文章信息
- 赵燕, 郝燕妮, 刘南京, 李婷, 吴小候, 罗春丽.
- ZHAO Yan, HAO Yan-ni, LIU Nan-jing, LI Ting, WU Xiao-hou, LUO Chun-li.
- miR-145通过下调PLCε抑制膀胱癌EMT和迁移及其机制研究
- Silence of PLCε Induced by miR-145 Inhibits EMT and Metastasis in Bladder Cancer
- 中国生物工程杂志, 2017, 37(3): 27-36
- China Biotechnology, 2017, 37(3): 27-36
- http://dx.doi.org/DOI:10.13523/j.cb.20170304
-
文章历史
- 收稿日期: 2016-09-27
- 修回日期: 2016-11-24
2. 重庆医科大学附属第一医院泌尿外科 重庆 400016
2. Department of Urinary Surgery, The First Affiliated Hospital of Chongqing, Medical University, Chongqing 400016, China
膀胱癌为泌尿生殖系统中最常见的恶性肿瘤,具有高侵袭性、易复发等生物学特点。在我国其发病率为6.69/10万, 死亡率为2.53/10万,并呈逐年上升趋势[1]。研究表明,多种分子机制参与膀胱癌侵袭转移过程,其中,上皮间质转化 (EMT) 被认为是肿瘤细胞发生侵袭转移的一个主要驱动力[2]。因此,探究膀胱癌发生EMT及转移的分子机制,对膀胱癌侵袭转移机制的深入研究是提高膀胱癌诊治效率的关键。
磷脂酰肌醇特异性磷脂酶Cepsilon (PLCε) 作为癌基因Ras的效应分子,受多种小G蛋白 (Ras、Rho、Rap) 调控[3-4]。近年来,随着对PLCε结构及其功能研究的不断深入,发现PLCε在人类恶性肿瘤的发生和进展中发挥着重要作用。期研究表明,PLCε在膀胱肿瘤组织和细胞中呈现高表达,且可以通过上调PKCα/β促进膀胱癌发生EMT和转移[5]。成熟的miRNA为全长含20~25个核苷酸的单链小分子RNA,它通过互补结合于其靶基因mRNA的3′端非编码区 (3′-UTR), 从而抑制靶基因的翻译或者是导致其靶基因mRNA的裂解[6]。越来越多的研究发现,miRNA在多种肿瘤中表达具有特异性, 并且在肿瘤的增殖、分化、侵袭转移及治疗中扮演重要角色。有文献报道,相对于正常膀胱组织,miR-145在膀胱癌中呈现低表达,过表达miR-145能抑制膀胱癌细胞系的生长[7]。在这里我们利用生物信息学工具预测可能调控PLCε的microRNA,为研究PLCε促肿瘤作用的机制提供新思路。
本研究以癌基因PLCε为切入点,探究PLCε癌基因对膀胱癌迁移和EMT的影响及分子机制;进一步通过在膀胱癌细胞中转入miR-145 mimic, 研究过表达miR-145后能否逆转PLCε诱导的迁移及EMT,从而为临床治疗膀胱肿瘤提供新靶点和新策略。
1 材料与方法 1.1 细胞及腺病毒人膀胱癌细胞株T24(重庆医科大学临床检验诊断学教育部重点实验室保存);空载腺病毒Ad-HK及PLCε的干扰腺病毒Ad-shPLCε由本课题组前期构建保存。
1.2 miR-145模拟物及其阴性对照物miR-145模拟物 (miR-145 mimics) 及其阴性对照物 (NC) 购自上海吉玛制药技术有限公司,序列信息见表 1。
| Mimics name | Mimics sequence (5′-3′) |
| hsa-miR-145 mimics | GUCCAGUUUUCCCAGGAAUCCCU |
| GGAUUCCUGGGAAAACUGGACUU | |
| Mimics negative control (miR-NC) | Sense:UUCUCCGAACGUGUCACGUTT |
| Antisense:ACGUGACACGUUCGGAGAATT |
1.3 主要试剂
细胞培养基RPMI1640和胎牛血清 (均购于Gibco公司);PCR引物合成 (Invitrogen公司);RNA提取试剂盒Trizol、RT-PCR试剂盒和Real-time PCR试剂盒 (TaKaRa生物技术有限公司);转染试剂lipofectamine 2000(Invitrogen公司);Transwell小室 (B. D公司);蛋白质提取试剂盒及Western blot相关试剂 (上海碧云天公司);PVDF膜 (美国Millipore公司);山羊抗人多克隆抗体PLCε(Santa cruz公司);兔抗人p-GSK-3β、GSK-3β抗体 (Cell Signaling Technology公司);兔抗人E-cadherin (Immunaway公司);兔抗人Snail、N-cadherin、Vimentin (Bioworld公司);GAPDH多克隆抗体、羊抗兔IgG (北京中杉金桥生物技术有限公司)。
1.4 方法 1.4.1 细胞培养膀胱癌细胞株T24常规培养于RPMI1640完全培养基 (含10%胎牛血清、100U/ml青霉素和100U/ml链霉素),置于37℃、5% CO2的湿度饱和培养箱中培养,待细胞贴壁且生长至80%~90%时以0.25%胰酶消化法进行传代。
1.4.2 不同组腺病毒感染和细胞转染膀胱癌细胞株T24传代后置37℃孵箱中,待细胞生长融合度达75%~85%,弃去原培养基,更换为2ml无血清RPMI1640培养基。然后分别用Ad-HK、Ad-shPLCε感染细胞,2h后更换为RPMI1640完全培养基,置于37℃、5%CO2孵箱中孵育,根据实验所需培养时间对细胞进行处理。生长良好的细胞接种至6孔板,培养过夜,转染时细胞融合度达50%左右,参照LipofectamineTM2000转染试剂说明书配比转染混合物:分别取miR-145 mimics、NC及转染试剂各5μl溶于250μl无血清RPMI1640培养基,常温孵育5min。将上述稀释好的miR-145 mimics、miR-NC分别与对应的转染试剂混合即配成转染混合物,常温孵育20min后逐滴顺时针加入对应孔中,轻轻混匀。转染后4h左右换液,细胞置于37℃、5% CO2培养箱中继续培养。根据实验要求转染时间进行后续实验。
1.4.3 PCR检测miR-145和PLCε的mRNA表达水平常规培养T24细胞,不同处理因素作用48h后,采用Triol法抽提细胞总RNA。利用反转录试剂盒将提取的RNA逆转录成cDNA, 其中,miR-145的cDNA采用miR-145的特异性RT引物构建反转录体系。以cDNA为模板进行qPCR (一般基因以β-antin为内参;miR-145以U6为内参) 和RT-PCR (以β-antin为内参) 检测。反应条件:预变性95℃ 3min;变性95℃ 10s;退火56℃ 30s;延伸72℃ 20s;共40个循环 (RT-PCR为30个循环)。qPCR结果,根据读取的CT值采用2-ΔΔCT法分析,mRNA相对表达量=2-[(CT处理-CT内参)-(CT对照-CT内参)]。RT-PCR结果用Quantity One软件分析条带的灰度值,mRNA相对表达量=目的条带灰度值/内参条带灰度值。所用引物序列见表 2。
| Gene | Forward primer (5′→ 3′) | Reverse primer (5′→3′) |
| miR-145 | GCGTCCAGTTTTCCCAGGA | GTGCAGGGTCCGAGGT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| PLCε(qPCR) | GGAGAATCCTCGGTAG | GGTTGTCAGCGTATGTCC |
| PLCε | CATGGAAGGATAAGCGTTGGT | CCCAAGTCCCGTGTTAAGA |
| E-cadherin | CGCATTGCCACATACAC | CCTTCCATGACAGACCC |
| Vimentin | ATGGCTCGTCACCTTCG | AGTTTCGTTGATAACCTGTCC |
| β-actin | GGGACCTGACTGACTACCTC | ACGAGACCACCTTCAACTCCAC |
| has-miR-145-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGGAT | |
1.4.4 Western blot检测蛋白质表达水平
常规培养T24细胞,不同处理因素作用48~72h后,按常规方法提取细胞总蛋白质, BCA法测定蛋白质浓度。蛋白质上样量约为30μg,经十二烷基硫酸钠-聚丙烯酰胺凝胶电泳 (sodium dodecylsulfate-polyacrylamide gelectrophoresis,SDS-PAGE) 分离后, 湿转移至聚偏氟乙稀 (polyvinylidene fluoride,PVDF) 膜, 5%封闭液室温封闭2h后加入相应一抗4℃孵育过夜;TBST洗涤5min×5次, 加入相应二抗室温孵育1h;TBST洗膜5min×5次, ECL暗室化学发光检测。实验结果采用Quantity One软件进行灰度定量分析, 并将目的蛋白灰度与内参GAPDH灰度的比值作为目的蛋白的相对表达量。
1.4.5 划痕实验检测细胞的迁移能力调整细胞密度并将其接种至新的6孔板中,待细胞融合至75%~85%,给予不同因素处理;待细胞完全长满6孔板后,在超净工作台内,用10μl无菌枪头在细胞表面十字交叉划线,PBS洗涤脱落的细胞,加入新鲜培养基,继续培养。划线后0h、24h、48h时间点于倒置显微镜下进行拍照。
1.4.6 Transwell实验检测细胞的侵袭能力每孔加入500μl含10%FBS的培养基于24孔板中,将8μm孔径的Transwell小室放于其中,小室的上室加入200μl浓度为5×104/ ml的细胞悬液,每组设置3个复孔。培养16h后取出小室,PBS冲洗2次,PBS浸湿棉签,轻轻擦去小室微孔膜上层细胞;室温下甲醇固定15min,结晶紫染液染色5min,蒸馏水冲洗。于倒置显微镜下,每孔随机选取5个视野进行计数,取平均值,以迁移至微孔膜下层的细胞数来评价其侵袭能力。
1.4.7 统计学分析实验结果采用SPSS 17.0软件进行统计分析。计量资料用均数±标准差 (x±s) 表示,多组间比较采用单因素方差分析,组间两两比较采用t检验。P < 0.05认为差异有统计学意义。
2 结果 2.1 感染Ad-shPLCε腺病毒抑制膀胱癌T24细胞内源性PLCε的表达RT-PCR和Western blot结果分别显示,转染Ad-shPLCε组,在mRNA和蛋白质水平PLCε的表达低于Ad-HK组 (P<0.01)(图 1);而空白对照组、Ad-HK组PLCε表达无统计学差异 (P>0.05)(图 1)。表明Ad-shPLCε腺病毒成功干扰T24细胞内源性PLCε的表达,为后续实验奠定了基础。
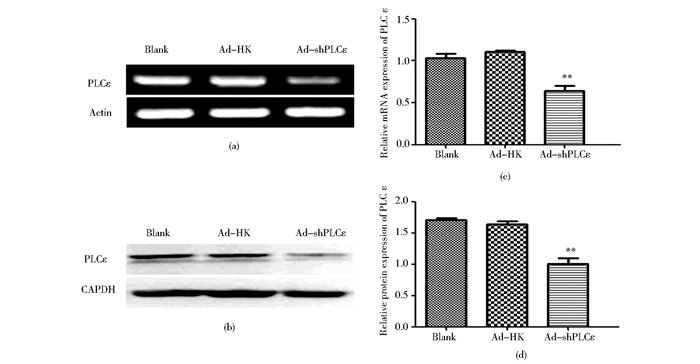
|
| 图 1 感染腺病毒后膀胱癌T24细胞内PLCε的表达 Figure 1 The expression of PLCε in T24 after treated with adenovirus (a) Expression of PLCε detected by RT-PCR (b) Expression of PLCε detected by Western blot (c)** P < 0.01 compared with blank control and Ad-HK control (d) ** P < 0.01 compared with blank control and Ad-HK control |
划痕实验结果表明,转染Ad-shPLCε组在24h、48h迁移的距离均小于Ad-HK组 (P<0.05)[图 2(a)];Transwell迁移实验结果显示,转染腺病毒24h后,相对于Ad-HK组,Ad-shPLCε组从小室上层穿到下层的细胞数明显减少 (P<0.01)[图 2(b)]。表明干扰PLCε表达可以抑制T24细胞的迁移。
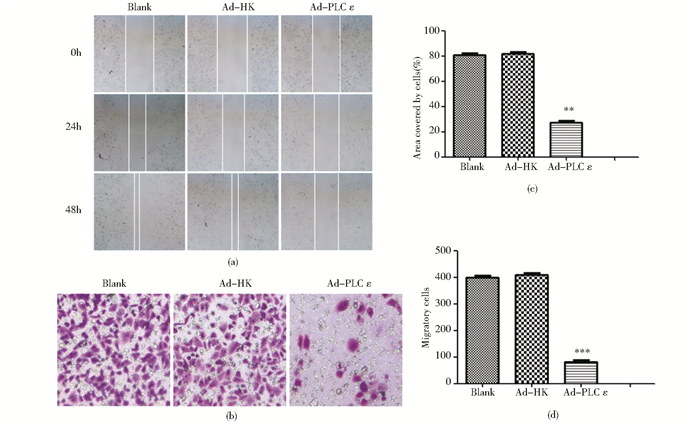
|
| 图 2 沉默PLCε对膀胱癌T24细胞迁移能力的影响 Figure 2 Downregulation of PLCε inhibits the migratory ability (a) Migratory capacity of T24 detected by wound-healing assay (×100) (b) The migratory cells detected by Transwell assay (×100) (c)** P < 0.01, compared with blank and Ad-HK control (d) *** P < 0.001, compared with the two control |
RT-PCR检测E-cadherin、N-cadherin、Vimentin在mRNA水平的表达量,结果显示Ad-shPLCε组E-cadherin明显高于对照组,而N-cadherin、Vimentin明显低于对照组,差异均有统计学意义 (P<0.05)[图 3(a)];Western blot检测以上基因的蛋白质表达量, 与PCR结果一致[图 3(b)]。以上实验结果表明,下调PLCε后能促进与上皮细胞特性相关分子E-cadherin的表达,而抑制与间质细胞特性相关分子N-cadherin和Vimentin的表达。
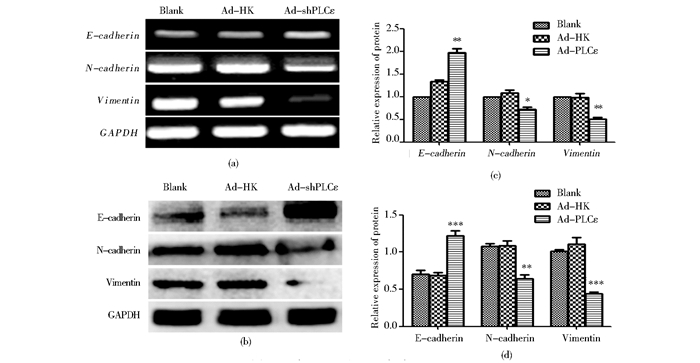
|
| 图 3 沉默PLCε后EMT标志分子的表达 Figure 3 The expression of EMT markers after downregulation of PLCε (a) E-cadherin、N-cadherin, Vimentin mRNA expression detected by RT-PCR (b) E-cadherin, N-cadherin, Vimentin protein expression detected by western blot (c)* P < 0.05, ** P < 0.01, compared with blank and Ad-HK control (d) ** P < 0.01, *** P < 0.001, compared with blank and Ad-HK control |
为了检测GSK-3β/Snail信号通路是否参与PLCε对EMT的上述作用,采用Western blot检测GSK-3β的磷酸化水平及Snail蛋白表达量。结果显示,与对照组相比,干扰PLCε表达后,p-GSK-3β(S9) 表达显著降低 (P<0.05),同时EMT相关的转录因子Snail的表达下降 (P<0.05)(图 4)。
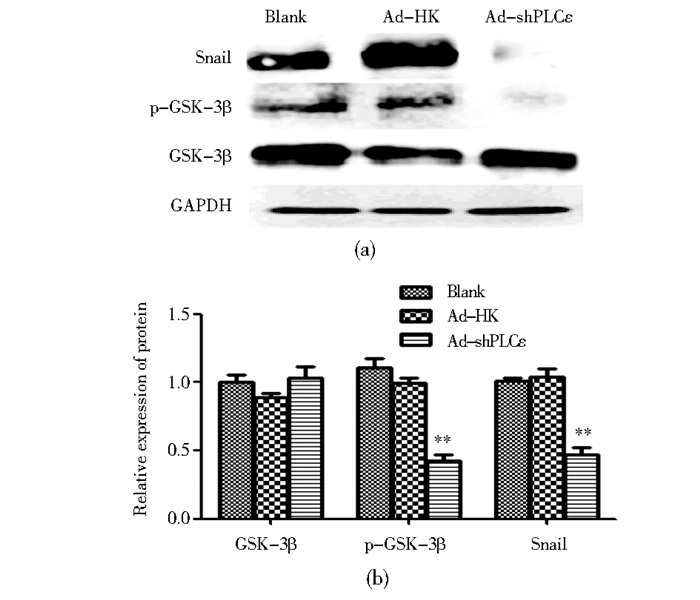
|
| 图 4 感染Ad-shPLCε对T24细胞中p-GSK-3β、Snail表达的影响 Figure 4 The effect of Ad-shPLCε on the expression of p-GSK-3β, Snail in T24 (a) The expression of Snail and p-GSK-3β was detected by Western blot (b) ** P < 0.01, compared with blank and Ad-HK control |
根据在线靶基因预测软件结果,miR-145为可能调控PLCε的潜在microRNA。将miR-145 mimics转染至T24细胞,qPCR结果显示,与阴性对照组相比,转染mimics可显著上调膀胱癌细胞T24中miR-145的表达,差异具有统计学意义 (P<0.01)[图 5(a)];为明确miR-145对PLCε的调控,采用qPCR和Western blot检测PLCε的表达,结果显示,PLCε在mRNA和蛋白质水平的表达较阴性对照组均下调 (P<0.05)[图 5(b)、(c)]。
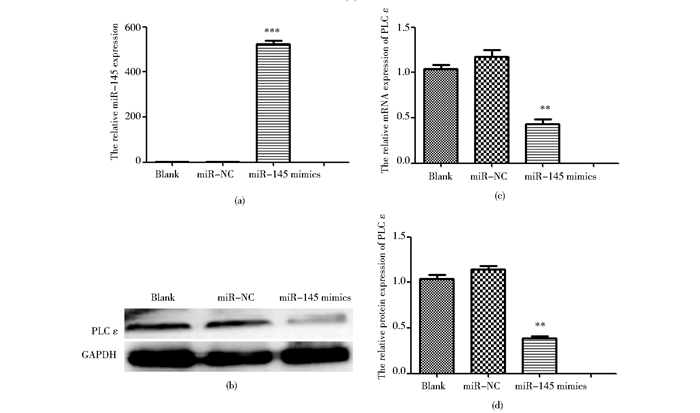
|
| 图 5 过表达miR-145抑制T24细胞中PLC ε的表达 Figure 5 The downregulation of PLCε by transferring miR-145 into T24 (a) Expression of miR-145 detected by qPCR *** P < 0.001, compared with NC group (b) Expression of PLCε detected by qPCR ** P < 0.01, compared with NC group (c) Expression of PLCε detected by Western blot (d) ** P < 0.01, compared with miR-NC group |
Western blot结果显示,转染miR-145 mimics后,膀胱癌细胞T24中GSK-3β的磷酸化 (S9位点) 水平降低 (P<0.05),同时Snail蛋白表达量减少 (P<0.05)。以上表明,过表达miR-145对GSK-3β磷酸化和Snail表达的影响,与干扰PLCε表达后的效果一致。
2.7 过表达miR-145抑制膀胱癌T24细胞的迁移及EMT划痕实验结果表明,miR-145 mimics组T24细胞迁移的距离较空白对照组和阴性对照组显著减小 (P<0.05)[图 7(a)];Transwell迁移实验结果显示,miR-145 mimics组从小室上层穿到下层的细胞数较空白对照组和阴性对照组显著减少 (P<0.05)[图 7(b)]。这说明,miR-145可以抑制膀胱癌细胞的迁移,为进一探究上述机制,转染miR-145 mimics至T24细胞,RT-PCR、Western blot检测E-cadherin、N-cadherin、Vimentin的表达。结果显示,在mRNA和蛋白质水平,miR-145 mimics组E-cadherin的表达均高于空白对照组和阴性对照组,N-cadherin、Vimentin的表达均低于对照组,差异有统计学意义 (P<0.05) (图 8)。
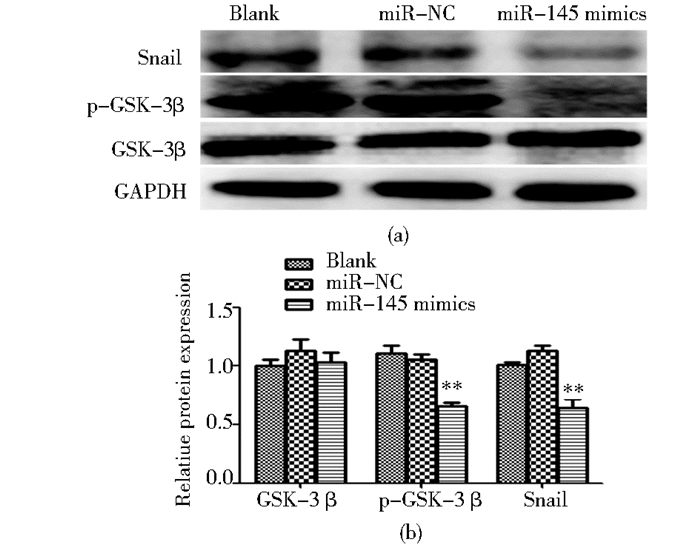
|
| 图 6 转染miR-145 mimics对T24细胞中p-GSK-3β,Snail蛋白表达的影响 Figure 6 The effect of miR-145 mimics on the expression of p-GSK-3β, Snail in T24 (a) Protein expression of p-GSK-3β, Snail detected by Western blot (b) ** P < 0.01, compared with miR-NC group |
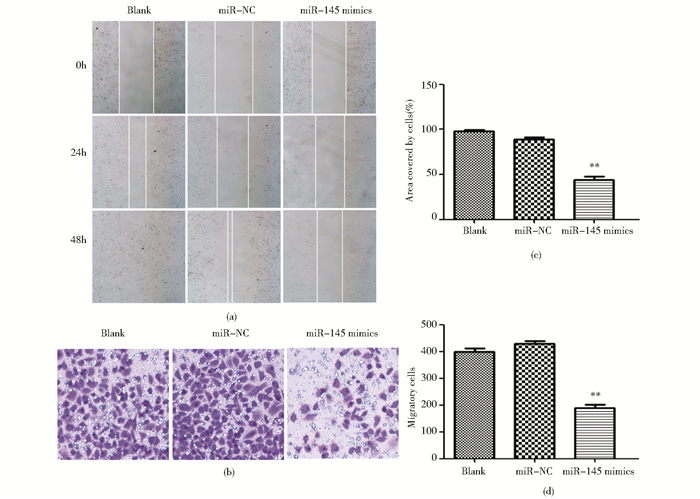
|
| 图 7 过表达miR-145抑制膀胱癌的迁移 Figure 7 Overexpression of miR-145 inhibits the migratory of T24 cell (a) Migratory ability of T24 detected by wound-healing assay (×100) (b) The migratory cells detected by Transwell assay (×100) (c) ** P < 0.01, compared with NC group (d) ** P < 0.01, compared with NC group |
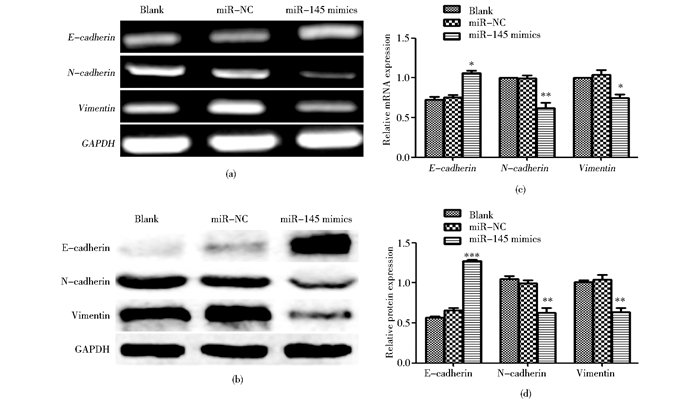
|
| 图 8 miR-145抑制T24细胞的EMT Figure 8 Overexpression of miR-145 inhibits EMT of T24 (a) E-cadherin, N-cadherin, Vimentin mRNA expression detected by RT-PCR (b) E-cadherin, N-cadherin, Vimentin protein expression detected by Western blot (c)* P < 0.05, ** P < 0.01, compared with miR-NC group (d) ** P < 0.01, *** P < 0.001, compared with miR-NC group |
PLCε是一种新近发现的与肿瘤发生密切相关的基因,位于人类染色体10q23[4]。被多种分子 (包括G蛋白、G蛋白偶联受体、生长因子、Ras家族、Rho家族) 激活后,可将细胞膜上的脂酰肌醇-4,5-二磷酸 (PIP2) 水解产生IP3和DAG两种重要的细胞内第二信使,二者分别激活IP3 /Ca2+和DG/PKC两种信号通路[8]。其中,PKC可使多种蛋白质 (如GSK-3β) 的丝氨酸、苏氨酸残基发生磷酸化,激活多条下游信号转导通路,从而参与调节细胞的增殖、分化,影响基因表达,以及肿瘤的发生、发展。近年来,本课题组对PLCε在泌尿系肿瘤发生发展中的作用展开了较为深入的研究,发现PLCε在膀胱癌、肾癌、前列腺癌中均表现为促癌作用[9-11]。其中,在膀胱癌组织和细胞中高表达,且表达水平与膀胱癌的临床分期有关,但与病理分级无关[11];利用干扰腺病毒沉默PLCε在膀胱癌中的表达后,有效抑制了膀胱癌细胞的增殖、侵袭、转移[3]。本课题在前期研究的基础上, 着重探究PLCε对EMT标志分子的影响,旨在明确PLCε在膀胱癌细胞T24发生侵袭转移过程中的作用及分子机制。
糖原合成酶激酶3β(GSK-3β) 是一种广泛存在于各种细胞的丝氨酸/苏氨酸蛋白激酶,在细胞中处于活化状态,它可以被上游激酶PKC、PKA、PKB、Akt等磷酸化其Ser9位点失去激酶活性,然而其活性的异常将会导致多种疾病的发生[12]。目前对GSK-3β的研究已经较为深入,发现它不仅可以抑制糖原的合成,还可以通过对多种底物 (包括转录因子、代谢和信号转导相关的蛋白质) 进行磷酸化来调控细胞的各种生物学功能[13]。值得关注的是GSK-3β对肿瘤的调节具有双向性,一方面,GSK-3β通过Wnt/β-catenin信号通路促使癌基因β-catenin发生磷酸化降解,从而发挥其肿瘤抑制作用[14];另一方面,GSK-3β使NF-κB信号通路中各分子发生磷酸化,进而促进NF-κB介导的基因表达,发挥其促肿瘤作用[15]。而GSK-3β对肿瘤作用的双向性可能与其所处的肿瘤微环境有关。鉴于GSK-3β的以上功能,我们将GSK-3β作为PLCε/PKC下游的一个靶点,探究PLCε/PKC/GSK-3β信号通路在膀胱癌发生EMT及转移中的作用。
Snail作为锌指蛋白转录因子,在肿瘤发生EMT及转移过程中发挥关键作用。研究发现,Snail与Smad相互作用蛋白 (SIP1) 竞争性结合E-cadherin基因启动子区域的E-box序列,从而抑制E-cadherin的表达,促进EMT发生;除此之外,Snail还可以促进基质金属蛋白酶MMP-9的表达[15], 以上均提示Snail可以作为肿瘤细胞发生转移的高相关因子。Biuyere等[16]发现,Snail在膀胱癌组织中的表达明显高于正常组织,且Snail蛋白的表达与膀胱癌的分级、分期及预后有关。在人Snail基因序列中存在2个GSK-3β的保守位点,与GSK-3β结合后使其发生磷酸化,来调节Snail的稳定性及细胞内定位。有研究报道[17]在EGF诱导的EMT中,PKC/GSK-3β信号通路调控了Snail的稳定性和转录。为此,本研究在沉默PLCε的表达后,采用Western blot检测GSK-3β与Snail表达变化及相关性,明确PLCε诱导膀胱癌发生EMT是否与GSK-3β失活介导的Snail的转录增加有关。
MicroRNA (miRNA) 作为基因表达调控的重要分子,在肿瘤侵袭转移中的作用日益突出。近年来,miRNA在侵袭转移中的作用成为膀胱癌的研究热点。已有研究发现,膀胱癌中miR-92b、miR-139、miR-429通过与其靶基因结合调控膀胱肿瘤EMT和转移[18-20]。本研究利用生物信息学数据库 (TargetScan、miRanda及miRDB) 分析作用于PLCε的miRNA,结合Ratert等[21]的膀胱癌miRNA表达谱芯片结果,筛选出miR-145。同时发现,miR-145的5′端关键位点可以与PLCε的3′-UTR端第96~103位的核苷酸互补结合。因此,在这里我们推测miR-145通过直接靶向作用抑制PLCε的表达,从而使膀胱癌中PLCε的促迁移作用减弱。本研究结果显示,在T24细胞中,过表达miR-145后,PLCε在mRNA和蛋白质水平的表达均减少,表明miR-145对PLCε有显著的抑制作用。miR-145与PLCε之间是否存在直接的靶向作用,有待利用荧光素酶报告实验进行下一步验证。
综上所述,采用腺病毒干扰技术,沉默癌基因PLCε的表达,研究其对膀胱癌迁移和EMT的影响及可能的分子机制,揭示了PLCε/GSK-3β/Snail这一新的信号通路,为膀胱癌的防治提供了新靶点和新策略;采用miRNA模拟物,恢复抑癌分子miR-145的功能,探究其与PLCε的关系及对膀胱癌EMT的影响。研究表明,过表达miR-145可以抑制癌基因PLCε的表达,其对膀胱癌细胞EMT的影响与沉默PLCε效果一致。因此,本研究为靶向癌基因治疗与恢复抑癌基因miRNA功能治疗的联合应用奠定了理论基础。
| [1] | Chen W, Zheng R, Baade P D, et al. Cancer statistics in China, 2015. CA:A Cancer Journal for Clinicians, 2016, 66(2) : 115–132. DOI:10.3322/caac.21338 |
| [2] | Ansieau S, Courtois-Cox S, Morel A P, et al. Failsafe program escape and EMT:a deleterious partnership. Seminars in Cancer Biology, 2011, 21(6) : 392–396. |
| [3] | Du H F, Ou L P, Yang X, et al. A new PKCalpha/beta/TBX3/E-cadherin pathway is involved in PLCepsilon-regulated invasion and migration in human bladder cancer cells. Cellular Signalling, 2014, 26(3) : 580–593. DOI:10.1016/j.cellsig.2013.11.015 |
| [4] | Song C, Hu C D, Masago M, et al. Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. The Journal of Biological Chemistry, 2001, 276(4) : 2752–2757. DOI:10.1074/jbc.M008324200 |
| [5] | Smrcka A V, Brown J H, Holz G G. Role of phospholipase Cepsilon in physiological phosphoinositide signaling networks. Cellular Signalling, 2012, 24(6) : 1333–1343. DOI:10.1016/j.cellsig.2012.01.009 |
| [6] | Calin G A, Croce C M. MicroRNA signatures in human cancers. Nature Reviews Cancer, 2006, 6(11) : 857–866. DOI:10.1038/nrc1997 |
| [7] | Noguchi S, Yasui Y, Iwasaki J, et al. Replacement treatment with microRNA-143 and-145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Letters, 2013, 328(2) : 353–361. DOI:10.1016/j.canlet.2012.10.017 |
| [8] | Zhang L, Malik S, Pang J, et al. Phospholipase Cepsilon hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell, 2013, 153(1) : 216–227. DOI:10.1016/j.cell.2013.02.047 |
| [9] | Du H F, Ou L P, Song X D, et al. Nuclear factor-kappaB signaling pathway is involved in phospholipase Cepsilon-regulated proliferation in human renal cell carcinoma cells. Molecular and Cellular Biochemistry, 2014, 389(1-2) : 265–275. DOI:10.1007/s11010-013-1948-4 |
| [10] | Wang Y, Wu X, Ou L, et al. PLCepsilon knockdown inhibits prostate cancer cell proliferation via suppression of Notch signalling and nuclear translocation of the androgen receptor. Cancer Letters, 2015, 362(1) : 61–69. DOI:10.1016/j.canlet.2015.03.018 |
| [11] | 郭永灿, 蒲军, 罗春丽, 等. 磷脂酶Cε基因在膀胱移行细胞癌中表达及其临床意义. 临床检验杂志, 2008, 26(1) : 52–54. Guo Y C, Pu J, Luo C L, et al. Expression and clinical significance of phospholipase C-epsilon gene in transitional cell carcinoma of bladder. Chinese Journal of Clinical Laboratory Science, 2008, 26(1) : 52–54. |
| [12] | Grimes C A, Jope R S. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Progress in Neurobiology, 2001, 65(4) : 391–426. DOI:10.1016/S0301-0082(01)00011-9 |
| [13] | Wu G, Huang H, Garcia Abreu J, et al. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One, 2009, 4(3) : e4926. DOI:10.1371/journal.pone.0004926 |
| [14] | Ding Q, He X, Xia W, et al. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Research, 2007, 67(10) : 4564–4571. DOI:10.1158/0008-5472.CAN-06-1788 |
| [15] | Kudo-Saito C, Shirako H, Takeuchi T, et al. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer cell, 2009, 15(3) : 195–206. DOI:10.1016/j.ccr.2009.01.023 |
| [16] | Bruyere F, Namdarian B, Corcoran N M, et al. Snail expression is an independent predictor of tumor recurrence in superficial bladder cancers. Urologic Oncology, 2010, 28(6) : 591–596. DOI:10.1016/j.urolonc.2008.11.005 |
| [17] | Liu Z C, Chen X H, Song H X, et al. Snail regulated by PKC/GSK-3beta pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell and Tissue Research, 2014, 358(2) : 491–502. DOI:10.1007/s00441-014-1953-2 |
| [18] | Huang J, Wang B, Hui K, et al. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncology Reports, 2016, 36(3) : 1693–1701. |
| [19] | Yonemori M, Seki N, Yoshino H, et al. Dual tumor-suppressors miR-139-5p and miR-139-3p targeting matrix metalloprotease 11(MMP11) in bladder cancer. Cancer Science, 2016, 107(9) : 1233–1242. DOI:10.1111/cas.13002 |
| [20] | Wu C L, Ho J Y, Chou S C, et al. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget, 2016, 7(18) : 26593–26603. |
| [21] | Ratert N, Meyer H A, Jung M, et al. miRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. JMD, 2013, 15(5) : 695–705. DOI:10.1016/j.jmoldx.2013.05.008 |
 2017, Vol. 37
2017, Vol. 37




