. Lactobacillus acidophilus ameliorates h. pylori-induced gastric inflammation by inactivating the Smad7 and NFκB pathways[J]. BMC Microbiol, 2012, 12: 38. DOI:10.1186/1471-2180-12-38 |
| [4] |
CHEN G, DENG C, LI YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation[J]. Int J Biol Sci, 2012, 8(2): 272-288. DOI:10.7150/ijbs.2929 |
| [5] |
LI H, YANG F, WANG Z, et al. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7[J]. Mol Med Rep, 2015, 12(1): 1561-1567. DOI:10.3892/mmr.2015.3497 |
| [6] |
YANG N, WANG G, HU C, et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis[J]. J Bone miner Res, 2013, 28(3): 559-573. DOI:10.1002/jbmr.1798 |
| [7] |
ALEXY T, ROONEY K, WEBER M, et al. TNF-α alters the release and transfer of microparticle-encapsulated miRNAs from endothelial cells[J]. Physiol Genomics, 2014, 46(22): 833-840. DOI:10.1152/physiolgenomics.00079.2014 |
| [8] |
DAMAVAND B, DERAKHSHANI S, SAEEDI N, et al. Intronic polymorphisms of the SMAD7 gene in association with colorectal cancer[J]. Asian Pac J Cancer Prev, 2015, 16(1): 41-44. DOI:10.7314/APJCP.2015.16.1.41 |
| [9] |
LAN HY. Transforming growth factor-β/Smad signaling in diabetic nephropathy[J]. Clin Exp Pharmacol Physio, 2012, 39(8): 731-738. DOI:10.1111/j.1440-1681.2011.05663.x |
| [10] |
LI Q, ZHANG D, WANG Y, et al. MiR-21/Smad7 signaling determines TGF-β1-induced CAF formation[J]. Sci Rep, 2013, 3: 2038. DOI:10.1038/srep02038 |
| [11] |
YANO M, INOUE Y, TOBIMATSU T, et al. Smad7 inhibits differentiation and mineralization of mouse osteoblastic cells[J]. Endocr J, 2012, 59(8): 653-662. DOI:10.1507/endocrj.EJ12-0022 |
| [12] |
LI N, LEE WY, LIN SE, et al. Partial loss of Smad7 function impairs bone remodeling, osteogenesis and enhances osteoclastogenesis in mice[J]. Bone, 2014, 67: 46-55. DOI:10.1016/j.bone.2014.06.033 |
| [13] |
ROBERTO VP, TIAGO DM, SILVA IA, et al. MiR-29a is an enhancer of mineral deposition in bone-derived systems[J]. Arch Biochem Biophys, 2014, 564: 173-183. DOI:10.1016/j.abb.2014.09.006 |
| [14] |
WANG X, GUO B, LI Q, et al. MiR-214 targets ATF4 to inhibit bone formation[J]. Nat Med, 2013, 19(1): 93-100. DOI:10.1038/nm.3026 |
| [15] |
ZHANG WB, ZHONG WJ, WANG L. A signal-amplification circuit between miR-218 and Wnt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation[J]. Bone, 2014, 58: 59-66. DOI:10.1016/j.bone.2013.09.015 |
| [16] |
SATO MM, NASHIMOTO M, KATAGIRI T, et al. Bone morphogenetic protein-2 down-regulates miR-206 expression by blocking its maturation process[J]. Biochem Biophys Res Commun, 2009, 383(1): 125-129. DOI:10.1016/j.bbrc.2009.03.142 |
| [17] |
QU B, XIA X, WU HH, et al. PDGF-regulated miRNA-138 inhibits the osteogenic differentiation of mesenchymal stem cells[J]. Biochem Biophys Res Commun, 2014, 448(3): 241-247. DOI:10.1016/j.bbrc.2014.04.091 |
| [18] |
HUANG CH, WEI JC, CHANG WC, et al. Higher expression of whole blood microRNA-21 in patients with ankylosing spondylitis associated with programmed cell death 4 mRNA expression and collagen cross-linked C-telopeptide concentration[J]. J Rheumatol, 2014, 41(6): 1104-1111. DOI:10.3899/jrheum.130515 |
| [19] |
LI H, WANG Z, FU Q, et al. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients[J]. Biomarkers, 2014, 19(7): 553-556. DOI:10.3109/1354750X.2014.935957 |
| [20] |
YOSHIDA K, MATSUZAKI K. Differential regulation of TGF-β/Smad signaling in hepatic stellate cells between acute and chronic liver injuries[J]. Front Physiol, 2012, 3: 53. DOI:10.3389/fphys.2012.00053 |
| [21] |
SHI Y, MASSAGUE J. Mechanisms of TGF-β signaling from cell membrane to the nucleus[J]. Cell, 2003, 113(6): 685-700. DOI:10.1016/S0092-8674(03)00432-X |
| [22] |
BRIONES-ORTA MA, TECALCO-CRUZ AC, SOSA-GARROCHO M, et al. Inhibitory Smad7:emerging roles in health and disease[J]. Curr Mol Pharmacol, 2011, 4(2): 141-153. DOI:10.2174/1874467211104020141 |
| [23] |
LI Q. Inhibitory SMADs:potential regulators of ovarian function[J]. Biol Reprod, 2015, 92(2): 50. DOI:10.1095/biolreprod.114.125203 |
| [24] |
YASUI T, KADONO Y, NAKAMURA M, et al. Regulation of RANKL-induced osteoclastogenesis by TGF-β through molecular interaction between Smad3 and Traf6[J]. J Bone miner Res, 2011, 26(7): 1447-1456. DOI:10.1002/jbmr.357 |
 2019, Vol. 48
2019, Vol. 48




