文章信息
- 曹莹莹, 邓盾, 张云, 孙爱君, 夏方亮, 胡云峰
- CAO Ying-ying, DENG Dun, ZHANG Yun, SUN Ai-jun, XIA Fang-liang, HU Yun-feng
- 南海深海新颖低温脂肪酶的克隆、表达及酶学性质鉴定
- Cloning, Expression and Characterization of a Novel Psychrophile Lipase from the Deep Sea of the South China Sea
- 中国生物工程杂志, 2016, 36(3): 43-52
- China Biotechnology, 2016, 36(3): 43-52
- http://dx.doi.org/DOI:10.13523/j.cb.20160307
-
文章历史
- 收稿日期: 2015-10-27
- 修回日期: 2015-11-18
2. 中国科学院大学 北京 100049;
3. 滨州市食品药品检验检测中心 滨州 256618
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Binzhou Food and Drug Inspection and Testing Center, Binzhou 256618, China
脂肪酶(EC 3.1.1 .3 )又称为三酰基甘油酯水解酶,能够将甘油三酯水解成甘油和脂肪酸。在动物、植物及微生物中广泛存在。脂肪酶属于α/β折叠水解酶超家族,催化中心由丝氨酸、天冬氨酸和组氨酸组成,少数脂肪酶由丝氨酸、谷氨酸和组氨酸组成[1]。脂肪酶能催化水解、醇解、酯化和转酯化等多种化学反应[2, 3, 4],是最重要的工业生物催化剂之一。例如,碱性脂肪酶被广泛用于洗涤剂工业[5]、香料香精工业需求酸性脂肪酶[6]、耐热脂肪酶适用于生物大分子和生物柴油的生产[7]、绿色工业需要低温脂肪酶[8]等。此外,脂肪酶还被广泛应用于近代的新材料、医药、食品加工、化学合成、生物能源等方面[9, 10, 11, 12, 13, 14, 15, 16, 17]。
但目前工业上使用的脂肪酶基本都是中温酶,酶活性的最适温度一般在50℃左右。低温脂肪酶的最适作用温度为20~40℃,在0℃左右仍有一定催化活性,低温脂肪酶同中温酶相比,具有高效、可低温下作用、周期短、能耗低等优势,在酒类酿造、面包加工、肉类软化、食品添加剂、洗涤添加剂、油脂工业、废纸脱墨、轻纺等许多方面都得到广泛的应用。目前,报道了很多关于低温脂肪酶产生菌的分离和酶学性质的研究[18, 19, 20, 21],低温脂肪酶能够在中、低温环境下高效地作用于底物,其水解机制和冷适应性也得到了研究[22]。
海洋微生物资源丰富,至今研究和鉴定的海洋微生物种类有限,从深海中获得脂肪酶产生菌成为一个新的研究热点。例如,Maharana和Pratima[18]从耐寒的Pseudomonas sp. AKM-L5克隆一种新型低温脂肪酶;Su等[23]利用宏基因组的方法从海绵微生物Ircinia sp.获得一种碱性脂肪酶;Bae等[24]从Pichia lynferdii Y-7723克隆出脂肪酶基因等[25, 26, 27, 28, 29]。目前关于深海放线菌中的脂肪酶的研究鲜有报道,本研究针对来自深海放线菌Pseudonocardia antitumoralis SCSIO 01299中一个脂肪酶,将该脂肪酶进行克隆,在大肠杆菌中实现重组表达,研究其基本酶学性质鉴定,为其进一步的工业化应用奠定良好的工作基础。
1 材料与方法 1.1 材 料 1.1.1 菌株、质粒大肠杆菌E.coli (DE3)、E.coli DH5α、E.coli BL21(DE3)菌株及表达载体pET28a(+)均为本实验室保存。Pseudonocardia antitumoralis SCSIO 01299来自中国南海120°0.975′E、19°0.6649′N,3 258m,pH7.8,2℃),由中国科学院南海海洋研究所分离保存。
1.1.2 试剂主要试剂:T4连接酶和FastPfu DNA PolyMerase购自北京全式金生物科技有限公司;细菌基因组提取试剂盒和限制性内切核酸酶购自Thermo Fisher Scientific Inc;Ni SephraoseTM 6 Fast Flow和PD-10脱盐柱购自GE Healthcare Life Sciences,UK; DNA胶回收试剂盒购自Omega bio-tek;质粒提取试剂盒购自上海捷瑞生物科技有限公司;其他试剂皆为分析纯。
1.1.3 主要仪器PCR仪:美国伯乐S1000TM Thermal Cycler;摇床:上海世平叠加式叠加式摇床PJS-2012R;冷冻离心机:德国Beckman Allegra X-30R;酶标仪:瑞士Tecan Infinite M200 Pro;高压灭菌锅:日本松下MLS-3781L-PC;超声破碎仪:宁波新芝生物科技股份有限公司SCIENTZ-ⅡD;pH计:德国Sartorius DB-10。
1.2 方 法 1.2.1 脂肪酶lipaseB5基因序列分析利用生物信息学手段对基因组进行注释,确定其中lipaseB5的开放阅读框,采用SignalP4.1Server(http://www.cbs.dtu.dk/services/SignalP/)来预测信号肽序列,Clustal W 1.81对测序后的基因进行拼接,软件DNAMAN分析同源性,软件MEGA 4.1分析进化树。脂肪酶基因的理论蛋白质分子质量及等电点采用Compute pI/MW (http://web.expasy.org/compute_pi/)来预测,预测氨基酸一致性分析采用PSI-BLAST (http://blast.ncbi.nlm.nih.gov)。
1.2.2 脂肪酶lipaseB5基因的克隆及载体构建LipaseB5成熟肽利用软件Primer Premier 5设计引物如下:上游引物:5′-CATGGATCCGTGAGCCGACACCTCGA TCC′(下划线部分为BamH I酶切位点);下游引物:5′- CCGCTCGAGTCAGTGTTCCTCGGTGTCGG′(下划线部分为Xho I酶切位点)。以Pseudonocardia antitumoralis SCSIO 01299的总DNA为模板,PCR扩增保守序列片段。具体过程:95℃预变性5min,95℃变性1min,55~65℃退火 30s,72v延伸120s,进行30个循环;于4℃下保存。将PCR产物电泳检测并割胶回收。PCR产物经BamH I和Xho I双酶切,回收片段与载体连接,转化至E.coli DH5α。将重组菌涂布筛选平板,37℃,24h挑选阳性克隆后测序。
1.2.3 脂肪酶lipaseB5表达和纯化将测序验证无误后的质粒pET28(+)- lipaseB5转入E. coli BL21(DE3)中,将E. coli BL21(DE3)涂布到100mg/ml卡那霉素的LB平板,37℃培养过夜后,挑取单菌落于LB液体培养基中进行培养。取样测定菌体浓度达到OD600为0.8左右时,加入0.2mmol/L IPTG在37℃诱导4~6h,4℃离心收集菌体。将菌体重新悬浮在含15ml预冷的50mmol/L pH7.5的磷酸盐缓冲液中,超声破碎,将破碎完的菌体裂解液在4℃下10 000r/min离心20min,收集上清液,将10ml上清液加到50mmol/L为pH7.5的Tris-HCl缓冲溶液预先平衡的镍柱中,用20~60mmol/L咪唑缓冲溶液梯度冲洗杂蛋白,用250mmol/L的咪唑缓冲溶液冲洗目的蛋白。取全菌体、上清液和沉淀用12%的 SDS-PAGE凝胶电泳检测。
1.2.4 脂肪酶lipaseB5的活性测定脂肪酶活性的测定采用比色法[30];1min内水解对硝基苯酚酯,释放1μmol对硝基苯酚所需的酶量定义为一个酶活单位。蛋白质浓度的测定方法采用Bradford法,以牛血清蛋白为标准。
1.2.5 脂肪酶lipaseB5底物特异性将不同长度酰基的对硝基苯酚酯(C2~C16)作为lipaseB5在标准条件下的测定酶活的反应底物,没有脂肪酶的作为平行对照组,在OD405下检测lipaseB5最适底物的相对活性。根据Lineweaver-Burk作图法,计算最适底物的Km和Vmax。
1.2.6 脂肪酶lipaseB5最适反应pH及稳定性在50mmol/L不同pH的缓冲液,即柠檬酸钠/柠檬酸(pH5.0~6.0)、Na2HPO4/NaH2PO4(pH6.0~7.5)、Tris-HCl (pH8.0~9.0)、Glycine-NaOH (pH9.0~10)中,在室温下反应,测定不同pH下的脂肪酶活力。在50mmol/L不同pH的缓冲液中,室温处理一定量的纯酶液1~2h,测定残余酶活力,考察脂肪酶pH稳定性。
1.2.7 脂肪酶lipaseB5的最适反应温度及稳定性将反应混合液分别置于pH7.5、50mmol/L Tris-HCl缓冲溶液(10~70℃)中,1h后,加入等量的脂肪酶lipaseB5分别在不同温度下反应3~5min,在OD405测定不同温度下脂肪酶的活力。将一定量的lipaseB5纯酶液于不同温度(10~60℃)下预处理不同的时间,每隔15min测定lipaseB5的酶活力,考察脂肪酶活性的温度稳定性。
1.2.8 金属离子及不同浓度对脂肪酶lipaseB5活性的影响以pH7.5、50mmol/L的Tris-HCl缓冲溶液为溶剂配制浓度为2mol/L、10mol/L的不同金属离子(Fe2+、Mn2+、Ni2+、Li+、Ca2+、Cu2+、Co2+、Zn2+、Ba2+、Mg2+)及浓度为0.1~1mol/L的NaCl溶液,在37℃下将脂肪酶lipaseB5在各种金属离子溶液中处理1h,来研究其对重组脂肪酶酶活力的影响。以未加入任何金属离子反应作为对照,并以此反应的酶活力为100%。计算加入金属离子及不同浓度NaCl溶液对lipaseB5酶活力的影响。
1.2.9 有机溶剂、变性剂、表面活性剂及EDTA对脂肪酶lipaseB5活性的影响在反应体系中分别加入不同浓度的有机溶剂(乙醇、环己烷、正庚烷、甲醇等)、变性剂(SDS、盐酸胍、β-巯基乙醇、尿素等)、EDTA、表面活性剂(Tween-80、Tween-20、TritonX-100等),在最适条件下测定酶活力,研究其对脂肪酶lipaseB5活力的影响。以未加入任何有机溶剂和化学试剂的反应作为对照,并以此反应的酶活力为100%。计算有机溶剂、变性剂、表面活性剂对酶活力的影响。
2 结果与讨论 2.1 脂肪酶lipaseB5序列分析对测序结果分析发现脂肪酶基因lipaseB5全长972bp,编码323个氨基酸,Primer Premier软件分析表明其G+C含量为68.0%。经ExPASy分析显示,该脂肪酶含有大小为30个氨基酸残基的信号肽。切除信号肽后的脂肪酶由293个氨基酸残基组成,理论蛋白质分子质量为30.5kDa,理论等电点为4.40。经NCBI比对结果显示:lipaseB5基因序列与来自Pseudonocardia sp. EC080625-04的脂肪酶基因有98%的相似性,与Pseudonocardia sp. P1脂肪酶有97%的相似性,与Pseudonocardia sp. AL041005-10、Pseudonocardia sp. HH130629-09、Pseudonocardia spinosispora、 Amycolatopsis sp. MJM2582、Kibdelosporangium sp. MJ126-NF4脂肪酸序列的相似性分别为85%、79%、72%、59%、52%。但这些序列均来自全基因组测序,并没有进行系统的酶学性质研究。对lipaseB5编码区进行蛋白质结构功能域分析结果如图 1所示,lipaseB5的73~296区段为羰基水解酶家族,序列中存在脂肪酶典型的特征序列:Gly122-Ile123-Ser124-Glu125-Gly126(GXSXG)[31, 32],它们是水解机制所必需的序列,也是丝氨酸水解酶中最保守的序列,该酶催化位点为Ser124、Asp222、His257,其中Ser124位于脂肪酸特征序列中。LipaseB5序列中包含PVVLVHG氧离子空穴区,为典型的催化中心[28],上述结构与Staphylococcus epidermidis AT2(ACF28538.1)、Janibacter sp. HTCC2649(EAP97825.1)、Acinetobacter sp. no.6(BAB68337.1)等[28, 33, 34]不同来源的低温脂肪酶序列相似。该蛋白质序列中低数量的精氨酸有利于形成一种更具柔性的三级结构,高数量的甘氨酸残基则有助于酶的构象改变以适应低温催化过程,表明该酶符合低温脂肪酶的结构特点。采用Mega4.1软件的邻接法对lipaseB5进行系统树分析,lipaseB5构建进化树如图 2所示。
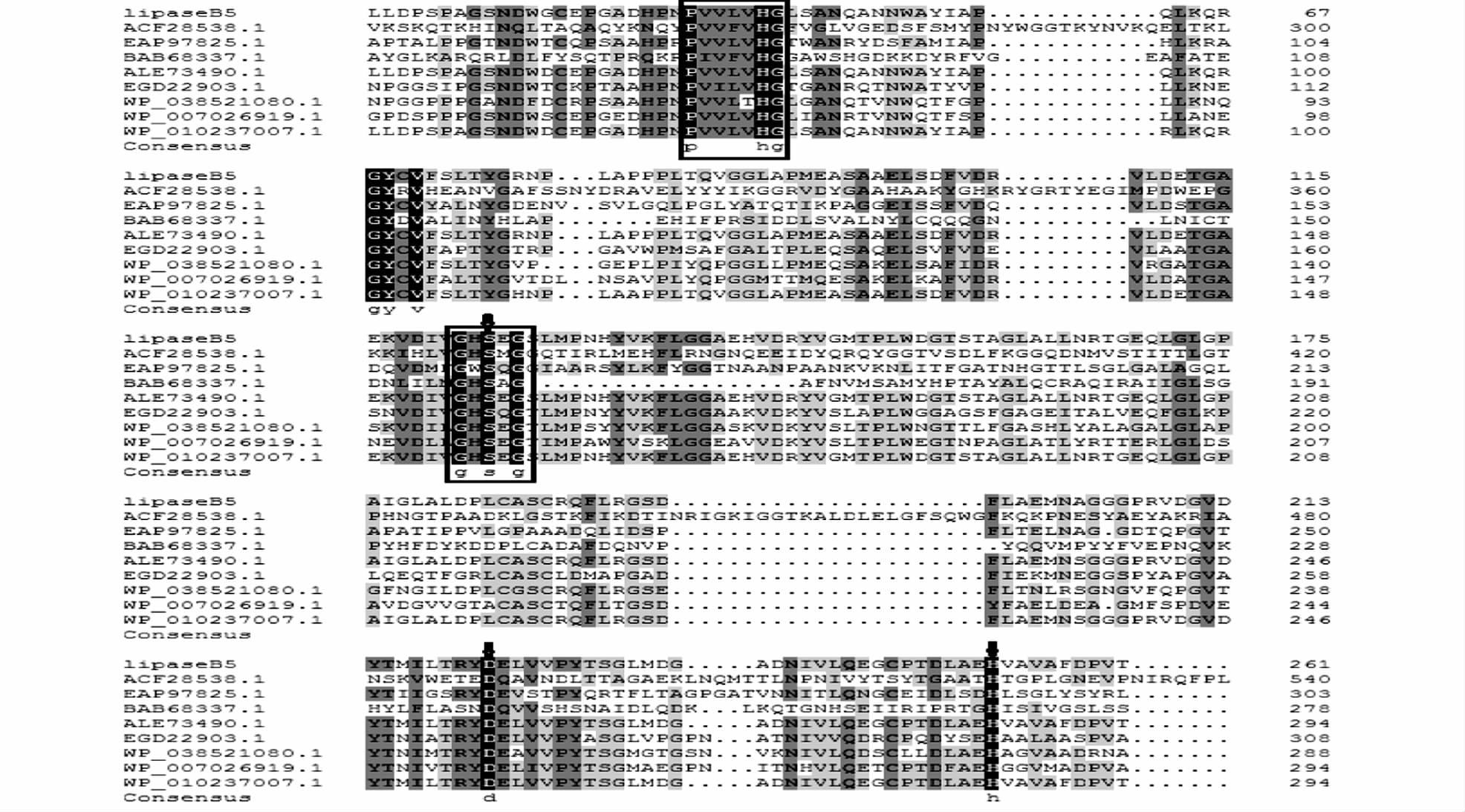 |
| 图 1 lipaseB5全序列比对图 Fig. 1 Multiple sequence alignment of lipaseB5Amino acid residues belonging to catalytic triad were presented as filled circles (↓). ACF28538.1,EAP97825.1 and BAB68337.1 are psychrotolerant lipases. These sequences share a conserved motif Gly-Ile-Ser-Tyr-Gly,containing a serine residue located at the putative active site. The aligned sequences are: LipaseB5; ACF28538.1:Staphylococcus epidermidis AT2;EAP97825.1:Janibacter sp.HTCC2649;BAB68337.1:Acinetobacter sp.NO.6; ALE73490.1: Pseudonocardia sp.EC080625-04; WP_010237007.1: Pseudonocardia sp.P1; WP_038521080.1: Amycolatopsis japonica; WP_007026919.1: Saccharomonospora paurometabolica |
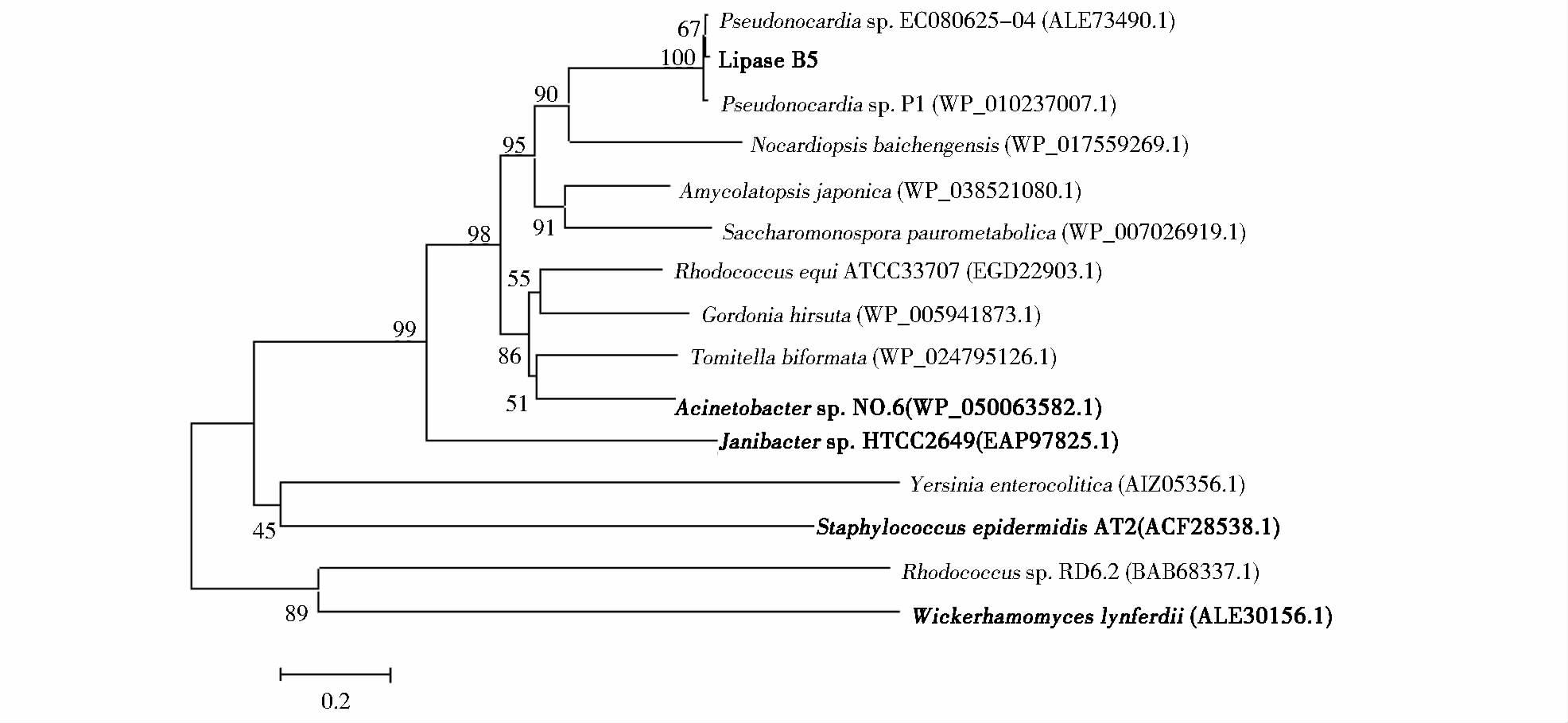 |
| 图 2 脂肪酶lipaseB5系统进化树 Fig. 2 Phylogenetic tree of lipases generated with MEGA v4.0 using the neighbor-joining meth The black parts are psychrotolerant lipases |
成功构建的表达载体pET28a(+)-lipaseB5,转入E.coli BL21(DE3),并实现在E.coli BL21(DE3)中的高效表达。通过Ni-NTA柱的纯化,获得纯脂肪酶lipaseB5的蛋白质含量为4.41μg/ml。SDS-PAGE检测显示单一目的蛋白质条带大约为30.5kDa(图 3),说明lipaseB5大小与理论大小一致。
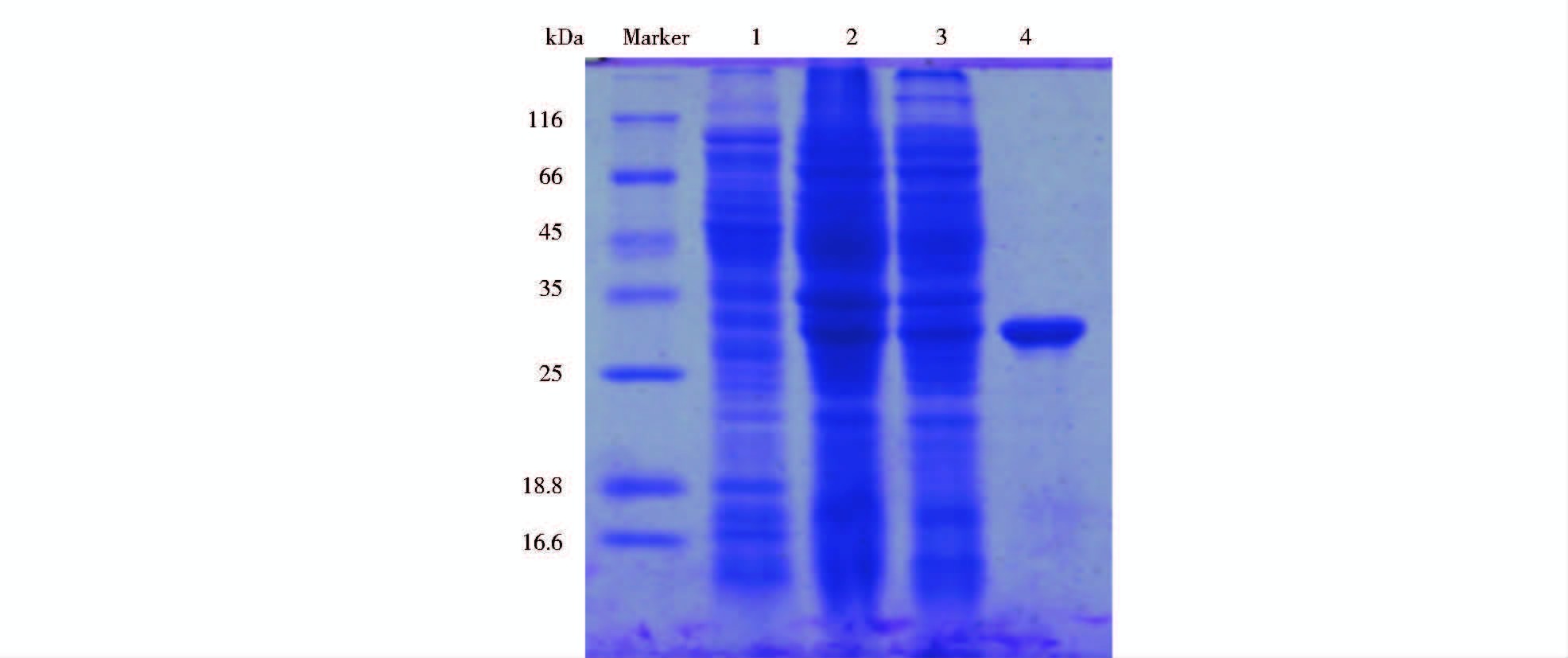 |
| 图 3 脂肪酶lipase7的蛋白质表达、纯化情况 Fig. 3 Expression and purification of lipaseB51: Total proteins before IPTG induction; 2: pET28a- (BL21)-lipaseB5 total proteins after IPTG induction; 3: Protein supernatants of lipaseB5 after IPTG induction; 4: Purified lipaseB5 |
LipaseB5催化不同长度酰基的对硝基苯酚酯(表 1),该酶能催化C2~C16链的对硝基苯酚酯,催化短链酯和中链酯的活性高于长链酯的活性。LipaseB5最佳的作用底物是对硝基苯酚癸酸酯C10(p-NPD)。在最适条件下,以p-NPD为底物,lipaseB5酶活性达到140.14U/mg,Km和Vmax分别为0.976mmol/L、109.8μmol/min。催化橄榄油的活力为32.019 7U/mg,判定该酶为脂肪酶。
| Substrate | U/mg | Relative activity (%)(x±s) |
| p -Nitrophenylacetate(C2) | 56.47 | 42.49±0.021 |
| p -Nitrophenylbutyrate(C4) | 103.18 | 73.63±0.360 |
| p -Nitrophenylcaproate(C6) | 112.42 | 80.22±0.946 |
| p -Nitrophenyloctanoate(C8) | 113.45 | 80.95±0.175 |
| p -Nitrophenyl decanoate (C10) | 140.14 | 100 |
| p -Nitrophenyl dodecanoate(C12) | 78.74 | 56.19±0.087 |
| p -Nitrophenyl myristate(C14) | 53.39 | 38.10±0.073 |
| p -Nitrophenylpalmitate(C16) | 18.48 | 13.19±0.15 |
pH对重组脂肪酶活性的影响见图 4,50mmol/L的Tris-HCl pH为7.5时lipaseB5酶活性最高,pH为7.0~8.5时lipaseB5有较高的酶活性。当pH低于7或高于9时,lipaseB5活性迅速降低。lipaseB5用不同pH的缓冲液处理1h后,pH在8.0时酶活性较稳定,pH在9.5时酶活性残余45.70%。而在过酸或过碱条件下,酶活丧失较为明显。
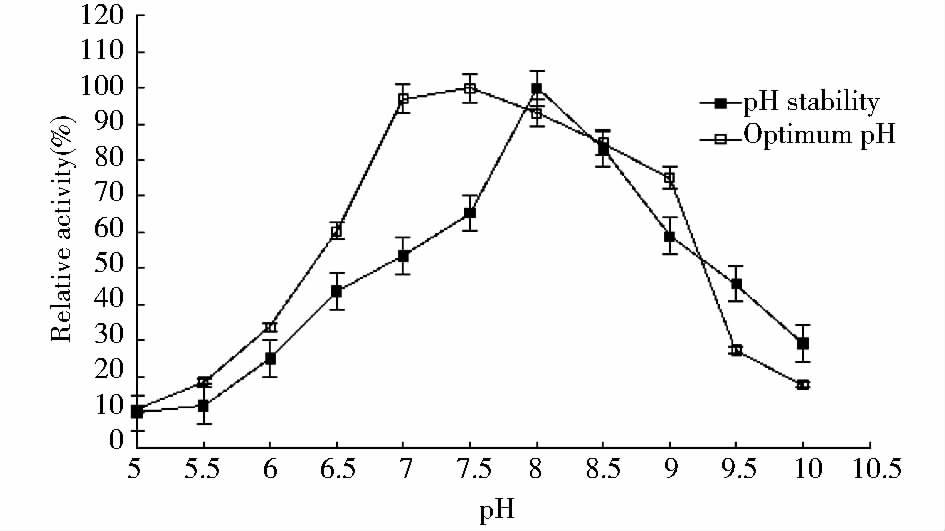 |
| 图 4 pH对lipaseB5酶活性的影响 Fig. 4 Effects of pH on the activity of lipase B5 and pH stability of lipaseB5 |
在最适反应pH条件下测得脂肪酶lipaseB5催化水解p-NPD反应最适温度为30℃(图 5),酶在4℃时残余酶活力达27.98%,在20℃时酶残余活力达到89.48%,温度大于30℃时酶活性急剧下降,这是菌株长期低温生活对环境的一种适应,也是低温酶的一个重要特征。lipaseB5在10~40℃经不同时间处理能保持稳定的酶活力,有较宽的温度适应范围。随着处理温度的升高,lipaseB5残余酶活力逐渐下降,当温度高于50℃时酶活性急剧降低,在60℃处理90min后,残余酶活力仍保持在20%以上。
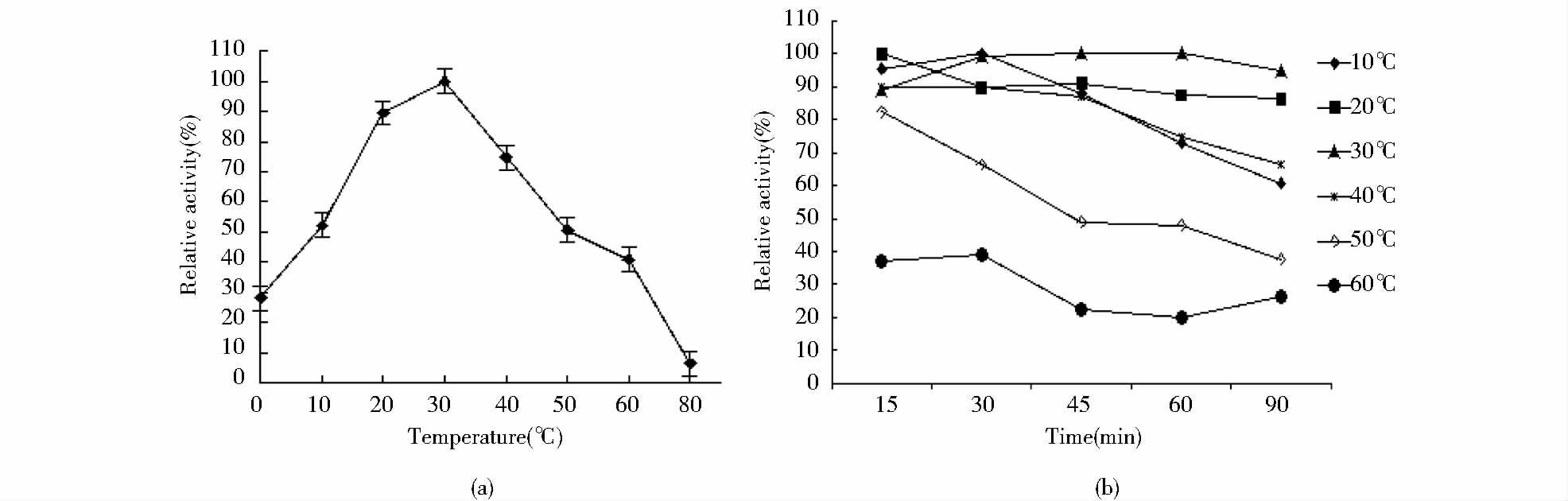 |
| 图 5 温度对lipaseB5酶活性的影响 Fig. 5 Effect of temperature on the activity of lipaseB5 (a)Effects of temperature and pH on the activity of lipaseB5 (b)Temperature dependence of lipaseB5 enzymes activity |
金属离子、不同浓度的NaCl溶液对酶活性的影响见表 2和图 6。在最适条件下测定酶活,结果如图 6所示,2mmol/L Li+、Ca2+、Mg2+对lipaseB5脂肪酶催化对硝基苯酚癸酸酯的活力分别达到对照的124.78%、128.38%、145.33%,当浓度达到10mmol/L时Li+、Ca2+、Mg2+对lipaseB5酶活性影响减弱。Cu2+、Ba2+、Zn2+、Mn2+对酶活性影响较小。lipaseB5在0.1mol/L的NaCl溶液中反应酶活残余为103.61%,当浓度升到0.2mol/L时酶活残余有所下降,但是酶活力稳定;在NaCl浓度为1mol/L时,lipaseB5酶活力残余为28.38%。说明lipaseB5是耐钠盐的脂肪酶[33]。
| Metalions | Relative activity (%)(x±s) | Metalions | Relative activity (%)(x±s) | ||
| 2mmol/L | 10mmol/L | 2mmol/L | 10mmol/L | ||
| Control | 100 | Control | 100 | ||
| Fe2+ | 15.82±0.021 | 8.82±0.046 | Ca2+ | 128.38±0.037 | 96.21±0.0447 |
| Mn2+ | 75.47±0.042 | 64.05±0.025 | Co2+ | 72.80±0.017 | 15.22±0.081 |
| Ni2+ | 19.02±0.03 | 10.71±0.03 | Zn2+ | 62.22±0.02 | 44.93±0.016 |
| Li+ | 124.78±0.011 | 70.89±0.028 | Ba2+ | 80.73±0.02 | 70.77±0.013 |
| Cu2+ | 90.491±0.027 | 86.391±0.014 | Mg2+ | 145.33±0.014 | 115.42±0.034 |
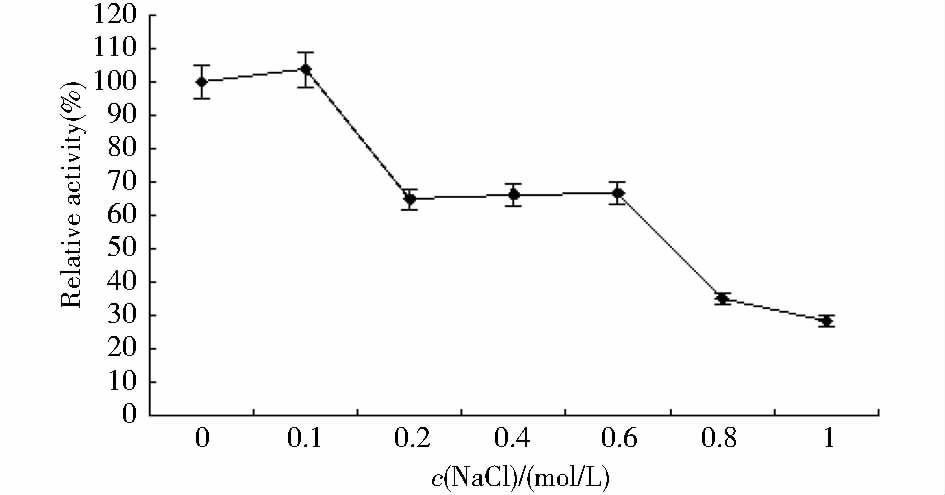 |
| 图 6 不同浓度的NaCl对lipaseB5酶活性的影响 Fig. 6 Effect of Different concentrations of NaCl to lipaseB5 enzyme activity |
由表 3、表 4可以得出,10%的乙醇、正丙醇、异丙醇、正丁醇、叔丁醇能有效地促进lipaseB5的活性,脂肪酶残余活性分别为(120.71±0.038)%、(126.4±0.040)%、(132.42±0.021)%、(146.93±0.142)%。甲醇、正戊醇、正己醇、正庚醇、正辛醇、正癸醇对lipaseB5活性有一定程度的抑制。所以短链的醇对lipaseB5活性有促进作用。当EDTA浓度为5mmol/L时,lipaseB5酶残余活力为(70.59±0.010)%。lipaseB5的酶活力对TritonX-100、Tween-80、Tween-20、十二烷基苯磺酸钠、三聚磷酸钠有稳定的耐受性,其中0.5%的Tween-80、Tween-20提高酶活性分别为(106.86±0.021)%、(120.25±0.061)%,1%的TritonX-100、Tween-20处理后,酶活力分别提高(101.75±0.006)%、(133.99±0.022)%,所以lipaseB5对部分表面活性剂具有很好的耐受性,lipaseB5这种耐有机溶剂和表面活性剂的特性在洗涤添加剂领域具有潜在的应用价值[35]。
| Organic solvents | Relative activity (%) | Organic solvents | Relative activity (%) | ||
| 5% | 10% | 5% | 10% | ||
| Control | 100 | Control | 100 | ||
| Methanol | 12.57±0.034 | 6.76±0.021 | 1-Heptanol | 17.24±0.009 | 45.40±0.029 |
| Ethanol | 155.97±0.050 | 120.71±.038 | n-Octanol | 84.72±0.0218 | 47.5±0.035 |
| n-propanol | 137.75±0.088 | 126.4±0.040 | n-Decyl alcohol | 103.08±0.082 | 63.81±0.038 |
| Isopropyl alcohol | 129.91±0.062 | 129.33±0.055 | DMSO | 98.36±0.0405 | 54.15±0.021 |
| n-Butanol | 41.88±0.102 | 32.42±0.021 | Ethyl acetate | 43.55±0.0602 | 25.19±0.0112 |
| 2-Methyi-1-propanol | 70.59±0.116 | 52.62±0.090 | n-Heptane | 89.27±0.066 | 83.28±0.146 |
| 2-Butanol | 119.72±0.005 | 41.88±0.142 | Cyclohexanone | 53.85±0.0194 | 41.11±0.0009 |
| Tert-butyl alcohol | 146.93±0.142 | 49.03±0.055 | n-Hexane | 39.74±0.0833 | 6.89±0.0033 |
| 1-Pentanol | 29.58±0.025 | 28.72±0.097 | Phenyl methyl ketone | 111.49±0.019 | 64.49±0.031 |
| 1-Hexanol | 1.84±0.096 | 51.00±0.057 | Cyclohexane | 24.61±0.403 | 2.67±0.003 |
| Chemical reagent | Relative activity (%) | Chemical reage | Relative activity (%) | |
| Control | 100% | Control | 100% | |
| detergents TritonX-100 | 0. 5% 88.22±0.11 | 1% 101.75±0.006 | Denaturing agents DTT | 5mmol/L 13.58±0.002 |
| Tween-80 | 106.86±0.021 | 80.6±0.010 | β-Mercaptoethanol | 68.51±0.010 |
| Tween-20 | 120.25±0.061 | 133.99±0.022 | Guanidine hydrochloride | 1.29±0.0023 |
| 5mmol/L | 10mmol/L | SDS | 0.383±0.0066 | |
| Sium tripolyphosphate | 44.04±0.023 | 51.59±0.04 | Urea | 1.51±0.008 |
| Sium decylbenzenesulfonate | 86.78±0.03 | 72.35±0.006 | EDTA | 70.59±0.01 |
低温脂肪酶具有低温高活性、耐碱和对热敏感等特点,目前研究的低温脂肪酶大多来自深海、极地和高寒等极端环境,主要来源于细菌和真菌,目前报道较多的是假单胞菌产生的低温脂肪酶,如Maharana和Pratima[18]、Panizza等[36]、Ji等[25]报道的一系列假单胞菌低温脂肪酶。
Pseudonocardia antitumoralis SCSIO 01299分离自中国南海深海,由于深海的高压、低温、低氧环境,克隆表达的微生物脂肪酶lipaseB5基因(G+C)%含量高。 lipaseB5最适反应温度为30℃,在4℃时,酶相对活力为27.98%,在10~30℃处理90min后酶活力仍保持在80%以上,但是lipaseB5对热比较敏感,在温度大于50℃时酶活力急剧下将,在60℃处理15min时,酶相对活力剩余36.96%,在60℃处理60min时,酶相对活力剩余20.11%,为热敏感低温脂肪酶。Naeem Rashid等[37]将Pseudomonas sp. Strain KB700脂肪酶在60℃处理5min,酶活力急剧降低至23%。低温脂肪酶对热敏感的这种特性可能与深海特殊的环境有关。lipaseB5最适pH为7.5,在pH7.5~8.5时酶活性稳定,在pH9.5时酶活性残余为45.70%,这与Yi等[38]报道的低温脂肪酶最适pH8.0有一定的差别。低浓度Li+、Ca2+、Mg2+处理后,脂肪酶lipaseB5催化p-NPD的活力分别达到对照的124.78%、128.38%、145.33%,对脂肪酶有明显的激活作用;lipaseB5在100mmol/L的NaCl溶液中的酶活力为103.61%,随着盐浓度的增加,酶残余活力降低,耐受盐浓度的范围较宽,与其菌株在深海高盐、高压、低温的生长环境相关,进一步说明lipaseB5具有良好的耐盐性,这与邱秀宝等[39]报道金属离子、NaCl对海洋低温碱性脂肪酶Bohaisea-9145酶活的影响结果,以及对产自适冷菌Yersinia enterocolitica strain KM1的低温脂肪酶影响结果相似[40]。其中Mg2+对lipaseB5酶活的促进作用优于Yesinia enterocolitica strain KM1对低温脂肪酶的活的促进作用。Cu2+、Ba2+、Zn2+、Mn2+、Co2+对脂肪酶lipaseB5的酶活力影响较小,所以在低浓度的金属离子的环境下脂肪酶lipaseB5可以保持酶活力的稳定性,在近代化工、环保、能源等工业领域中具有良好的应用价值。lipaseB5对短链醇及多种有机溶剂具有稳定的耐受性。目前在脂肪酶法合成生物柴油应用中存在的主要问题有:脂肪酶对短链醇的转化率低,且短链醇对酶有一定毒性,使得酶的使用寿命短[41]。而lipaseB5具有对有机溶剂和金属离子良好的耐受性,因此,lipaseB5在生物柴油工业、食品加工工业等方面都具有潜在的应用价值[15],这也是lipaseB5工业应用潜力挖掘的重点。在洗涤剂工业中,所使用的酶制剂要有良好的碱稳定性、热稳定性,以及金属离子、表面活性剂的耐受性[42],LAS、STTP、AES等也是洗涤剂常添加的表面活性剂,在洗涤剂中应用广泛。脂肪酶lipaseB5在0.5%、1%的Tween-80、Tween-20、TritonX-100中酶活力得到提高,在5mmol/L的LAS、STTP等部分非离子表面活性剂反应体系中保持稳定的酶活力。因此,lipaseB5具有成为优良洗涤剂添加酶的潜力。Seo等[42]利用宏基因组克隆表达得到的低温脂肪酶对Tween-80、Tween-20、TritonX-100的耐受性结果与lipaseB5影响结果相近,在近期的低温脂肪酶报道中,Maharanaa和Pratima[18]研究的一个来源于耐冷假单胞菌的新颖低温脂肪酶Pseudomonas sp.AKM-L5具有良好的有机溶剂耐受性,其结果与lipaseB5对表面活性剂、有机溶剂的耐受性结果相近,进一步证实了低温脂肪酶lipaseB5在洗涤添加剂领域的潜在应用价值。
综上所述,脂肪酶lipaseB5作为一种新型的低温脂肪酶,具有耐低温、耐金属离子、耐盐、耐多种短链醇及多种表面活性剂等特性,具有良好的产业化应用前景,为深海微生物脂肪酶资源的利用了奠定良好的基础。
| [1] | Treichel H,Débora M,Marcio A,et al. A review on microbial lipases production. Food and Bioprocess Technology, 2009,3(2):182-196. |
| [2] | 念保义,黄志华,罗菊香,等.脂肪酶转酯化和水解反应拆分薄荷醇的研究进展.化工进展, 2011,30(6):1320-1325. Nian B Y,Huang Z H,Luo J X,et al. Research progress of racemic menthol by lipase kinetic resolution. Chemical Industry and Engineering Progress,2011,30(6):1320-1325. |
| [3] | 蒋振华,于敏,任立伟,等.有机相中固定化脂肪酶催化合成植物甾醇酯. 催化学报, 2013,34(12): 2255-2262. Jiang Z H,Yu M,Ren L W, et al. Synthesis of phytosterol esters catalyzed by immobilized lipase inorganic media. Chinese Journal of Catalysis,2013,34(12):2255-2262. |
| [4] | 赵天涛, 高静, 张丽杰,等.有机相中脂肪酶催化合成乳酸乙酯.催化学报,2006,27(6):537-540. Zhao T T,Gao J,Zhang L J, et al. Ethyl lactate synthesis by lipase in organic media. Chinese Journal of Catalysis, 2006,27(6):537-540. |
| [5] | Rohit Sharmaa Y C,Uttam Chand B. Production,purification,characterization and applications of lipases. Biotechnology Advances, 2001,19(8):627-662. |
| [6] | Mhetras N C,Bastawde K B,Gokhale D V,et al. Purification and characterization of acidic lipase from Aspergillus niger NCIM 1207. Journal of Bioscience and Bioengineering, 2009,102(12):82-89. |
| [7] | Jin Kyu R, Dae Gyun A, Yeon Gu K,et al. New thermophilic and thermostable esterase with sequence similarity to the hormone-sensitive lipase family,cloned from a metagenomic library. Applied and Environmental Microbiology, 2005,71(2):817-825. |
| [8] | 康建波,胡士恒,王亚男,等.脂肪酶催化玉米油合成脂肪酸乙酯工艺研究.现代化工, 2012,31(7):47-49. Kang J B,Hu S H,Wang Y N,et al. Optimization of lipase-catalyzed synthesis of fatty acid ethyl ester from corn oil. Modern Chemical Industry, 2012,31(7):47-49. |
| [9] | Zhao X B, Qi F, Yuan C L,et al. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renewable and Sustainable Energy Reviews,2015,44:182-197. |
| [10] | Tomke P D,Rathod V K.Ultrasound assisted lipase catalyzed synthesis of cinnamyl acetate via transesterification reaction in a solvent free medium. Ultrasonics Sonochemistry, 2015,27:241-246. |
| [11] | 李阳,韦伟,曹茜. 脂肪酶固定化新材料.中国粮油学报, 2014,29(7):122-128. Li Y,Wei W, Cao Q,et al. New materials for immobilized lipase. Journal of Chinese Cereals and Oils Association, 2014,29(7):122-128. |
| [12] | 骆晓敏,霍丽斯.脂肪酶在食品工业中的应用.广东化工, 2014,41(15):140-141. Luo X M,Huo L S. The application of lipase in food industry. Guangdong Chemical Industry, 2014,41(15): 140-141. |
| [13] | 朱浩.新型磁性纳米材料的设计合成及固定化脂肪酶研究.兰州:兰州大学,化工学院,2013. Zhu H. Design and Synthesis of Novel Magnetic Nanomaterials tor Lipase Imniobilization.Lanzhou: Lanzhou University, Institute of Technology, 2013. |
| [14] | 刘海洲,刘均洪,张媛媛,等.微生物脂肪酶的最新应用研究进展.食品研究与开发, 2009,30(1):141-143. Liu H Z,Liu J H,Zhang Y Y,et al. The letest exploratory development trends on microbial lipase. Food Research and Development,2009,30(1):141-143. |
| [15] | Su E Z,Zhang J G,Huang M G,et al. Optimization of the lipase-catalyzed irreversible transesterification of Pistacia chinensis Bunge seed oil for biodiesel production. Russian Chemical Bulletin, 2015,63(12):2719-2728. |
| [16] | Khan N R,Jadhav S V,Rathod V K,et al. Lipase catalysed synthesis of cetyl oleate using ultrasound: Optimisation and kinetic studies. Ultrasonics Sonochemistry,2015,27:522-529. |
| [17] | Montiel M C, Serrano M, Maximo M F,et al. Synthesis of cetyl ricinoleate catalyzed by immobilized Lipozyme (R) CalB lipase in a solvent-free system. Catalysis Today,2015,255:49-53. |
| [18] | Maharana A, Pratima R. A novel cold-active lipase from psychrotolerant Pseudomonas sp.AKM-L5 showed organic solvent resistant and suitable for detergent formulation. Journal of Molecular Catalysis B: Enzymatic,2015,120:173-178. |
| [19] | 王春雨,迟乃玉,张庆芳,等. 低温脂肪酶的分离纯化及酶学性质.食品与生物技术学报,2013,32(8):809-813. Wang C Y,Chi N Y,Zhang Q F,et al. Purification and enzymatic properties of cryogenic lipase. Journal of Food Science and Biotechnology,2013,32(8): 809-813. |
| [20] | 杨帆,李福英,何雄飞,等. 深海产低温脂肪酶菌株Dspro004的诱变育种.生物学杂志,2013,30(3):20-23. Yang F,Li F Y,He X F,et al. The mutagenic breeding of a cold-adapted lipase producing strain Dspro004 from deep sea. Journal of Biology,2013,30(3):20-23. |
| [21] | 衣丹,林学政,沈继红,等.南极产低温脂肪酶菌株Psychrobacter sp.G发酵条件优化研究.海洋科学,2011,29(4): 504-510. Yi D,Lin X Z,Shen J H.et al.Study on optimization fermentation conditions for producing low-temperature lipase by Psychrobacter sp.G.Advances In Marine Science, 2011,29(4): 504-510. |
| [22] | Rabbani G, Ahmad E,Khan M V,et al. Impact of structural stability of cold adapted candida antarctica lipase B (CaLB): in relation to pH, chemical and thermal denaturation. Rsc Advances,2015,5(26): 20115-20131. |
| [23] | Su J, Zhang F L,Sun W,et al. A new alkaline lipase obtained from the metagenome of marine Sponge ircinia sp. World Journal of Microbiology Biotechnology, 2015,31(7): 1093-1102. |
| [24] | Bae J H,Kwon M H,Kim I H,et al. Purification and characterization of a cold-active lipase from Pichia lynferdii Y-7723: pH-dependant activity deviation. Biotechnology and Bioprocess Engineering,2014,19(5): 851-857. |
| [25] | Ji X L,Li S,Lin L B,et al. Gene cloning,sequence analysis and heterologous expression of a novel cold-active lipase from Pseudomonas sp. PF16. Technology and Health Care,2015,13(5): 109-117. |
| [26] | Yu D H,Margesin R. Partial characterization of a crude cold-active lipase from Rhodococcus cercidiphylli BZ22. Folia Microbiologica,2014,59(5): 439-445. |
| [27] | Ugur A,Boran R. Production and characterization of a cold-active and n-hexane activated lipase from a newly isolated Serratia grimesii RB06-22. Biocatalysis and Biotransformation,2014,32(4): 222-230. |
| [28] | Yuan D J, Lan D M, Xin R P, et al. Biochemical properties of a new cold-active mono- and diacylglycerol lipase from marine member Janibacter sp.strain HTCC2649. International Journal of Molecular Sciences, 2014,15(6): 10554-10566. |
| [29] | Maiangwa J, Mohamad Shukuri Mohamad S, Abu Bakar R,et al.Adaptational properties and applications of cold-active lipases from psychrophilic bacteria. Extremophiles,2014,19(2): 235-247. |
| [30] | Marianne K, Birgit H, Dietmar S,et al. Extracellular Lipase of Pseudomonas sp. strain ATCC 21808:purification, characterization, crystallization, and preliminary X-ray diffraction data. Journal of Bacteriology,1991,173(15): 4836-4841. |
| [31] | Kumari A,Gupta R.Functional characterisation of novel enantioselective lipase TALipA from Trichosporon asahii MSR54: sequence comparison revealed new signature sequence AXSXG among yeast lipases. Applied Biochemistry and Biotechnology,2015,175(1): 360-371. |
| [32] | Martini V P, Glogauer A, Muller-Santos M, et al. First co-expression of a lipase and its specific foldase obtained by metagenomics. Microbial Cell Factories,2014,13:171-184. |
| [33] | Kamarudin N H A, Abd Rahman R N Z R,Salleh A,et al. Unscrambling the effect of C-terminal tail deletion on the stability of a cold-adapted, organic solvent stable lipase from Staphylococcus epidermidis AT2. Molecular Biotechnology, 2014,56(8):747-757. |
| [34] | Suzuki T, Nakayama T, Kurihara T,et al.Cold-active lipolytic activity of psychrotrophic Acinetobacter sp. strain No. 6. Journal of Bioscience and Bioengineering, 2001,92(2):144-148. |
| [35] | Grbavcic S. Development of an environmentally acceptable detergent formulation for fatty soils based on the lipase from the indigenous extremophile Pseudomonas aeruginosa strain. Journal of Surfactants and Detergents, 2015,18(3):383-395. |
| [36] | Panizza P, Cesarini S, Diaz P, et al.Saturation mutagenesis in selected amino acids to shift Pseudomonas sp. acidic lipase Lip I.3 substrate specificity and activity. Chemical Communications,2015,51(7):1330-1333. |
| [37] | Naeem Rashid Y S, Satoshi E,Haruyuki A, et al.Low-temperature lipase from psychrotrophic Pseudomonas sp. strain KB700A. Applied and Environmental Microbiology,2001,67(9): 4064-4069. |
| [38] | Yi Z W,Chan Z H,Yang F, et al.Optimization of cold-active lipase production by CALIP1 from deep-sea sediment-derived metagenomic library. Research Journal of Biotechnology,2015,10(4): 91-97. |
| [39] | 邱秀宝 邵孙谧,郑王.海洋低温碱性脂肪酶研究. 微生物学报, 2004,44(6):789-793. Qiu X B,Shao S Y,Zheng W,et al.Bohaisea-9145 study on low-temperature alkaline lipase from Bohaisea-9145.Acta Microbiologica Sinica,2004,44(6):789-793. |
| [40] | Ji X L, Chen G Y, Zhang Q, et al.Purification and characterization of an extracellular cold-adapted alkaline lipase produced by psychrotrophic bacterium Yersinia enterocolitica strain KM1. Journal of Basic Microbiology, 2015, 55(6): 718-728. |
| [41] | 杨建军,马晓迅,陈斌.生物柴油合成中脂肪酶的应用研究进展. 化工进展,2008, 27(11):1777-1781. Yang J J,Ma X X,Chen B,et al. Progress of enzymatic synthesis of biodiesel by lipase. Chemical Industry and Engineering Progress,2008, 27(11):1777-1781. |
| [42] | Niyonzima F N. Biochemical properties of the alkaline lipase of Bacillus flexus XJU-1 and its detergent compatibility. Biologia, 2014, 69(9):1108-1117. |
| [42] | Seo S,Lee Y,Soon S H.Characterization of a novel cold-active esterase isolated from swamp sediment metagenome. World Journal of Microbiology Biotechnology, 2014, 30(3):879-886. |
 2016, Vol. 36
2016, Vol. 36




