文章信息
- 沈鹏飞, 王斌, 谢子康, 郑冲, 瞿玉兴.
- SHEN Peng-fei, WANG Bin, XIE Zi-kang, ZHENG Chong, QU Yu-xing.
- 软骨寡聚基质蛋白过表达对BMP-2诱导骨髓间充质干细胞分化的影响
- Effects of Cartilage Oligomeric Matrix Protein Overexpression on BMP-2 Induced Cell Differentiation of Bone Marrow Mesenchymal Stem Cells
- 中国生物工程杂志, 2016, 36(10): 1-7
- CHINA BIOTECHNOLOGY, 2016, 36(10): 1-7
- http://dx.doi.org/DOI:10.13523/j.cb.20161001
-
文章历史
- 收稿日期: 2016-04-05
- 修回日期: 2016-04-25
软骨组织工程给软骨损伤治疗带来希望,其中,利用基因修饰技术干预干细胞增殖和成软骨分化,从而特异性、长效地调控软骨修复,在软骨组织工程领域具有广阔的应用前景[1]。软骨寡聚基质蛋白(cartilage oligomeric matrix protein,COMP)属于凝血酶敏感蛋白家族,是软骨、韧带、肌腱等组织细胞外基质中最主要的非胶原蛋白之一,在细胞黏附、增殖、分化中扮演重要角色[2-4]。BMP-2(bone morphogenetic protein 2)可诱导多种干细胞增殖及成软骨分化,促进软骨细胞增殖与成熟;同时也可诱导骨髓间充质干细胞(mesenchymal stem cells,MSCs)成骨分化,因此促进BMP-2诱导的干细胞成软骨分化、抑制其成骨分化,对于软骨修复至关重要[5-7]。本研究在体外通过脂质体转染含人COMP基因的质粒使MSCs过表达COMP,用BMP-2诱导骨髓MSCs分化,观察COMP过表达对MSCs成骨分化和成软骨分化的影响。
1 材料与方法 1.1 试剂与仪器SD大鼠骨髓间充质干细胞、干细胞完全培养基购自广州赛业生物科技,载有人COMP基因的pIRES-hrGFP-1a质粒购自上海生工公司;10%胎牛血清、DMEM培养基(Gibco),real-time PCR试剂盒、质粒抽提试剂盒、2×Taq PCR MasterMix PCR扩增试剂(TaKaRa),人重组BMP-2(Peprotech,美国),COMP抗体(Abcam),RUNX2抗体、SOX9抗体(CST公司,美国),Ⅱ型胶原抗体、骨钙蛋白抗体(Millipore),β-actin抗体(博士德公司,武汉),羊抗兔二抗、羊抗鼠二抗、细胞裂解液(碧云天公司),茜素红和阿利新蓝染料(广州赛业生物科技公司),BCA蛋白浓度检测试剂盒、预染蛋白Marker(美国ThermoFisher Scientific公司),引物合成委托上海生工公司。
1.2 细胞转染与分组实验设计为三个组别:对照组为正常培养的骨髓间充质干细胞,空载体组为转染空载质粒的骨髓间充质干细胞,过表达组为转染过表达COMP重组质粒的骨髓间充质干细胞。
培养在干细胞完全培养基中的第五代对数期骨髓间充质干细胞,于转染前12 h种植细胞培养六孔板,使细胞汇合度在转染时达到40%~50%,脂质体转染步骤严格按照说明书进行,转染时长为6 h,转染后三组均更换含50 ng/ml BMP-2、10 % FBS的DMEM培养基继续诱导分化培养至各后续实验检测所需的时间,每隔2~3天换液。
1.3 Real-time PCR分析mRNA表达水平各组处理后收集细胞,六孔板每孔添加适量Trizol和氯仿,提取总RNA,去基因组后检测RNA纯度,纯度合格且无蛋白质污染,用MMLV逆转录酶进行逆转录。GAPDH上游引物:GAAGGTGAAGGTCGGAGTC,下游引物:GAAGATGGTGATGGGATTTC;COMP上游引物:CCCAACTCAGACCAGAAGGA,下游引物:GTCACAAGCATCTCCCACAA;SOX9上游引物:GACGTGCAAGCTGGGAAAGT,下游引物:CGGCAGGTATTGGTCAAACTC;RUNX2上游引物:GCGTCCTATCAGTTCCCAAT,下游引物:CAGCGTCAACACCATCATTC;蛋白聚糖上游引物:TGGCATTGAGGACAGCGAAG,下游引物:TCCAGTGTGTAGCGTGTGGAAATAG;Ⅰ型胶原上游引物:GACATGTTCAGCTTTGTGGACCTC,下游引物:GGGACCCTTAGGCCATTGTGTA;Ⅱ型胶原上游引物:CGCCACGGTCCTACAATGTC,下游引物:GTCACCTCTGGGTCCTTGTTCAC;X型胶原上游引物:CATGCCTGATGGCTTCATAAA,下游引物:AAGCAGACACGGGCATACCT。逆转录成cDNA后,进行定量分析,反应条件为预变性95 ℃,5 min,变性94 ℃,30 s,目的基因均退火60 ℃,30 s,(内参GAPDH退火55 ℃,30 s),延伸72 ℃,1 min,40个循环,72 ℃,6 min。PCR产物进行琼脂糖凝胶电泳,紫外灯下观察DNA条带,凝胶成像系统拍照保存。
1.4 Western blotting分析蛋白表达情况各组处理后收集细胞,冰上加裂解液充分裂解细胞,离心收集上清,使用BCA法测定样品蛋白浓度,分装成30 μg每小管,加上样缓冲液后高温变性,样品经SDS-PAGE电泳后电转移至PVDF膜,TBST配制的5 %脱脂奶粉常温封闭1~2 h,TBST洗膜3次后孵育一抗,COMP、RUNX2、SOX9、Ⅱ型胶原、骨钙蛋白、X型胶原一抗稀释比例均为1:1 000,β-actin稀释比例为1:2 000,一抗4 ℃摇床孵育过夜,TBST洗膜3次后孵育对应的HRP标记的羊抗小鼠或羊抗兔二抗,稀释比例为1:5 000,常温孵育2 h;TBST洗膜后暗室添加适量ECL试剂后压片、显影、定影,胶片扫描后蛋白条带用Image J软件进行灰度分析,半定量检测蛋白表达。
1.5 成骨染色培养至第21天时弃去培养基,PBS清洗细胞2遍,体积分数4 %的多聚甲醛固定30 min,用PBS清洗2遍,加人0.1 %茜素红染液,染色5 min,用PBS清洗2遍,置于倒置显微镜系统成像。
1.6 成软骨染色培养至第21天时,弃去培养基,用PBS清洗细胞2遍,体积分数4 %的多聚甲醛固定30 min,加入l %阿利新蓝染液室温染色30 min,用PBS清洗2遍后置于倒置显微镜系统成像。
1.7 统计处理数据以均数±标准差(x±s)表示,使用SPSS 16.0分析数据,多组间比较行单因素方差分析(One Way ANOVA),P < 0.05为差异具有统计学意义。
2 结果 2.1 骨髓间充质干细胞COMP基因过表达检测细胞转染7天(168 h)后,过表达组目的基因COMP mRNA水平均显著高于对照组和空载体组(P < 0.05),COMP蛋白表达明显多于对照组和空载体组,见图 1。
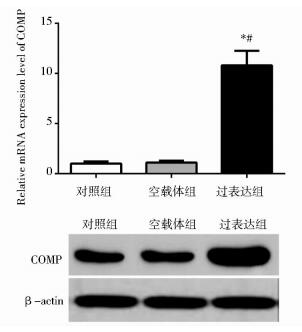
|
| 图 1 骨髓间充质干细胞中COMP过表达效果验证 Figure 1 The validation of COMP overexpression effect in MSCs by RT-PCR and Western blotting Compared with control group, * P < 0.05; Compared with empty vector group, # P < 0.05 |
COMP过表达组细胞转染7天(168 h)后,成骨标记基因RUNX2、Ⅰ型胶原(Col1a1)mRNA水平均显著低于对照组和空载体组(P < 0.05),见图 2;细胞转染后7天(168 h),Western blot结果显示过表达组细胞成骨标记基因RUNX2、骨钙蛋白(Osteocalcin)的蛋白表达水平均明显低于对照组和空载体组(P < 0.05),见图 3。茜素红染色反映矿化结节的生成情况,COMP过表达组细胞转染21天(504 h),茜素红染色深度明显弱于对照组和空载体组,见图 4。以上结果说明骨髓间充质干细胞COMP基因过表达抑制其成骨分化。
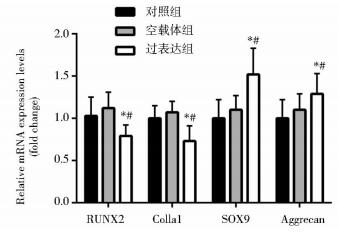
|
| 图 2 PCR分析COMP基因过表达对骨髓间充质干细胞成骨和成软骨标记基因表达的影响 Figure 2 The effects of COMP overexpression on osteogenesis and chondrogenesis related gene expression in MSCs by RT-PCR Compared with control group, * P < 0.05; Compared with empty vector group, # P < 0.05 |
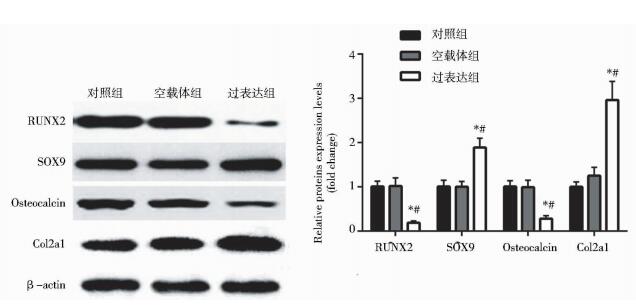
|
| 图 3 Western blot检测COMP基因过表达对骨髓间充质干细胞成骨和成软骨标记基因表达的影响 Figure 3 The effects of COMP overexpression on osteogenesis and chondrogenesis related gene protein expression in MSCs by Western blotting Compared with control group, * P < 0.05; Compared with empty vector group, # P < 0.05 |
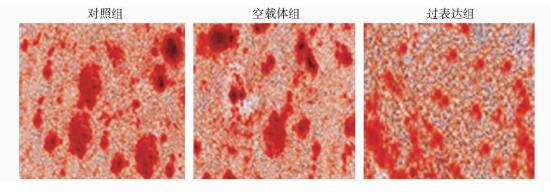
|
| 图 4 茜素红染色结果(×40) Figure 4 The results of alizarin red staining |
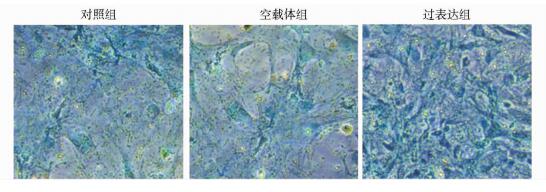
COMP过表达组细胞转染7天(168 h)后,成软骨标记基因SOX9、蛋白聚糖(Aggrecan)mRNA水平均显著高于对照组和空载体组(P < 0.05),见图 2;细胞转染后7天(168 h),Western blot结果显示过表达组细胞成软骨标记基因SOX9、Ⅱ型胶原(Col2a1)的蛋白表达均明显多于对照组和空载体组(P < 0.05),见图 3。阿利新蓝染色反映细胞基质蛋白多糖的合成情况,COMP过表达组细胞转染21天(504 h),阿利新蓝染色深度、密度明显强于对照组和空载体组,见图 5。结果说明骨髓间充质干细胞COMP基因过表达诱导其成软骨分化。
|
| 图 5 阿利新蓝染色结果(×100) Figure 5 The results of alcian blue staining |
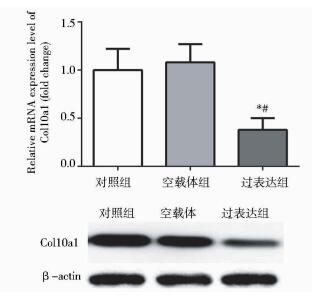
COMP过表达组细胞转染14天(336 h)后,基因Col10a1 mRNA水平均显著低于对照组和空载体组(P < 0.05),Col10a1蛋白表达也明显少于对照组和空载体组,见图 6。结果提示骨髓间充质干细胞COMP基因过表达可抑制软骨细胞的成熟与肥大,维持软骨细胞表型。
|
| 图 6 RT-PCR和Western blotting分析Col10a1基因表达 Figure 6 The gene expression of Col10a1 in MSCs by RT-PCR and Western blotting Compared with control group, * P < 0.05; Compared with empty vector group, # P < 0.05 |
组织移植是治疗软骨损伤的主要方法之一,它可在短期内改善临床症状,但长期疗效还不明确。软骨供给不足及对供体产生损害,且再生能力有限,这些缺点限制了软骨移植等组织移植的应用[1, 8]。应用MSCs的软骨组织工程技术治疗软骨损伤与退变是目前的研究热点,多种生长因子在MSCs分化中发挥重要作用,但作用有限且无特异性[9]。COMP为软骨特异的胞外基质蛋白,研究表明,通过基因修饰技术过表达COMP有利于维持软骨表型[10];COMP可通过C端与BMP-2结合,抑制其成骨诱导作用,进而减少血管内皮细胞钙化[11],这些研究提示COMP对细胞命运产生作用,且该作用可能与BMP-2有关[12-13]。BMP-2具有诱导干细胞成骨分化和成软骨分化的双重作用。目前研究较少关注BMP-2双面效应的调节,且关于COMP过表达是否会对BMP-2诱导干细胞分化的方向产生影响,也还不清楚。本研究将两者结合起来,探讨COMP过表达对BMP-2诱导MSCs分化的影响。
结果显示,COMP过表达组目的基因COMP mRNA和蛋白表达水平明显高于对照组,说明目的基因成功转染并顺利表达。RUNX2是与成骨分化相关的关键转录因子,在调节骨细胞成熟过程中发挥重要作用;BMP-2可促进RUNX2表达,后者又能上调成骨相关基因的表达,如I型胶原、骨钙蛋白等[14-15]。本研究结果还显示,过表达COMP在成骨诱导中晚期,COMP过表达组成骨基因的表达较对照组均显著下调,这可能与过表达COMP减弱BMP-2成骨诱导作用,抑制RUNX2及下游成骨相关基因表达有关。为观察成骨分化过程中矿化程度,使用茜素红对钙结节进行染色了解骨化进程,本研究中细胞转染21天后,COMP过表达组茜素红染色强度明显弱于对照组,提示COMP过表达使干细胞成骨分化进程中钙结节形成受到抑制。
SOX9是BMP-2诱导干细胞成软骨分化进程中的关键转录因子,可被BMP-2信号通路活化,促进其诱导的成软骨分化,同时抑制其诱导的成骨分化[16-17]。Ⅱ型胶原和蛋白聚糖是细胞外基质的重要成分,被认为是软骨分化的重要分子指标[18]。BMP-2诱导干细胞成软骨分化过程中,晚期阶段会出现软骨细胞的肥大、凋亡及基质钙化,破坏正常软骨细胞表型,X型胶原为晚期软骨分化标记物[19-20]。本研究结果显示,过表达COMP在诱导成软骨分化过程中,成软骨相关指标SOX9、Ⅱ型胶原及蛋白聚糖的基因表达水平显著上升。COMP本身可以活化BMP-2促进其成软骨诱导作用,上调成软骨相关基因表达;SOX9单独作用可促进蛋白聚糖基因表达,另一方面,COMP上调SOX9表达后,也进一步促进蛋白聚糖表达。阿利新蓝染色结果同样说明过表达COMP可促进细胞外基质蛋白多糖的合成。此外,本文研究指出过表达COMP可显著下调X型胶原的mRNA和蛋白表达水平,抑制诱导生成的软骨细胞成熟和肥大,维持软骨细胞表型。
综上所述,过表达骨髓间充质干细胞COMP基因可抑制BMP-2诱导MSCs成骨分化,促进BMP-2诱导MSCs成软骨分化,这可能为软骨组织工程提供一个新的方向和靶点。
| [1] | 虞冀哲, 董学海, 陈海丹, 等. 间充质干细胞应用于软骨组织工程的研究进展. 生物骨科材料与临床研究 , 2015, 12 (4) : 60–64. Yu J Z, Dong X H, Chen H D, et al. Reviewed the research progress of cartilage tissue engineering using mesenchymal stem cells. Orthop Biomech Mater Clin Study , 2015, 12 (4) : 60–64. |
| [2] | Liang Y, Fu Y, Qi R, et al. Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood , 2015, 126 (7) : 905–914. DOI:10.1182/blood-2015-01-621292 |
| [3] | Kluzek S, Bay-Jensen A C, Spector T, et al. Higher serum levels of cartilage oligomeric matrix protein (comp) are associated with self-reported knee pain. Osteoarthr Cartilage , 2014, 22 (3) : S74. |
| [4] | Kluzek S, Bay-Jensen A C, Judge A, et al. Serum cartilage oligomeric matrix protein and development of radiographic and painful knee osteoarthritis. A community-based cohort of middle-aged women. Biomarkers , 2015, 20 (8) : 557–564. DOI:10.3109/1354750X.2015.1105498 |
| [5] | Kim M J, Lee B, Yang K, et al. BMP-2 peptide-functionalized nanopatterned substrates for enhanced osteogenic differentiation of human mesenchymal stem cells. Biomaterials , 2013, 34 (30) : 7236–7246. DOI:10.1016/j.biomaterials.2013.06.019 |
| [6] | Barati D, Shariati S R, Moeinzadeh S, et al. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J Controll Release , 2016, 223 : 126–136. DOI:10.1016/j.jconrel.2015.12.031 |
| [7] | Guzzo R M, Gibson J, Xu R H, et al. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem , 2013, 114 (2) : 480–490. DOI:10.1002/jcb.v114.2 |
| [8] | 朱瑜琪, 王金荣, 王智耀. 间充质干细胞促进关节软骨的修复与再生. 中国组织工程研究 , 2015, 19 (50) : 8195–8200. Zhu Y Q, Wang J R, Wang Z Y. Mesenchymal stem cells promote articular cartilage repair and regeneration. Chin J Tissue Eng Res , 2015, 19 (50) : 8195–8200. |
| [9] | 曲峰, 袁邦拓, 齐玮, 等. Wnt3a对骨髓间充质干细胞成软骨分化的影响. 中国矫形外科杂志 , 2016, 24 (2) : 155–159. Qu F, Yuan B T, Qi W, et al. Effect of Wnt3a on chondrogenic differentiation of nesenchymal stem cells. Orthop J China , 2016, 24 (2) : 155–159. |
| [10] | Dharmavaram R M, Liu G, Tuan R S, et al. Stable transfection of human fetal chondrocytes with a type II procollagen minigene: expression of the mutant protein and alterations in the structure of the extracellular matrix in vitro. Arthritis Rheum , 1999, 42 (7) : 1433–1442. DOI:10.1002/(ISSN)1529-0131 |
| [11] | Du Y, Wang Y, Wang L, et al. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ Res , 2011, 108 (8) : 917–928. DOI:10.1161/CIRCRESAHA.110.234328 |
| [12] | Ishida K, Acharya C, Christiansen B A, et al. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone , 2013, 55 (1) : 23–35. DOI:10.1016/j.bone.2013.03.007 |
| [13] | Guo P, Shi Z L, Liu A, et al. Effects of cartilage oligomeric matrix protein on bone morphogenetic protein-2-induced differentiation of mesenchymal stem cells. Orthop Surg , 2014, 6 (4) : 280–287. DOI:10.1111/os.12135 |
| [14] | Wang W J, Sun C, Liu Z, et al. Transcription factor Runx2 in the low bone mineral density of girls with adolescent idiopathic scoliosis. Orthop Surg , 2014, 6 (1) : 8–14. DOI:10.1111/os.2014.6.issue-1 |
| [15] | Ge C, Cawthorn W P, Li Y, et al. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARγ transcription factors. J Cell Physiol , 2016, 231 (3) : 587–596. DOI:10.1002/jcp.v231.3 |
| [16] | Liu C F, Lefebvre V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res , 2015, 43 (17) : 8183–8203. DOI:10.1093/nar/gkv688 |
| [17] | Gurusinghe S, Young P, Michelsen J, et al. Suppression of differentiation and hypertrophy in canine chondrocytes through lentiviral vector expression of Sox9 and induced pluripotency stem cell factors. Biotechnol Lett , 2015, 37 (7) : 1495–1504. DOI:10.1007/s10529-015-1805-5 |
| [18] | 王冠, 陈星星, 薛鑫, 等. Ⅱ型胶原体外促进大鼠骨髓间充质干细胞向软骨细胞分化. 第三军医大学学报 , 2015, 37 (9) : 886–890. Wang G, Chen X X, Xue X, et al. Collagen Ⅱ promotes differentiation of rat bone marrow mesenchymal stem cells into chondrocytes in vitro. J Third Mil Med Univ , 2015, 37 (9) : 886–890. |
| [19] | Lu Y, Ding M, Li N, et al. Col10a1-Runx2 transgenic mice with delayed chondrocyte maturation are less susceptible to developing osteoarthritis. Am J Transl Res , 2014, 6 (6) : 736–745. |
| [20] | Gu J, Lu Y, Li F, et al. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis , 2014, 5 (10) : e1469. DOI:10.1038/cddis.2014.444 |
 2016, Vol. 36
2016, Vol. 36




