2. 中国海洋大学化学化工学院,山东 青岛 266100;
3. 江汉大学环境与健康研究院,湖北 武汉 430056
汞是一种可随食物链富集的有毒痕量金属元素,具有较高生态风险。来源于自然释放和人为排放的汞经大气传输可进行长距离传输,使局域汞污染扩散至全球,导致汞污染成为全球关注的环境问题[1]。河口和滨海湿地在汞循环中起着十分重要的作用。河流输运的汞可在河口迅速沉积至沉积物,使河口地区成为汞重要的“汇”[2]。滨海湿地是甲基汞的重要产生场所,其较为活跃的微生物活动、丰富的有机质和复杂的氧化还原条件等可显著促进汞的甲基化[3-4]。例如,研究表明滨海湿地甲基汞浓度比潮间带泥滩沉积物高出近百倍[5];同时,滨海湿地产生的甲基汞可随食物链富集在生物体中,进而对人体健康产生威胁[3]。
福建省漳江口红树林地区是我国重要的滨海湿地类型保护区[6],存在一定程度的环境污染问题[7-8]。目前对于漳江口汞研究主要集中于沉积物中汞污染情况与来源。研究表明漳江口红树林湿地沉积物中汞的含量为82~343 μg/kg,部分采样点存在轻度汞污染[9]。此外,漳江口水产养殖区域沉积物中汞含量高于红树林沉积物,表明养殖活动增加了附近区域的汞污染风险[10]。目前,对漳江口汞的研究多侧重于沉积物介质,而对水体汞浓度、分布及其影响因素尚无报道,限制了对该区域汞循环的理解和认识。
为探究漳江口水体不同形态汞的空间分布特征及影响因素,于2018年9月在漳江入海口附近采集了表层水,测定了总汞(THg)、溶解态汞(DTHg)、颗粒态汞(PTHg)、总甲基汞(TMeHg)、溶解态甲基汞(DMeHg)和颗粒态甲基汞(PMeHg)的浓度,分析了其分布特征及影响因素,并监测了漳江口水中不同形态汞的24 h时间序列变化。
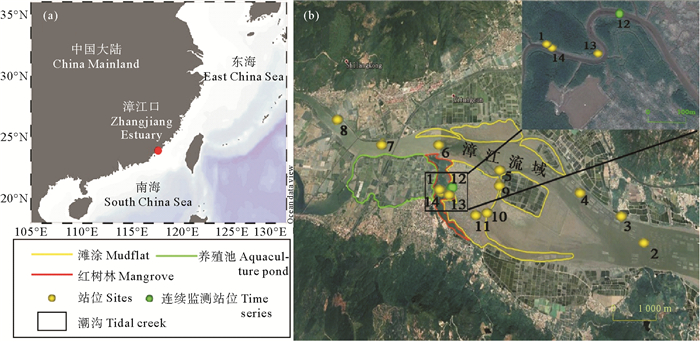
1 材料与方法 1.1 样品采集及保存本研究采样地点位于福建省漳州市云霄县漳江入海口附近(见图 1),支流汇集处即竹塔村附近存在养殖区和红树林区域,潮沟分布于红树林区域内,在漳江流域附近存在较多滩涂区域。于2018年9月采集了14个站位表层水样品,并在潮沟内的站位12进行24 h连续监测。采用Niskin(5L)采水器(General Oceanics,USA)采集表层水样品后,分别将25和45 mL未过滤水样转移至硼硅玻璃瓶中,加入一定体积的盐酸至0.5% (v/v)和0.4% (v/v),用于测定THg和TMeHg。另取一份水样经0.40 μm聚碳酸酯膜(德国Merck公司)过滤后,分别加入盐酸至0.5% (v/v)和0.4%(v/v)用于测定DTHg和DMeHg。样品测定前-20 ℃冷冻保存。分别通过THg和DTHg的差值及TMeHg和DMeHg的差值求得PTHg和PMeHg浓度。
|
( 沿潮沟采样站位12为时间序列站。Time series measurements were performed at site 12, in the tidal creek. ) 图 1 福建省云霄县漳州市漳江口采样点分布图 Fig. 1 Sampling sites at the Zhangjiang Estuary |
水中THg及DTHg的测定参照EPA 1631方法[11]。向25 mL未过滤/过滤的海水中加入125 μL的0.2 mol/L的氯化溴,消解12 h后加入62.5 μL 30%(w/v)的盐酸羟胺除去过量的氯化溴,然后加入125 μL 20%(w/v)氯化亚锡将二价汞还原为挥发态的零价汞,采用汞自动分析仪(Brooks Rand Laboratory,Seattle,WA,USA)检测。
水中TMeHg及DMeHg的测定参照EPA 1630方法[12]。向特氟龙蒸馏管内加入45 mL样品,加入200 μL 1% 吡咯烷二硫代氨基甲酸铵(APDC)后,在(125±3)℃条件下通高纯氮气((90±10)mL/min)蒸馏约2.5~4 h。将蒸馏后的样品转移至气泡瓶内,加入超纯水至100 mL,加入2 mL 2 mol/L醋酸缓冲液调节样品pH至4.9,加入50 μL 1%的NaBEt4反应15 min后,通高纯氮气(200 mL/min)吹扫15 min,将乙基化衍生产物捕集至Tenax管上。Tenax管上吸附的乙基化衍生物在200 ℃加热解析,然后经填充柱气相色谱(OV-3,70 ℃)分离和800 ℃热裂解为Hg0,并采用冷原子荧光光谱仪(Model Ⅲ,Brooks Rand Laboratory,Seattle,WA,USA)检测[13]。
1.3 质量控制为保证数据质量,每批次(不超过20个)样品设置2个方法空白和2个加标回收,并随机抽取一个样品平行分析三次做精密度检验。THg和MeHg的方法空白分别为0.4和0.02 ng/L,满足EPA方法要求(THg方法空白小于0.5 ng/L,MeHg方法空白小于0.03 ng/L)。THg及MeHg标准添加回收率分别为89%~112%和93%~110%,也满足EPA方法的要求(THg:75%~125%;MeHg:65%~135%)。THg和MeHg分析相对标准偏差(RSD)为1.3%~10.3%和3.9%~13.3%,也在方法要求范围内(RSD<15%)。
1.4 数据分析平面分布图采用Ocean Data View(AWI,Germany)绘制。Spearman相关性分析(用来检验THg、TMeHg、DTHg和DMeHg与环境参数的相关性是否显著)使用SPSS 16.0(SPSS Inc.,Chicago,IL)软件。
2 结果与讨论 2.1 漳江口水中THg浓度及平面分布漳江口表层水中THg、DTHg和PTHg浓度分别为(8.4±3.5) ng/L(3.8~16.8 ng/L)、(2.1±1.9) ng/L(0.6~6.7 ng/L)和(6.3±2.5) ng/L(2.4~12.8 ng/L)(见表 1)。与目前已报道的河口比较,漳江口水中总汞含量在国内处于较低水平(如低于长江口的31.0 ng/L[14]以及珠江口的(4.0~47.0) ng/L[15])。与国外河口比较,漳江口水中总汞浓度高于Scheldt River Estuary的0.5~5.0 ng/L[16]和Sinnamary Estuary的(2.2±0.6) ng/L[17],但低于Berry’s Creek Estuary的42.0 ng/L[18]、Elbe Estuary的2.3~102.4 ng/L[19]和San Francisco Bay Estuary的0.15~88 ng/L[20]。不同系统总汞浓度差异可能主要受到汞输入的不同和氧化还原等迁移转化过程的影响。漳江口DTHg浓度在国内河口处于较低水平(如长江口的5.0~25.0 ng/L[14]和小清河河口的3.9~45.0 ng/L[21]),而高于Sinnamary Estuary[17]和Elbe Estuary[19],可能主要受到总汞含量水平的控制。漳江口PTHg浓度低于长江口的28.0 ng/L[14]和Elbe Estuary的1.6~99.1 ng/L[19],与Sinnamary Estuary相比含量较高[17]。
|
|
表 1 漳江口水体THg、DTHg、PTHg浓度及与其它河口比较 Table 1 Comparison of concentrations of various Hg species in Zhangjiang Estuary with other estuaries |
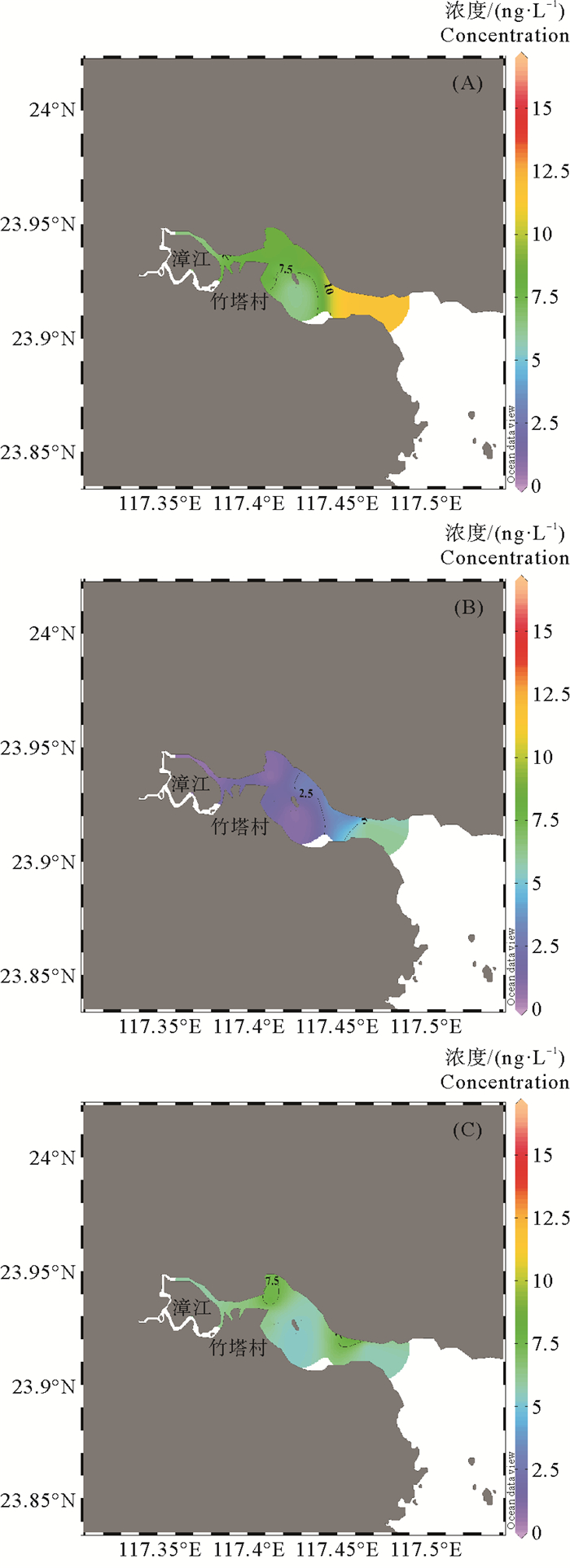
平面分布上,漳江口表层水中THg高值区主要分布在竹塔村、北部近岸区域以及漳江入海口处附近,低值区主要位于漳江支流及南岸区域(见图 2(A))。DTHg高值区主要分布在北部近岸区域及入海口附近(见图 2(B))。PTHg高值区分布在竹塔村和漳江入海口附近(见图 2(C))。支流汇集处上游城镇分布较为密集,来自周围城镇的陆源污染物输入可能导致支流汇集处THg浓度相对较高。竹塔村及入海口附近存在较多的养殖区,这可能是竹塔村及入海口处THg和PTHg含量较高的原因。此外,红树林分布在竹塔村附近,红树林区域向漳江输出大量的溶解有机质。由于溶解有机质与汞具有强络合作用,红树林区域也可能是漳江口水体汞的重要潜在来源[22]。
|
图 2 漳江口表层水THg(A)、DTHg(B)和PTHg(C)(ng/L)浓度平面分布 Fig. 2 The spatial distribution of THg (A), DTHg (B) and PTHg (C) (ng/L) in the surface water of Zhangjiang Estuary |
漳江口表层水中TMeHg、DMeHg和PMeHg浓度分别为(0.13±0.08) ng/L(0.06~0.33 ng/L)、(0.06±0.03) ng/L(0.01~0.11 ng/L)和(0.07±0.07) ng/L(0.01~0.28 ng/L)(见表 2)。相较于国内(长江口的(0.94±0.17) ng/L[23])及国外其他沿海河口,漳江口TMeHg浓度处于较低水平(如Scheldt River Estuary的0.64~8.80 ng/L[16],Sinnamary Estuary的(0.74±0.20) ng/L[17],Berry’s Creek Estuary的0.4~2.8 ng/L[18]和San Francisco Bay Estuary的<0.01~0.46 ng/L[20]),这可能是由于漳江口总汞浓度较低和不同系统汞甲基化和去甲基化能力的差异导致的。DMeHg浓度高于Scheldt River Estuary的0.02~0.08 ng/L[16],而低于小清河河口的0.12~0.75 ng/L[21]和Sinnamary Estuary的(0.40±0.18) ng/L[17]。PMeHg浓度低于Scheldt River Estuary的0.62~8.70 ng/L[16]和Sinnamary Estuary的(0.36±0.24) ng/L[17],而与报道的Elbe Estuary(0.05~0.19 ng/L)[19]结果相近。
|
|
表 2 漳江口水体总TMeHg、DMeHg、PMeHg浓度及与其它河口比较 Table 2 Comparison of concentrations of various MeHg species in Zhangjiang Estuary with other estuaries |
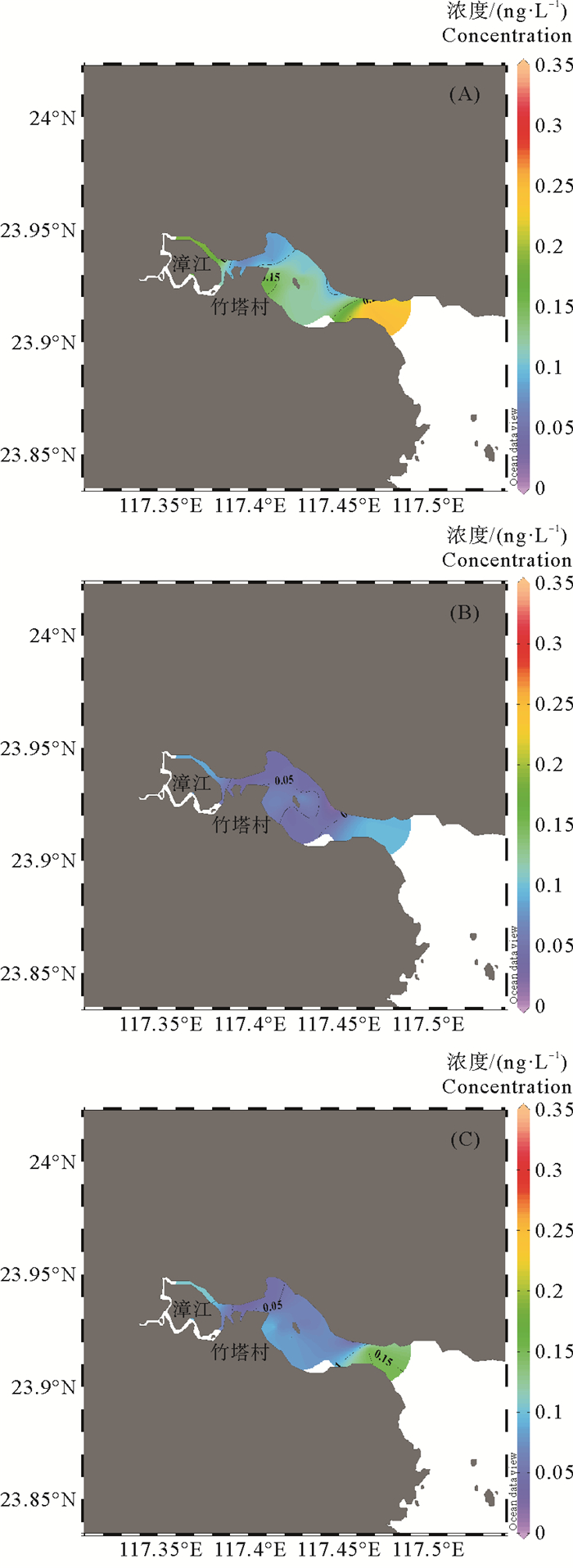
平面分布上,TMeHg、DMeHg以及PMeHg分布均较为相似(见图 3),高值区主要位于漳江北部支流、竹塔村附近和入海口处。由于竹塔村附近分布着红树林,湿地长期缺氧及存在大量营养物质等条件有助于汞的甲基化[24],因此红树林湿地可能是MeHg的重要来源,产生的MeHg可通过潮汐和地下水进入周围水域[25]。研究表明,在河口及近海体系中,由沉积物扩散到水体中的甲基汞占比较大[26-27]。养殖活动产生大量的有机质可通过沉降增加表层沉积物有机质含量,有机质可促进微生物活动从而提高汞的甲基化[28-29]。因此,入海口处存在的养殖活动会增加沉积物汞的甲基化能力,导致该区域MeHg浓度较高。
|
图 3 漳江口表层水MeHg(A)、DMeHg(B) 和PMeHg(C)浓度(ng/L)平面分布 Fig. 3 The spatial distribution of MeHg (A), DMeHg (B) and PMeHg (C) (ng/L) in Zhangjiang Estuary water |
漳江口表层水中TMeHg/THg、DTHg/THg和DMeHg/TMeHg平均值分别为(1.7±0.8)%(0.8%~2.9%)、(23.3±15.0)%(6.7%~56.8%)和(48.8±31.0)%(3.1%~99.2%)。漳江口TMeHg/THg比值高于长江口的(0.7±0.2)%[23]。DTHg/THg比值高于长江口的9.7%[14]和Berry’s Creek Estuary的<10%[18],而低于Sinnamary Estuary的54.5%[17](见表 1)。DMeHg/TMeHg比值与Sinnamary Estuary的51.4%[17]相当,而高于Scheldt River Estuary的3.1%[16]和Berry’s Creek Estuary的<10%[18](见表 2)。红树林湿地丰富的有机质与营养盐、活跃的微生物活动和缺氧条件有助于汞的甲基化[24],潮汐作用可能进一步促进了这一区域THg与TMeHg的交换[22]。
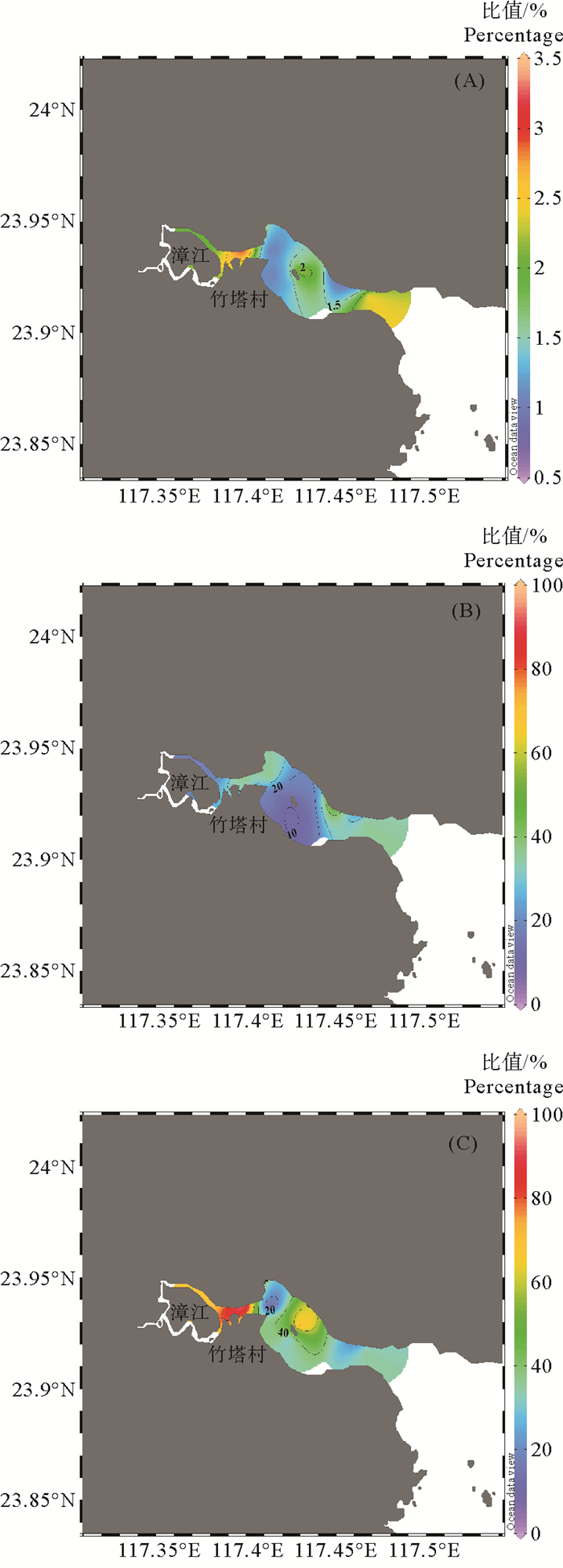
平面分布上,漳江口水中TMeHg/THg高值区主要集中在漳江支流汇集处以及漳江入海口处(见图 4(A))。TMeHg/THg值可代表系统汞的甲基化潜力[30]。这一结果说明该区域汞甲基化能力较强。DMeHg/TMeHg高值区主要分布在漳江口支流汇集处(见图 4(C))。DTHg/THg高值区主要分布在漳江北部近岸支流汇集处及入海口处(见图 4(B)),其分布可能主要受到水中颗粒物含量的影响。
|
图 4 漳江口表层水中MeHg/THg(A)、DTHg/THg(B) 及DMeHg/MeHg(C)(%)的平面分布 Fig. 4 Spatial distribution of MeHg/THg (A), DTHg/THg (B) and DMeHg/MeHg (C) (%) in the surface water of Zhangjiang Estuary |
为识别影响漳江口水中THg、DTHg、TMeHg以及DMeHg分布的关键影响因素,通过Spearman相关性分析探讨了与THg、DTHg、TMeHg以及DMeHg相关的环境参数(如温度、盐度以及pH)(p<0.05)。结果表明(见表 3),水中THg和TMeHg均与温度(R=-0.69,p<0.01;R=-0.63,p<0.05)呈显著的负相关。DTHg与温度(R=-0.57,p<0.05)呈显著负相关,与THg呈显著正相关(R=0.68,p<0.01)。TMeHg和THg也具有显著正相关。
|
|
表 3 漳江口水中THg、DTHg、TMeHg和DMeHg与环境参数的关系 Table 3 The relationships of THg, DTHg, TMeHg and DMeHg with environmental parameters in Zhangjiang Estuary |
漳江口水中THg与温度呈明显负相关,这可能是由于高温可促进水中汞的还原[31],从而降低水中汞的浓度。水中THg与TMeHg呈显著正相关(p<0.01),原因可能是水中的无机汞是汞甲基化过程的重要底物,无机汞浓度高时其系统生成的甲基化产物也相应增高[32]。
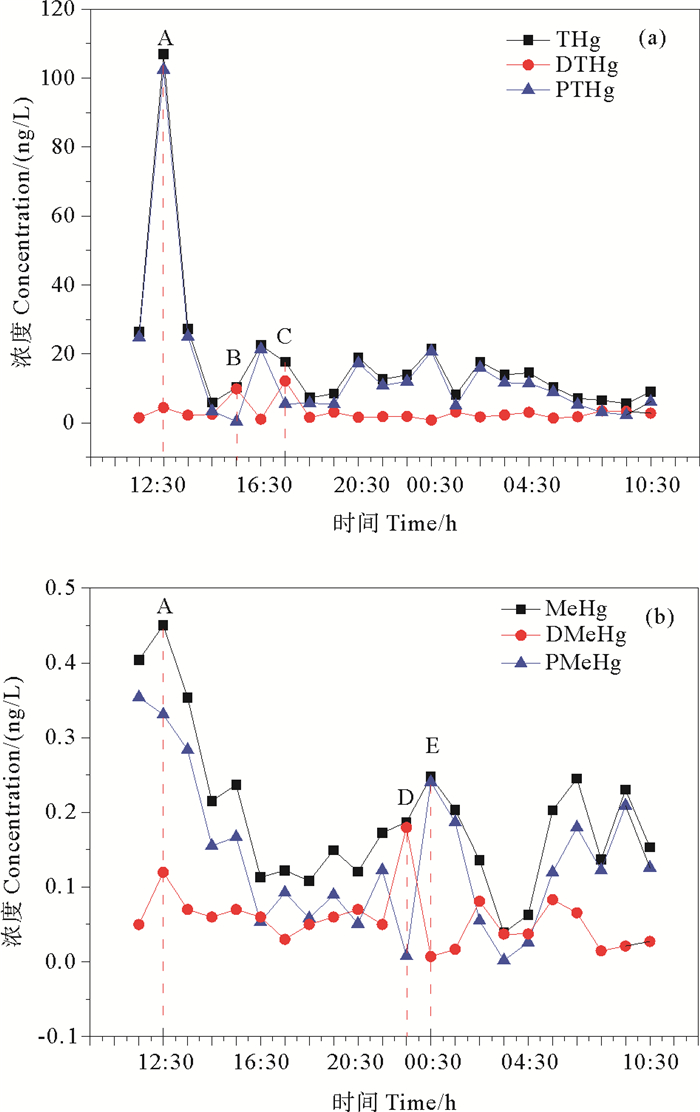
2.5 漳江口水中不同形态汞的24 h连续时间变化24 h时间序列监测站位THg浓度为(17.8±20.9) ng/L(5.6~106.9 ng/L),PTHg浓度为(14.8±20.9) ng/L(0.4~102.4 ng/L),DTHg浓度为(3.1±2.7) ng/L(0.8~12.1 ng/L);TMeHg浓度为(0.19±0.10) ng/L(0.11~0.45 ng/L),PMeHg浓度为(0.14±0.10) ng/L(0.00~0.35 ng/L),DMeHg浓度为(0.06±0.04) ng/L(0.01~0.18 ng/L)。
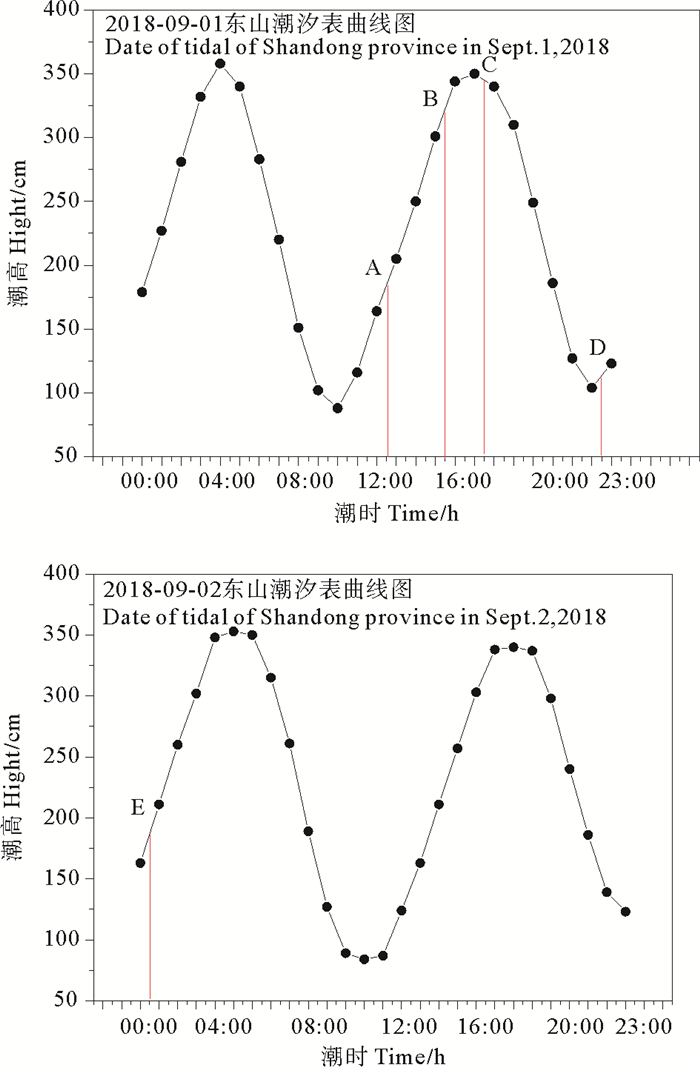
如图 5(a)所示,PTHg与THg呈现相似的时间变化趋势,浓度高值点出现在A点。此时正处于周围养殖塘翻整阶段(8月下旬至9月中旬为养殖区域翻整的阶段,为下一步生产而翻整养殖区底部泥土)[33],翻塘可能导致上覆水中悬浮颗粒物的增加与沉积物中汞的释放,增加了水体中THg和PTHg的浓度。DTHg浓度高值点出现在B、C点,浓度最高点为C点。对比当日潮汐曲线图(见图 6)可以发现,DTHg高值点出现在高潮位点附近,提示潮汐对DTHg的影响较为显著。研究表明,在水深较浅的环境中,潮汐等物理扰动会导致底部沉积物发生再悬浮[34],在扰动下,重金属易发生解吸重新释放至水体中[35-36]。TMeHg和DMeHg浓度高值点在排放养殖泥浆水的A点以及潮位较低的E点(TMeHg)和D点(DMeHg)(见图 5(b)),表明养殖活动以及潮汐可能是影响水中MeHg浓度的重要因素。潮汐可促进红树林与沿海水域的物质交换[22],低潮时潮沟地下水的渗出[37]可能是导致MeHg浓度高值点出现在潮位较低点的主要原因。
|
图 5 THg、PTHg、DTHg(a)和MeHg、PMeHg、DMeHg(b)(ng/L)浓度变化 Fig. 5 Variations of THg, PTHg, DTHg (a) and MeHg, PMeHg, DMeHg (b)(ng/L)with time |
|
( 潮汐数据引自中国海事服务网。Data of tidal were from the China Maritime Service Network. ) 图 6 连续观测期间红树林潮汐变化 Fig. 6 Tidal change in the mangrove area during the continuously monitoring peoriod |
2018年9月漳江口水中THg、DTHg、PTHg浓度分别为(8.4±3.5) ng/L(3.8~16.8 ng/L)、(2.1±1.9) ng/L(0.6~6.7 ng/L)、(6.3±2.5) ng/L(2.4~12.8 ng/L),DTHg占THg的比例(DTHg/THg)为(23.3±15.0)%;TMeHg、DMeHg和PMeHg浓度分别为(0.13±0.08) ng/L(0.06~0.33 ng/L)、(0.06±0.03) ng/L(0.01~0.11 ng/L)、(0.07±0.07) ng/L(0.01~0.28 ng/L),MeHg占THg的比例(TMeHg/THg)和DMeHg占TMeHg的比例(DMeHg/TMeHg)分别为(1.7±0.8)%和(48.8±31.0)%。漳江口水体中,THg高值区主要分布在竹塔村、北部近岸区域以及漳江入海口处附近,提示陆源输入和养殖活动对漳江口水中THg分布影响明显。TMeHg高值区主要位于漳江北部支流、竹塔村附近和入海口处,红树林输入和入海口处存在的养殖活动可能是影响MeHg浓度的重要因素。24 h不同形态汞监测结果表明养殖活动和潮汐可能是影响漳江口水中汞分布的重要因素。
| [1] |
O'Driscoll N J, Rencz A, Lean D R S. The Biogeochemistry and Fate of Mercury in the Environment[M]. England: Taylor & Francis Ltd, 2005: 221-238.
(  0) 0) |
| [2] |
Cossa D, Coquery M, Gobeil C, et al. Mercury Fluxes at the Ocean Margins[M]. United States of America: Kluwer Academic Publishers, 1996: 229-247.
(  0) 0) |
| [3] |
Marvin-Dipasquale M, Agee J, Bouse R, et al. Microbial cycling of mercury in contaminated pelagic and wetland sediments of San Pablo Bay, California[J]. Environmental Geology, 2003, 43(3): 260-267. DOI:10.1007/s00254-002-0623-y (  0) 0) |
| [4] |
Hall B D, Aiken G R, Krabbenhoft D P, et al. Wetlands as principal zones of methylmercury production in southern Louisiana and the Gulf of Mexico region[J]. Environmental Pollution, 2008, 154(1): 124-134. DOI:10.1016/j.envpol.2007.12.017 (  0) 0) |
| [5] |
O'Driscoll N J, Canário J, Crowell N, et al. Mercury speciation and distribution in coastal wetlands and tidal mudflats: Relationships with sulphur speciation and organic carbon[J]. Water Air & Soil Pollution, 2011, 220(1-4): 313-326. (  0) 0) |
| [6] |
徐宗焕, 方柏州, 陈家金, 等. 福建漳江口红树林生长与气象条件的关系[J]. 中国农学通报, 2007, 23(8): 532-535. Xu Z H, Fang B Z, Chen J J, et al. A correlation research between mangrove growth and meteorological conditions of Zhangjiang Estuary in Yunxiao, Fujian province[J]. Chinese Agricultural Science Bulletin, 2007, 23(8): 532-535. DOI:10.3969/j.issn.1000-6850.2007.08.116 (  0) 0) |
| [7] |
Pan Z, Sun Y, Liu Q L, et al. Riverine microplastic pollution matters: A case study in the Zhangjiang River of Southeastern China[J]. Marine Pollution Bulletin, 2020, 159: 111516. DOI:10.1016/j.marpolbul.2020.111516 (  0) 0) |
| [8] |
吴敏兰, 吴锦城, 谢映勤, 等. 漳江口红树林湿地自然保护区非点源污染研究[J]. 集美大学学报(自然科学版), 2013, 18(2): 102-108. Wu M L, Wu J C, Xie Y Q, et al. Study on non-point source pollution in the natural mangrove wetland reserve in Zhangjiang Estuary[J]. Journal of Jimei University (Natural Science), 2013, 18(2): 102-108. DOI:10.3969/j.issn.1007-7405.2013.02.004 (  0) 0) |
| [9] |
马晓霞, 姜兆玉, 王永飞, 等. 福建漳江口红树林沉积物重金属汞(Hg)的分布特征[J]. 生态科学, 2019, 38(2): 9-17. Ma X X, Jiang Z Y, Wang Y F, et al. Distribution of mercury (Hg) in mangrove sediments in Zhangjiang Estuary, Fujian province[J]. Ecological Science, 2019, 38(2): 9-17. (  0) 0) |
| [10] |
Wu H, Liu J L, Bi X Y, et al. Trace metals in sediments and benthic animals from aquaculture ponds near a mangrove wetland in Southern China[J]. Marine Pollution Bulletin, 2017, 117(1-2): 486-491. DOI:10.1016/j.marpolbul.2017.01.026 (  0) 0) |
| [11] |
US EPA: Method 1631: Revision E, Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry[M]. VSA: United States Environmental Protection Agency, 2002: 1-33.
(  0) 0) |
| [12] |
US EPA: Method 1630: Methylmercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and CVAFS[M]. Washington: Pennsylvania Avenue NW, 2001: 1-41.
(  0) 0) |
| [13] |
Chen L F, Li Y B, Liu C, et al. Wet deposition of mercury in Qingdao, a coastal urban city in China: Concentrations, fluxes, and influencing factors[J]. Atmospheric Environment, 2018, 174: 204-213. DOI:10.1016/j.atmosenv.2017.11.059 (  0) 0) |
| [14] |
郑舜琴, 张淑美. 长江下游及长江口海域中汞的分布[J]. 海洋科学, 1986, 10(6): 22-24. Zheng S Q, Zhang S M. The distribution of mercury in downstream and estuary of Changjiang River[J]. Marine Sciences, 1986, 10(6): 22-24. (  0) 0) |
| [15] |
张亚南, 贺青, 陈金民, 等. 珠江口及其邻近海域重金属的河口过程和沉积物污染风险评价[J]. 海洋学报, 2013, 35(2): 178-186. Zhang Y N, He Q, Chen J M, et al. Heavy metals' process in water and pollution risk assessment in surface sediments of the Zhujiang River Estuary[J]. Acta Oceanologica Sinica, 2013, 35(2): 178-186. DOI:10.3969/j.issn.0253-4193.2013.02.019 (  0) 0) |
| [16] |
Leermakers M, Galletti S, Galan S D, et al. Mercury in the Southern North Sea and Scheldt estuary[J]. Marine Chemistry, 2001, 75(3): 229-248. DOI:10.1016/S0304-4203(01)00039-1 (  0) 0) |
| [17] |
Muresan B, Cossa D, Coquery M, et al. Mercury sources and transformations in a man-perturbed tidal estuary: The Sinnamary Estuary, French Guiana[J]. Geochimica et Cosmochimica Acta, 2008, 72(22): 5416-5430. DOI:10.1016/j.gca.2008.08.021 (  0) 0) |
| [18] |
Cardona-Marek T, Schaefer J, Ellickson K, et al. Mercury speciation, reactivity, and bioavailability in a highly contaminated estuary, Berry's Creek, New Jersey Meadowlands[J]. Environmental Science & Technology, 2007, 41(24): 8268-8274. (  0) 0) |
| [19] |
Coquery M, Cossa D. Mercury speciation in surface waters of the North Sea[J]. Netherlands Journal of Sea Research, 1995, 34(4): 245-257. DOI:10.1016/0077-7579(95)90035-7 (  0) 0) |
| [20] |
Conaway C H, Squire S, Mason R P, et al. Mercury speciation in the San Francisco Bay estuary[J]. Marine Chemistry, 2003, 80(2-3): 199-225. DOI:10.1016/S0304-4203(02)00135-4 (  0) 0) |
| [21] |
Jiang T, Skyllberg U, Björn E, et al. Characteristics of dissolved organic matter (DOM) and relationship with dissolved mercury in Xiaoqing River-Laizhou Bay estuary, Bohai Sea, China[J]. Environmental Pollution, 2017, 223: 19-30. DOI:10.1016/j.envpol.2016.12.006 (  0) 0) |
| [22] |
Bergamaschi B A, Krabbenhoft D P, Aiken G R, et al. Tidally driven export of dissolved organic carbon, total mercury, and methylmercury from a mangrove-dominated estuary[J]. Environmental Science & Technology, 2012, 46(3): 1371-1378. (  0) 0) |
| [23] |
Liu C, Chen L F, Liang S K, et al. Distribution of total mercury and methylmercury and their controlling factors in the East China Sea[J]. Environmental Pollution, 2020, 258: 113667. DOI:10.1016/j.envpol.2019.113667 (  0) 0) |
| [24] |
Tjerngren I, Meili M, Björn E, et al. Eight boreal wetlands as sources and sinks for methyl mercury in relation to soil acidity, C/N ratio, and small-scale flooding[J]. Environmental Science & Technology, 2012, 46(15): 8052-8060. (  0) 0) |
| [25] |
Mitchell C P J, Gilmour C C. Methylmercury production in a Chesapeake Bay salt marsh[J]. Journal of Geophysical Research-Biogeosciences, 2008, 113(G2): G00C04. (  0) 0) |
| [26] |
Mason R P, Lawson N M, Lawrence A L, et al. Mercury in the Chesapeake Bay[J]. Marine Chemistry, 1999, 65(1-2): 77-96. DOI:10.1016/S0304-4203(99)00012-2 (  0) 0) |
| [27] |
Choe K Y, Gill G A, Lehman R D, et al. Sediment-water exchange of total mercury and monomethyl mercury in the San Francisco Bay-Delta[J]. Limnology and Oceanography, 2004, 49(5): 1512-1527. DOI:10.4319/lo.2004.49.5.1512 (  0) 0) |
| [28] |
Holmer M, Marba N, Terrados J, et al. Impacts of milkfish (Chanos chanos) aquaculture on carbon and nutrient fluxes in the Bolinao area, Philippines[J]. Marine Pollution Bulletin, 2002, 44(7): 685-696. DOI:10.1016/S0025-326X(02)00048-6 (  0) 0) |
| [29] |
冯新斌, 仇广乐, 付学吾, 等. 环境汞污染[J]. 化学进展, 2009, 21(2/3): 436-457. Feng X B, Qiu G L, Fu X W, et al. Mercury pollution in the environment[J]. Progress in Chemistry, 2009, 21(2/3): 436-457. (  0) 0) |
| [30] |
Kim E, Noh S, Lee Y G, et al. Mercury and methylmercury flux estimation and sediment distribution in an industrialized urban bay[J]. Marine Chemistry, 2014, 158(20): 59-68. (  0) 0) |
| [31] |
Ci Z J, Zhang X H, Yin Y G, et al. Mercury redox chemistry in waters of the eastern Asian seas: From polluted coast to clean open ocean[J]. Environmental Science & Technology, 2016, 50(5): 2371-2380. (  0) 0) |
| [32] |
Kucharzyk K H, Deshusses M A, Porter K A. Relative contributions of mercury bioavailability and microbial growth rate on net methylmercury production by anaerobic mixed cultures[J]. Environmental Science: Processes & Impacts, 2015, 17(9): 1568-1577. (  0) 0) |
| [33] |
谭芳林, 黄丽, 潘辉, 等. 福建漳江口湿地人类活动状况调查[J]. 湿地科学, 2006, 4(3): 40-45. Tan F L, Huang L, Pan H, et al. Investigation on human activities condition in wetlands of the Zhangjiang River estuary, Fujian province[J]. Wetland Science, 2006, 4(3): 40-45. (  0) 0) |
| [34] |
Sanford L P. Wave-forced resuspension of upper Chesapeake Bay Muds[J]. Estuaries, 1994, 17(1): 148-165. DOI:10.2307/1352564 (  0) 0) |
| [35] |
Hatje V, Birch G F, Hill D M. Spatial and temporal variability of particulate trace metals in Port Jackson estuary, Australia[J]. Estuarine, Coastal and Shelf Science, 2001, 53(1): 63-77. DOI:10.1006/ecss.2001.0792 (  0) 0) |
| [36] |
Zwolsman J J G, van Eckb G. Geochemistry of major elements and trace metals in suspended matter of the Scheldt estuary, southwest Netherlands[J]. Marine Chemistry, 1999, 66(1-2): 91-111. DOI:10.1016/S0304-4203(99)00026-2 (  0) 0) |
| [37] |
Zhang H, Moffett K B, Windham M L, et al. Hydrological controls on methylmercury distribution and flux in a tidal marsh[J]. Environmental Science & Technology, 2014, 48(12): 6795-6804. (  0) 0) |
2. College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China;
3. Institute of Environment and Health, Jianghan University, Wuhan 430056, China
 2022, Vol. 52
2022, Vol. 52


