2. 复旦大学公共卫生学院, 国家卫生健康委员会卫生技术评估重点实验室(复旦大学), 上海 200032
手术部位感染(surgical site infection,SSI)是患者在住院过程中发生的最常见的医源性感染,病原微生物能够在植入皮肤和体内的缝合线表面定植,成为SSI的重要致病因素。SSI是住院时间延长和术后再次入院的重要原因[1],可能增加患者的经济负担、降低其生活质量甚至危及生命[2-3]。含三氯生涂层的缝线是旨在降低SSI发生率的抗菌缝线。2002年第1款三氯生涂层缝线薇乔(Vicryl Plus)得到美国食品药品监督管理局的批准后上市。2018年世界卫生组织发布《手术部位感染的预防指南》第2版[4],建议使用三氯生涂层缝线以降低SSI风险,且缝线的使用不受手术类型影响。然而,因缺乏高质量的卫生技术评估报告,三氯生涂层缝线的临床应用仍面临挑战。随着三氯生涂层缝线临床应用证据的不断增加,决策者需要最新且整合了我国临床试验的系统化证据。现通过评估卫生技术的有效性,探讨三氯生涂层缝线预防不同类型手术切口感染的效果,为手术部位感染的科学防控提供科学证据。
1 资料与方法 1.1 文献纳入与排除标准 1.1.1 文献检索以“缝线”“缝合”“薇乔”“PGLA910”“PDS”“单乔”“三氯生”“浸渍”“感染”“抗菌”“消炎”“消肿”“防粘连”“杀菌”“灭菌”“抑菌”“防腐”“抗生素”为检索词,在万方数据、中国知网、维普检索中文文献;以“surgical wound infection”“surgical wound dehiscence” “surgical site infection” “SSI” “surgical infection” “post-operative wound infection” “post-operative wound dehiscence” “sutures” “Polyglactin 910” “Vicryl” “Vicryl Plus” “polyglactin” “Monocryl” “Monocryl Plus” “Glycolide E-caprolactone copolymer” “Polydioxanone” “PDS” “PDS Plus” “Polydioxanone” “triclosan” “antimicrobial*” “antiseptic*” “antibiotic*” “antibacterial*” “biocide*”为检索词,在PubMed、Embase、Cochrane Central Register of Controlled Trials、Web of Science、Current Nursing and Allied Health Literature检索英文文献。根据Cochrane临床随机对照试验(randomized controlled trial,RCT)高敏感度检索式[5]对研究类型进行限定。
1.1.2 纳入排除标准纳入标准:研究类型为RCT;研究对象为接受手术的任何疾病患者,无年龄限制;干预措施为使用三氯生涂层缝线,对照组使用不含抗菌剂的缝线;结果指标为SSI发生率;以全文形式发表,不限语种;文献发表于2000年1月1日—2020年8月31日。排除标准:未报告SSI例数或发生率的文献;个人观点、评论、重复发表的文献;二次研究文献。
1.2 数据提取与文献质量评价对符合纳入标准的文献提取数据,包括:①研究基本信息,即论文发表信息、研究开展的机构和国家;②研究对象的社会人口学特征,即年龄、接受的手术部位和手术类型、手术切口分级(Ⅰ类为清洁切口、Ⅱ类为清洁-污染切口、Ⅲ类为污染切口、Ⅳ类为污秽-感染切口)[6];③研究的方法学信息;④结果数据,即各组SSI发生例数和研究对象总例数。联系通信作者补充文献中不确切的信息。采用Cochrane偏倚风险评价工具评价纳入文献的质量[5]。
1.3 统计学分析用R 4.0.3软件按照文献发表的年份进行累积meta分析[7]。将SSI发生率的相对危险度(relative risk,RR)及其95%置信区间(confidence interval,CI)作为合并效应量,在meta分析时采用倒方差计算权重[8]。用I2判断统计学异质性:I2 < 50%,则纳入分析的资料具有同质性,可采用固定效应模型分析;I2≥50%,则纳入分析的资料具有异质性,分析原因并用随机效应模型分析。根据手术切口的污染程度[6]进行亚组分析。
2 结果 2.1 纳入文献及研究共获得文献2 254篇,将其中35篇[9-43]纳入meta分析,研究对象合计14 739例。文献筛选流程见图 1。
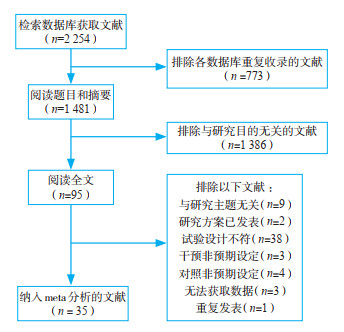
|
图 1 文献筛选流程 |
纳入的研究最早于2008年开展,2011年及以后的研究合计33项(占94%);14项在亚洲开展(我国7项),18项在欧洲开展,2项在美洲开展,1项在澳洲开展;研究涉及各外科科室,其中2项研究涉及多个科室,各项研究涉及的科室包括胃肠外科(17项)、心胸外科(5项)、骨关节外科(5项)、甲乳外科(4项)、血管外科(2项)、藏毛窦手术(2项),脑外科、整形外科和口腔外科各1项,另有1项仅提及腹部手术。
2.2 质量评价22项研究具有高度偏倚风险,其中3项有选择性偏倚、8项有实施偏倚、4项有检测偏倚、11项有损耗性偏倚、7项因受厂商资助可能产生其他偏倚风险(图 2)。根据GRADE证据质量评级,Ⅰ类切口的证据质量低,其他切口的证据质量中等。
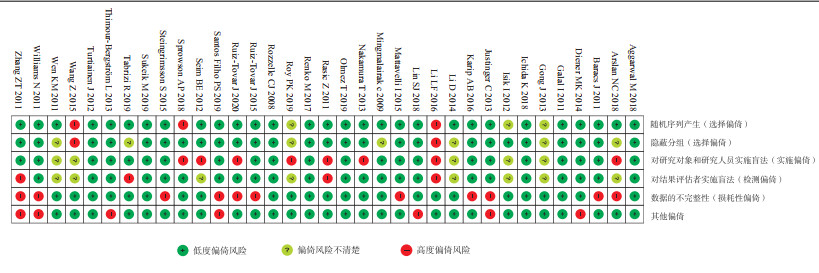
|
图 2 35项研究的偏倚风险 |
图 3(a)为2008—2020年发表的35项RCT中三氯生涂层缝线预防所有类型切口SSI的有效性评价结果,从第4项RCT开始累积SSI的组间差异有统计学意义,三氯生涂层缝线相对于普通缝线发生SSI的合并RR为0.71(95%置信区间:0.64~0.78),提示三氯生涂层缝线能够降低29%的SSI发生率。
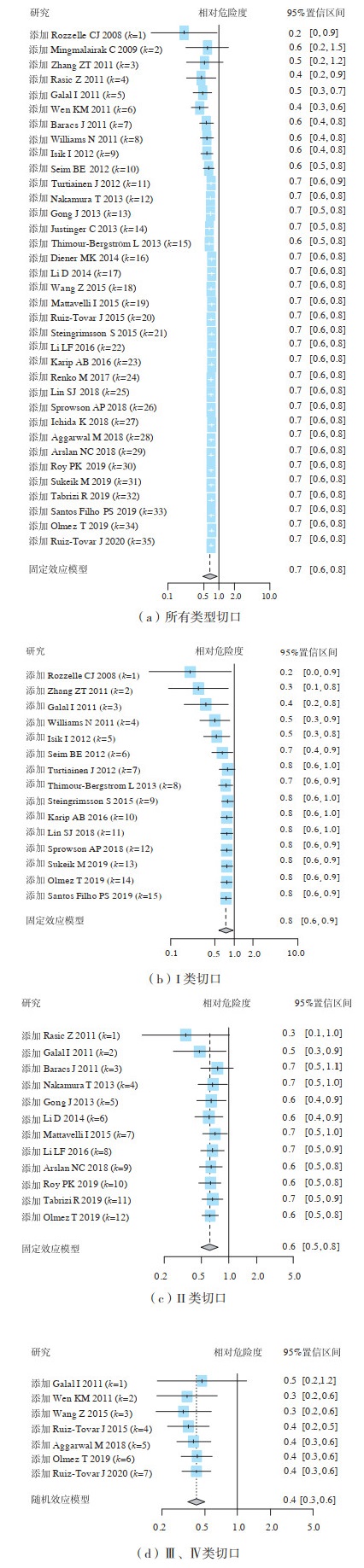
|
图 3 35项研究三氯生涂层缝线预防各类切口手术部位感染的有效性评价 |
三氯生涂层缝线在预防I、Ⅱ类切口发生SSI方面有一定的效果,合并RR分别为0.75(95%置信区间:0.62~0.91)、0.62(95%置信区间:0.51~0.76),见图 3(b)、图 3(c)。三氯生涂层缝线预防Ⅲ、Ⅳ类切口发生SSI的效果最佳,从第2项RCT(即2011年)开始的累积meta分析结果证实三氯生涂层缝线预防Ⅲ、Ⅳ类切口发生SSI的有效性,合并RR为0.42(95%置信区间:0.31~0.56),三氯生涂层缝线可降低58%的SSI发生率,见图 3(d)。
2.3.3 基于我国临床证据的SSI发生率对来自我国7项RCT进行了亚组分析,结果表明,三氯生涂层缝线能够预防SSI的发生,合并RR为0.25(95%置信区间:0.15~0.42)。
3 讨论卫生技术评估以政策为导向,可为政策制定提供科学证据。运用卫生技术有效性评估的方法,对三氯生涂层缝线预防SSI的效果进行了客观评估。卫生技术的有效性评估需借助系统评价,可被视作累积多项独立研究的高等级证据[44]。本研究基于系统评价和累积meta分析较为全面地评估了三氯生涂层缝线预防SSI的效果,可为外科手术的感染防控、循证制定科学的干预策略及改善患者预后提供科学证据。
选用的累积模式按照年份排序,以显示干预效应随时间变化的趋势。累积meta分析可在效应量的逐次累积合并过程中比较评估结果的动态变化,还能分析新加入的研究对总体结果的影响,弥补了传统卫生技术有效性评估无法识别单项研究结果对总体结果影响的局限性[45-46]。2013年发表的有效性评估证据[47]证实,含抗菌剂缝线可降低SSI发生率(比值比=0.16,95% 置信区间:0.37~0.99)。本研究表明,三氯生涂层缝线可预防SSI。累积的森林图显示,随着年份的演进和病例数的增加,统计学检验效能增加,有效性评估的精确度增加。基于手术切口分类的亚组分析同样显示,三氯生涂层缝线可预防SSI,且预防Ⅲ、Ⅳ类切口SSI的效果最佳。I、Ⅱ类切口的SSI发生率显著低于Ⅲ、Ⅳ类切口[48],会导致三氯生涂层缝线预防I、Ⅱ类切口SSI的效果不及预防Ⅲ、Ⅳ类切口SSI的效果。本研究对我国的研究进行了亚组分析,证实了该缝线的临床应用价值,弥补了既往发表的有效性评估论文缺乏我国原始研究数据的不足。
世界卫生组织和美国疾病预防控制中心发布的手术部位感染预防指南[4, 49]均对含抗菌剂缝线的临床有效性作出中等推荐。英国国家卫生与临床优化研究所最新发布的指南[50]明确指出,在应用缝线时可考虑使用三氯生涂层缝线,以降低SSI风险。《中国手术部位感染预防指南》[51]建议,在各类手术中使用抗菌涂层缝线以预防SSI(有条件推荐,中等质量证据)。可见,含抗菌剂缝线尤其是三氯生涂层缝线可预防SSI已成为共识。
4 结论三氯生涂层缝线能够预防各类污染切口的手术部位感染,对Ⅲ、Ⅳ类切口的预防效果最佳。在手术过程中使用三氯生涂层缝线有助于保障患者安全、避免医疗资源浪费。
·作者声明本文无实际或潜在的利益冲突
| [1] |
JENKS P J, LAURENT M, MCQUARRY S, et al. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital[J]. J Hosp Infect, 2014, 86(1): 24-33. DOI:10.1016/j.jhin.2013.09.012 |
| [2] |
OHNO M, SHIMADA Y, SATOH M, et al. Evaluation of economic burden of colonic surgical site infection at a Japanese hospital[J]. J Hosp Infect, 2018, 99(1): 31-35. DOI:10.1016/j.jhin.2017.12.013 |
| [3] |
BADIA J M, CASEY A L, PETROSILLO N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries[J]. J Hosp Infect, 2017, 96(1): 1-15. DOI:10.1016/j.jhin.2017.03.004 |
| [4] |
WORLD HEALTH ORGANIZATION. Global guidelines for the prevention of surgical site infection, second edition[R]. Geneva: World Health Organization, 2018.
|
| [5] |
HIGGINS J P T, THOMAS J, CHANDLER J, et al. Cochrane handbook for systematic reviews of interventions version 6.1[R]. Chichester: Cochrane Collaboration, 2020.
|
| [6] |
抗菌药物临床应用指导原则修订工作组. 抗菌药物临床应用指导原则: 2015年版[M]. 北京: 人民卫生出版社, 2015: 1-5.
|
| [7] |
BALDUZZI S, RÜCKER G, SCHWARZER G. How to perform a meta-analysis with R: a practical tutorial[J]. Evid Based Ment Health, 2019, 22(4): 153-160. DOI:10.1136/ebmental-2019-300117 |
| [8] |
NYAGA V N, ARBYN M, AERTS M. Metaprop: a STATA command to perform meta-analysis of binomial data[J]. Arch Public Health, 2014, 72(39): 1-10. |
| [9] |
ROZZELLE C J, LEONARDO J, LI V. Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial[J]. J Neurosurg Pediatr, 2008, 2(2): 111-117. DOI:10.3171/PED/2008/2/8/111 |
| [10] |
MINGMALAIRAK C, UNGBHAKORN P, PAOCHAROEN V. Efficacy of antimicrobial coating suture coated polyglactin 910 with triclosan (vicryl plus) compared with polyglactin 910 (vicryl) in reduced surgical site infection of appendicitis, double blind randomized control trial, preliminary safety report[J]. J Med Assoc Thai, 2009, 92(6): 770-775. |
| [11] |
ZHANG Z T, ZHANG H W, FANG X D, et al. Cosmetic outcome and surgical site infection rates of antibacterial absorbable (Polyglactin 910) suture compared to Chinese silk suture in breast cancer surgery: a randomized pilot research[J]. Chin Med J (Engl), 2011, 124(5): 719-724. |
| [12] |
RASIĆ Z, SCHWARZ D, ADAM V N, et al. Efficacy of antimicrobial triclosan-coated polyglactin 910 (vicryl plus) suture for closure of the abdominal wall after colorectal surgery[J]. Coll Antropol, 2011, 35(2): 439-443. |
| [13] |
GALAL I, EL-HINDAWY K. Impact of using triclosan-antibacterial sutures on incidence of surgical site infection[J]. Am J Surg, 2011, 202(2): 133-138. DOI:10.1016/j.amjsurg.2010.06.011 |
| [14] |
文坤明, 曾庆良, 冯国丽, 等. 抗菌薇乔缝线预防胃肠急诊手术切口感染的临床研究[J]. 中国普外基础与临床杂志, 2011, 18(9): 969-972. |
| [15] |
BARACS J, HUSZÁR O, SAJJADI S G, et al. Surgical site infections after abdominal closure in colorectal surgery using triclosan-coated absorbable suture (PDS plus) vs. uncoated sutures (PDS Ⅱ): a randomized multicenter study[J]. Surg Infect, 2011, 12(6): 483-489. DOI:10.1089/sur.2011.001 |
| [16] |
WILLIAMS N, SWEETLAND H, GOYAL S, et al. Randomized trial of antimicrobial-coated sutures to prevent surgical site infection after breast cancer surgery[J]. Surg Infect, 2011, 12(6): 469-474. DOI:10.1089/sur.2011.045 |
| [17] |
ISIK I, SELIMEN D, SENAY S, et al. Efficiency of antibacterial suture material in cardiac surgery: a double-blind randomized prospective study[J]. Heart Surg Forum, 2012, 15(1): E40-E45. DOI:10.1532/HSF98.20111106 |
| [18] |
SEIM B E, TØNNESSEN T, WOLDBAEK P R. Triclosan-coated sutures do not reduce leg wound infections after coronary artery bypass grafting[J]. Interact Cardiovasc Thorac Surg, 2012, 15(3): 411-415. DOI:10.1093/icvts/ivs266 |
| [19] |
TURTIAINEN J, SAIMANEN E I, MÄKINEN K T, et al. Effect of triclosan-coated sutures on the incidence of surgical wound infection after lower limb revascularization surgery: a randomized controlled trial[J]. World J Surg, 2012, 36(10): 2528-2534. DOI:10.1007/s00268-012-1655-4 |
| [20] |
NAKAMURA T, KASHIMURA N, NOJI T, et al. Triclosan-coated sutures reduce the incidence of wound infections and the costs after colorectal surgery: a randomized controlled trial[J]. Surgery, 2013, 153(4): 576-583. DOI:10.1016/j.surg.2012.11.018 |
| [21] |
龚健, 孟红波, 宋振顺, 等. 抗菌缝线在胃肠道肿瘤手术腹壁切口缝合中应用效果[J]. 中华实用诊断与治疗杂志, 2013, 27(4): 392-393. |
| [22] |
JUSTINGER C, SLOTTA J E, NINGEL S, et al. Surgical-site infection after abdominal wall closure with triclosan-impregnated polydioxanone sutures: results of a randomized clinical pathway facilitated trial (NCT00998907)[J]. Surgery, 2013, 154(3): 589-595. DOI:10.1016/j.surg.2013.04.011 |
| [23] |
THIMOUR-BERGSTRÖM L, ROMAN-EMANUEL C, Scherstén H, et al. Triclosan-coated sutures reduce surgical site infection after open vein harvesting in coronary artery bypass grafting patients: a randomized controlled trial[J]. Eur J Cardiothorac Surg, 2013, 44(5): 931-938. DOI:10.1093/ejcts/ezt063 |
| [24] |
DIENER M K, KNEBEL P, KIESER M, et al. Effectiveness of triclosan-coated PDS plus versus uncoated PDS Ⅱ sutures for prevention of surgical site infection after abdominal wall closure: the randomized controlled PROUD trial[J]. Lancet, 2014, 384(9938): 142-152. DOI:10.1016/S0140-6736(14)60238-5 |
| [25] |
李丹, 庄競, 刘永刚, 等. 可吸收缝线全筋膜与丝线间断缝合腹部切口: 效果及生物相容性的比较[J]. 中国组织工程研究, 2014, 18(43): 6996-7000. DOI:10.3969/j.issn.2095-4344.2014.43.018 |
| [26] |
王哲, 宋展, 万春. 抗菌薇乔缝线在预防胃肠急诊手术切口感染中的应用研究[J]. 中华医院感染学杂志, 2015, 25(4): 904-906. |
| [27] |
MATTAVELLI I, REBORA P, DOGLIETTO G, et al. Multi-center randomized controlled trial on the effect of triclosan-coated sutures on surgical site infection after colorectal surgery[J]. Surg Infect, 2015, 16(3): 226-235. |
| [28] |
RUIZ-TOVAR J, ALONSO N, MORALES V, et al. Association between triclosan-coated sutures for abdominal wall closure and incisional surgical site infection after open surgery in patients presenting with fecal peritonitis: a randomized clinical trial[J]. Surg Infect, 2015, 16(5): 588-594. DOI:10.1089/sur.2014.072 |
| [29] |
STEINGRIMSSON S, THIMOUR-BERGSTRÖM L, Roman-Emanuel C, et al. Triclosan-coated sutures and sternal wound infections: a prospective randomized clinical trial[J]. Eur J Clin Microbiol Infect Dis, 2015, 34(12): 2331-2338. DOI:10.1007/s10096-015-2485-8 |
| [30] |
李利发, 张井潇, 陈小波, 等. 三种不同缝合线对腹壁手术切口愈合质量影响的研究[J]. 中国普外基础与临床杂志, 2016, 23(4): 445-449. |
| [31] |
KARIP A B, ÇELIK K, AYDIN T, et al. Effect of triclosan-coated suture and antibiotic prophylaxis on infection and recurrence after karydakis flap repair for pilonidal disease: a randomized parallel-arm double-blinded clinical trial[J]. Surg Infect, 2016, 17(5): 583-588. DOI:10.1089/sur.2015.207 |
| [32] |
RENKO M, PAALANNE N, TAPIAINEN T, et al. Triclosan-containing sutures versus ordinary sutures for reducing surgical site infections in children: a double-blind, randomized controlled trial[J]. Lancet Infect, 2017, 17(1): 50-57. |
| [33] |
LIN S J, CHANG F C, HUANG T W, et al. Temporal change of interleukin-6, c-reactive protein, and skin temperature after total knee arthroplasty using triclosan-coated sutures[J]. Biomed Res Int, 2018, 2018: 1-9. |
| [34] |
SPROWSON A P, JENSEN C, PARSONS N, et al. The effect of triclosan-coated sutures on the rate of surgical site infection after hip and knee arthroplasty: a double-blind randomized controlled trial of 2 546 patients[J]. Bone Joint J, 2018, 100B(3): 296-302. |
| [35] |
ICHIDA K, NODA H, KIKUGAWA R, et al. Effect of triclosan-coated sutures on the incidence of surgical site infection after abdominal wall closure in gastroenterological surgery: a double-blind, randomized controlled trial in a single center[J]. Surgery, 2018, 164(1): 91-95. DOI:10.1016/j.surg.2017.12.020 |
| [36] |
AGGARWAL M, KUMAR A, PANDOVE P K, et al. Triclosan coated polydiaxanone suture versus noncoated polydiaxanone suture in preventing surgical site infection in perforation peritonitis: a comparitive study[J]. Int J Surg Med, 2018, 4(3): 118-122. |
| [37] |
ARSLAN N C, ATASOY G, ALTINTAS T, et al. Effect of triclosan-coated sutures on surgical site infections in pilonidal disease: prospective randomized study[J]. Int J Colorectal Dis, 2018, 33(10): 1445-1452. DOI:10.1007/s00384-018-3138-z |
| [38] |
ROY P K, KALITA P, LALHLENMAWIA H, et al. Comparison of surgical site infection rate between antibacterial coated surgical suture and conventional suture: a randomized controlled single centre study for preventive measure of postoperative infection[J]. Int J Pharm Sci Res, 2019, 10(5): 2385-2391. |
| [39] |
SUKEIK M, GEORGE D, GABR A, et al. Randomized controlled trial of triclosan coated vs uncoated sutures in primary hip and knee arthroplasty[J]. World J Orthop, 2019, 10(7): 268-277. DOI:10.5312/wjo.v10.i7.268 |
| [40] |
TABRIZI R, MOHAJERANI H, BOZORGMEHR F. Polyglactin 910 suture compared with polyglactin 910 coated with triclosan in dental implant surgery: randomized clinical trial[J]. Int J Oral Maxillofac Surg, 2019, 48(10): 1367-1371. DOI:10.1016/j.ijom.2019.01.011 |
| [41] |
SANTOS FILHO P S, SANTOS M, Colafranceschi A S, et al. Effect of using triclosan-impregnated polyglactin suture to prevent infection of saphenectomy wounds in CABG: a prospective, double-blind, randomized clinical trial[J]. Braz J Cardiovasc Surg, 2019, 34(5): 588-595. |
| [42] |
OLMEZ T, BERKESOGLU M, TURKMENOGLU O, et al. Effect of triclosan-coated suture on surgical site infection of abdominal fascial closures[J]. Surg Infect, 2019, 20(8): 658-664. DOI:10.1089/sur.2019.052 |
| [43] |
RUIZ-TOVAR J, LLAVERO C, JIMENEZ-FUERTES M, et al. Incisional surgical site infection after abdominal facial closure with triclosan-coated barbed suture vs triclosan-coated polydioxanone loop suture vs polydioxanone loop suture in emergent abdominal surgery: a randomized clinical trial? Check for updates[J]. J Am Coll Surg, 2020, 230(5): 766-774. DOI:10.1016/j.jamcollsurg.2020.02.031 |
| [44] |
POGUE J, YUSUF S. Overcoming the limitations of current meta-analysis of randomised controlled trials[J]. Lancet, 1998, 351(9095): 47-52. DOI:10.1016/S0140-6736(97)08461-4 |
| [45] |
JÜNI P, NARTEY L, REICHENBACH S, et al. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis[J]. Lancet, 2004, 364(9450): 2021-2029. DOI:10.1016/S0140-6736(04)17514-4 |
| [46] |
LAU J, ANTMAN E M, JIMENEZ-SILVA J, et al. Cumulative meta-analysis of therapeutic trials for myocardial infarction[J]. N Engl J Med, 1992, 327(4): 248-254. DOI:10.1056/NEJM199207233270406 |
| [47] |
SAJID M S, CRACIUNAS L, SAINS P, et al. Use of antibacterial sutures for skin closure in controlling surgical site infections: a systematic review of published randomized, controlled trials[J]. Gastroenterol Rep, 2013, 1(1): 42-50. |
| [48] |
ORTEGA G, RHEE D S, PAPANDRIA D J, et al. An evaluation of surgical site infections by wound classification system using the ACS-NSQIP[J]. J Surg Res, 2012, 174(1): 33-38. |
| [49] |
BERRÍOS-TORRES S I, UMSCHEID C A, BRATZLER D W, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017[J]. JAMA Surg, 2017, 152(8): 784-791. |
| [50] |
THE NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Surgical site infections: prevention and treatment[R]. London: The National Institute for Health and Care Excellence, 2019.
|
| [51] |
中华医学会外科学分会外科感染与重症医学学组, 中国医师协会外科医师分会肠痿外科医师专业委员会. 中国手术部位感染预防指南[J]. 中华胃肠外科杂志, 2019, 22(4): 301-314. |
 2022, Vol. 25
2022, Vol. 25


