鱼类生活的水环境中富含病原,其皮肤、鳃、肠等黏膜相关组织是病原感染鱼体时最先接触的部位,黏膜组织及黏液是鱼类抵御外界病原入侵的第一道防线,因此,鱼类黏膜免疫相比于陆生动物具有更重要的意义[1-3]。分泌型抗体(SIgs)是黏膜免疫系统的主要组成成分,能够保护细胞免受病原体的粘附、入侵,并将病原体排至黏膜分泌物中,其有效分泌对黏膜免疫防御至关重要。多聚免疫球蛋白受体(Polymeric immunoglobulin receptor,pIgR)是黏膜免疫系统的另一重要成分,介导多聚免疫球蛋白(pIg)的转胞吞作用穿过上皮细胞至黏膜表面形成SIgs,发挥免疫防御功能。
多聚免疫球蛋白受体是一种跨膜糖蛋白受体,属于免疫球蛋白超家族的成员。哺乳动物中,pIgR在黏膜上皮细胞和外分泌腺导管细胞中合成。每一分子免疫球蛋白的转运需要合成一分子pIgR,缺乏pIgR会影响SIgs的分泌。目前,硬骨鱼类中红旗东方鲀(Takifugu rubripes)[4]、鲤鱼(Cyprinus carpio)[5]、斜带石斑鱼(Epinephelus coioides)[6]、虹鳟(Oncorhynchus mykiss)[7]、大西洋鲑(Salmo salar)[8]、牙鲆(Paralichthys olivaceus)[9]、大菱鲆(Scophthalmus maximus)[10]、斑马鱼(Danio rerio)[11]、大西洋鳕鱼(Gadus morhua)[12]、海鲈(Lateolabrax japonicus)[13]、鲫鱼(Carassius auratus)[14]、泥鳅(Misgurnus anguillicaudatus)[15]等的pIgR基因已被克隆表达,并有研究发现pIgR的分泌成分(Secretory component,SC)与免疫球蛋白以复合物的形式存在于鱼类皮肤、鳃、肠黏液及胆汁等外分泌物中[4, 7, 16],但鱼类pIgR对pIg的转胞吞作用及其表达调节机制尚不明确。本文综述了哺乳动物pIgR的功能、调节因子与相关通路以及硬骨鱼类pIgR的相关研究进展,以加深对鱼类pIgR在黏膜免疫防御中作用的了解,为探究鱼类pIgR表达的调节机制提供基础。
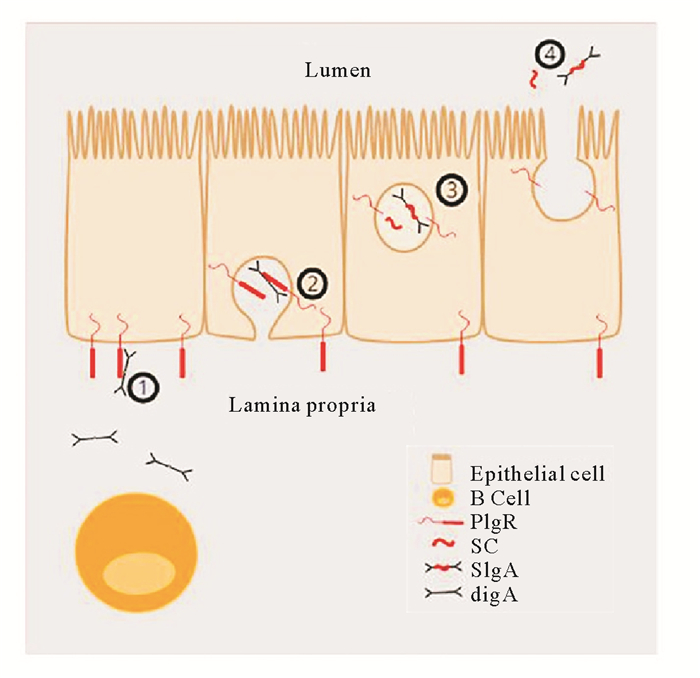
1 多聚免疫球蛋白受体的功能 1.1 哺乳动物pIgR介导的转胞吞作用哺乳动物pIgR的主要功能是从黏膜固有层转运二聚体IgA或多聚体IgM穿过上皮细胞到达黏膜表面,这一过程称作转胞吞作用[17-18]:(1)pIgR在分泌型上皮细胞中合成后,被运输至基底侧,与在固有层中形成的pIg结合(主要是pIgA,若IgM存在也可结合);(2)pIgR-pIg复合物经内吞作用进入细胞,然后通过转胞吞作用到达黏膜上皮细胞顶侧;(3)pIgR与pIg的结合域被蛋白酶裂解,pIgR的胞外区与pIgA以复合物形式释放到外分泌液中,形成分泌型IgA(SIgA);而空载的pIgR也可发生转运,释放游离的SC到分泌物中;(4)释放出的SC和SIgA扩散进入黏液层(见图 1)。因此,pIgR在SIgs形成过程中起着关键作用,并作为SIgs的一部分或者以游离SC形式发挥防御功能。
|
(①多聚免疫球蛋白(pIg)在黏膜固有层中形成,在上皮细胞基部与多聚免疫球蛋白受体(pIgR)结合; ②pIgR-pIg复合物从基底侧经内吞和转胞吞作用到达黏膜细胞顶侧;③pIgR经蛋白酶裂解释放出SC和SIgs;④SC和SIgs释放到黏膜表面。①pIg made in the lamina propria bind to polymeric immunoglobulin receptor (pIgR). ②Endocytosis and transcytosis of the pIg-pIgR complex from the basolateral to the apical side of the mucosal epithelium. ③Intracellular proteolytic cleavage of pIgR creating secretory component (SC) and SIgA. ④Release of SC and SIgA to the mucosal surface.) 图 1 哺乳动物多聚免疫球蛋白受体转运多聚免疫球蛋白至黏膜表面的过程[18] Fig. 1 Transport of polymeric immunoglobulins (pIg) to the mucosal surface[18] |
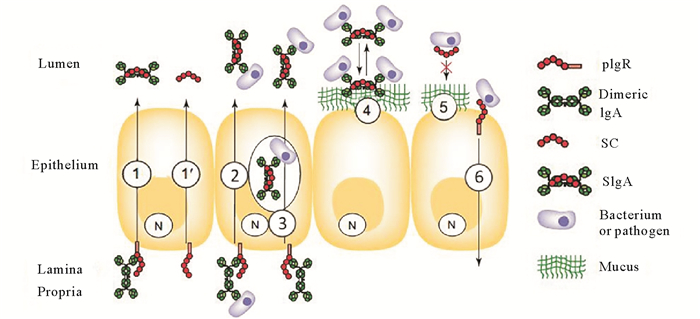
介导黏膜抗体分泌。pIgR在哺乳动物的多种组织中表达,特别是黏膜免疫相关组织,其主要功能是介导多聚体IgA和IgM穿胞转运,其中IgA是哺乳动物主要的黏膜抗体,经上述转胞吞作用过程形成SIgA或游离SC到外分泌液中(见图 2中的
|
图 2 多聚免疫球蛋白受体(pIgR)转胞吞作用及分泌成分(SC)功能展示模型[17] Fig. 2 Multi-faceted functions of the polymeric Ig receptor (pIgR) and secretory component (SC) obtained in various in vitro and in vivo experimental models[17] |
免疫清除抗原与中和细胞内病原体功能。哺乳动物中研究发现,黏膜固有层中形成的pIgA在遇到抗原后,形成pIgA-抗原复合物,在细胞基底部与pIgR结合,通过与黏膜抗体同样的转胞吞作用途径,将pIgA-抗原复合物外排至黏膜表面,清除进入到黏膜固有层中的抗原(见图 2中的②)[28-32]。因此,黏膜pIgA可作为上皮细胞的备用防线,捕获第一道防线中SIgA遗漏的抗原。另外,pIgR在转运pIgA的过程中,可中和黏膜上皮细胞内的病原体,进而消灭或清除病原体(见图 2中的③)。研究发现缺少J链的小鼠感染轮状病毒后,病毒衣壳蛋白VP2和VP6特异性IgA抗体由于缺乏J链而不能被pIgR转运,无法限制病毒的入侵,表明细胞内病原的中和作用机制需要pIgR介导pIgA转运支持[33]。最近研究发现,牙鲆pIgR也具有转运固有层IgM-抗原复合物进入肠黏液的功能。但是,目前尚未见鱼类pIgR介导的pIgs在细胞内中和病原的相关报道。
在黏膜表面维持机体稳态。哺乳动物中,被转运到黏膜表面的SIgA与病原结合,可以有效防止黏膜表面吸附病原,进而阻止病原吸附上皮细胞(见图 2中的④)。空载的pIgR裂解后产生的游离SC在黏膜表面能够与细菌成分结合(见图 2中的⑤),如:游离SC与产肠毒素性大肠杆菌(Enterotoxingenic Escherichia coli)及艰难梭菌(Clostridium difficile)毒素A结合可减少感染,降低发病率[24]。SC还能够直接参与机体保护,其各种糖基是细菌的重要配体,可作为微生物的重要清除因子,保护上皮免受侵袭,发挥非特异性免疫功能。在黏膜分泌液中,足够数量的SC能够减弱细菌毒素对细胞的伤害,也可限制细菌增殖从而降低感染率[35]。结合形式的SC可以保护SIgA,防止蛋白酶降解pIgA,提高pIgA的稳定性进而提高黏膜免疫的效率。硬骨鱼类研究发现,虹鳟皮肤和肠黏液中SC可识别并结合多种病原菌,表明鱼类pIgR同哺乳动物一样,在维持机体稳态中发挥着重要作用[34]。
尽管pIgR为机体黏膜免疫提供众多贡献,但也能让某些病原通过上皮屏障进入体内(见图 2中的⑥)。研究表明,肺炎链球菌能“利用”pIgR胞外区域ILD3和ILD4,经转胞吞作用进入上皮细胞[36]。但通常情况下,pIgR介导微生物由顶面进入细胞内的现象较少发生。
2 多聚免疫球蛋白受体表达的调节机制 2.1 多聚免疫球蛋白受体表达的调节因子在哺乳动物中,pIgR的上调表达可促进SIgA的分泌,pIgR表达量对于SIgA的有效分泌至关重要。微生物、细胞因子、激素等均可通过与哺乳动物PIGR基因特定的位点结合,激活相关转录因子的信号通路来调节PIGR基因的表达水平(见表 1)。
|
|
表 1 细胞因子等因素对pIgR表达的调节作用 Table 1 Regulators of pIgR expression |
细胞因子干扰素-γ(IFN-γ)、肿瘤坏死因子(TNF-α)、白介素-1(IL-1)及白介素-4(IL-4)可在哺乳动物的多种黏膜上皮细胞中上调pIgR的表达量,且存在协同作用[37, 41, 44, 46]。研究显示,使用IFN-γ刺激人肠上皮细胞系(HT-29细胞),pIgR分泌量与INF-γ剂量在一定浓度范围内呈剂量依赖关系[37]。使用10 ng/mL TNF-α处理HT-29细胞,测得PIGR mRNA水平在48 h达到峰值,表达量随TNF-α作用时间的延长而增多[59]。利用IL-1β刺激HT-29细胞,pIgR阳性细胞率上升,pIgR表达量与IL-1β存在剂量和时间依赖关系[44]。
微生物及其代谢产物也能调节pIgR表达。丁酸盐、脂多糖、大肠杆菌、双链RNA、呼吸道肠道病毒及鲍氏酵母菌均可在人的肠黏膜上皮细胞系中上调pIgR表达[55-58]。作为细菌发酵产物及结肠上皮细胞重要能量来源的丁酸盐,可提高HT-29细胞中细胞因子的产生并上调pIgR表达[54]。当多形拟杆菌(一种人与鼠的肠道共生菌)感染无菌小鼠后,其pIgR表达量显著上升,表明共生菌在pIgR表达调节中起重要作用[56]。采用脂多糖刺激体外培养的牛乳腺上皮细胞,PIGR基因的表达量增加[55]。激素(如糖皮质激素和泌乳刺激素)的刺激可上调PIGR基因的表达水平[50]。在山羊乳腺PIGR基因表达及其调控的研究中发现,妊娠3个月后,PIGR基因的表达呈增量调节,分娩3天后显著增加,在泌乳期间PIGR基因表达量达到峰值[52]。
目前关于鱼类pIgR表达调节的研究较少,只有少量关于病原感染引起pIgR表达变化方面的资料。通过灭活鳗弧菌(Vibrio anguillarum)免疫刺激牙鲆,可以上调pIgR基因的表达;注射免疫引起的黏膜组织中pIgR基因表达时间差别不大,而浸泡免疫能更早地上调黏膜免疫相关组织中pIgR表达[16]。使用灭活鳗弧菌免疫大菱鲆,黏膜组织中pIgR基因相对表达量也显著上调,并且在黏膜组织中上调幅度较高[10]。感染嗜水气单胞菌、小瓜虫后,泥鳅鳃和皮肤中pIgR表达量均显著上调[15]。斑马鱼感染海豚链球菌(Streptococcus iniae)后,后肠和脾中pIgR表达量显著性上调,在4 h达到高峰[11]。
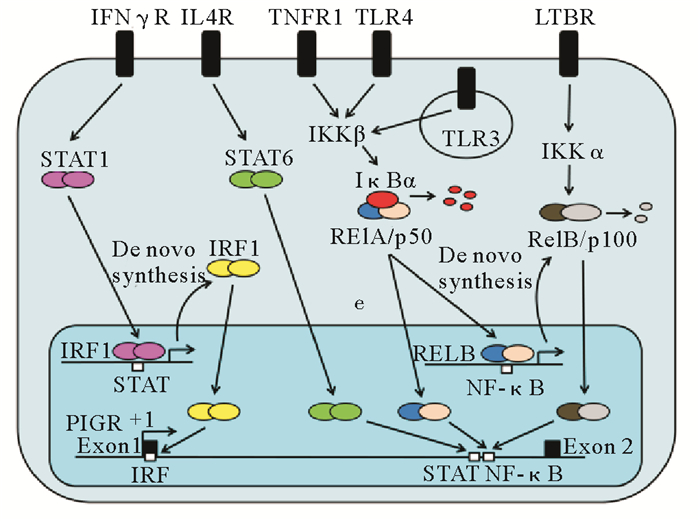
2.2 多聚免疫球蛋白受体表达的调节通路IFN-γ、IL-4和IL-1被证明可以调节pIgR的转录[30]。IFN-γ刺激人肠上皮细胞系HT-29(见图 3),IFN-γ与上皮细胞上的受体结合,激活细胞内的信号传导及转录激活因子1(STAT1),导致转录因子STAT-1(信号传感器和活化剂)的磷酸化和核移位,与靶基因IRF1上的特定位点结合,并诱发IRF1基因的转录,随后IRF1与外显子1处的高保守ISRE结合促进PIGR基因的转录[38, 60]。而酪氨酸磷酸化抑制剂可抑制STAT-1的活化,从而抑制IFN-γ上调细胞pIgR表达量的能力[61]。IFN-γ除了通过IRF1通路诱导pIgR表达,还可直接激活NF-κB,因此IFN-γ对pIgR的调节作用与IRF1和NF-κB通路相关。IL-4通过其受体激活信号传导及转录激活因子6(STAT6),后者与pIgR 2号外显子上的相应位点结合,调节pIgR的表达;而IL-1被证明可以活化一个MyD88依赖性信号通路,经由NF-κB途径对pIgR的转录进行调节[62]。
TNF-α对pIgR的调节作用与NF-κB通路有关[63]。TNF-α与受体结合后,快速激活IKKβ,IKKβ的活化使IκBα磷酸化,磷酸化的IκBα从NF-κB二聚体上脱落,被蛋白酶降解,而NF-κB由抑制状态被激活,移位至细胞核与PIGR外显子1处的结合位点结合,诱导PIGR基因转录。此外还存在旁路途经,NF-κB被激活后,RELB是Rel/p50的靶基因之一,Rel/p50与RELB结合,从头合成RelB/p100二聚体(NF-κB的二聚体之一),RelB/p100移位至细胞核,与PIGR上的NF-κB结合位点结合,促进PIGR转录[64]。人PIGR基因的5’-端区域及外显子1处均含有NF-κB结合位点,但它们都各自发挥诱导功能,并不会相互协调以促进TNF-α对pIgR诱导表达[65-66]。研究发现人PIGR基因的5’-端区域NF-κB的结合位点发生突变后,出现了pIgR对于TNF刺激的启动子活性降低现象,证明TNF诱导的经典NF-κB途径可以直接诱导PIGR转录。
微生物及其代谢物对PIGR基因的上调作用主要是通过Toll样受体(TLR)信号通路[67],TLRs可诱导RelB从头合成,与PIGR外显子1处的NF-κB结合位点结合,激活PIGR基因转录。同时TLR3和TLR4可上调IRF-3的合成,IRF-3又可与NF-κB相互作用激活TLR通路上的多个目的基因,这可与RelB相互作用以促进PIGR基因转录。在分析了多种促炎基因后,研究者发现TLR3信号比TLR4信号途径引起的炎性反应更强[67]。但是,目前激素对pIgR调节的机制还不清楚。
3 结语与展望鱼类持续暴露在富含微生物的水环境中,其黏膜上皮屏障相比于陆生动物在应对环境微生物方面面临更大的威胁,因此其黏膜免疫系统在免疫防御中具有更重要的作用,但是目前对鱼类黏膜免疫系统的了解较少。pIgR是黏膜免疫系统的重要组成成分,能够转运pIgs跨上皮细胞运输到外分泌液中形成SIgs,细胞内pIgR水平对SIgs发挥免疫保护和维持机体稳态功能至关重要。尤其是在目前鱼类疾病频发、缺乏有效治疗药物的情况下,疫苗接种成为最有效的预防措施,随着越来越多的鱼类疫苗被开发利用,人们愈加关注到黏膜免疫在鱼类免疫保护中的重要作用。相比于哺乳动物,鱼类pIgR研究起步较晚,迄今为止,多种硬骨鱼类的pIgR基因已被克隆表达,初步发现鱼类pIgR也有类似哺乳动物pIgR的功能,可以转运分泌型IgT和IgM进入肠道、鳃和皮肤黏液,在黏液中能够结合微生物,但尚缺乏鱼类pIgR对IgM和IgT转胞吞作用的研究资料,细胞因子、激素及微生物对pIgR表达的调节作用研究也较少,也亟待开展疫苗免疫后鱼类pIgR及黏膜Igs获得性免疫应答规律,以及黏膜抗体清除抗原与中和病原体等方面的研究。本文在综述哺乳动物pIgR的功能和调节机制的基础上,介绍了硬骨鱼类相关研究进展,为探究鱼类pIgR表达的调节因素及调节机制,提高细胞内pIgR水平,促进黏膜抗体分泌研究奠定基础,也可加深人们对鱼类黏膜免疫系统的了解。
| [1] |
Cain K D, Jones D R, Raison R L. Characterisation of mucosal and systemic immune responses in rainbow trout (Oncorhynchus mykiss) using surface plasmon resonance[J]. Fish & Shellfish Immunology, 2000, 10(8): 651-666.
(  0) 0) |
| [2] |
Santos N M, Tavernethiele J J, Barnes A C, et al. The gill is a major organ for antibody secreting cell production following direct immersion of sea bass (Dicentrarchus labrax, L.) in a Photobacterium damselae ssp. piscicida bacterin: An ontogenetic study[J]. Fish & Shellfish Immunology, 2001, 11(1): 65-74.
(  0) 0) |
| [3] |
Xu D H, Klesius P H, Shelby R A. Cutaneous antibodies in excised skin from channel catfish, Ictalurus punctatus Rafinesque, immune to Ichthyophthirius multifiliis[J]. Journal of Fish Diseases, 2010, 25(1): 45-52.
(  0) 0) |
| [4] |
Hamuro K, Suetake H, Saha N R, et al. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin[J]. Journal of Immunology, 2007, 178(9): 5682-5689. DOI:10.4049/jimmunol.178.9.5682
(  0) 0) |
| [5] |
Rombout J H, Sj V D T, Yang G, et al. Expression of the polymeric Immunoglobulin Receptor (pIgR) in mucosal tissues of common carp (Cyprinus carpio L.)[J]. Fish & Shellfish Immunology, 2008, 24(5): 620-628.
(  0) 0) |
| [6] |
Feng L N, Lu D Q, Bei J X, et al. Molecular cloning and functional analysis of polymeric immunoglobulin receptor gene in orange-spotted grouper (Epinephelus coioides)[J]. Comparative Biochemistry & Physiology Part B Biochemistry & Molecular Biology, 2009, 154(3): 282-289.
(  0) 0) |
| [7] |
Zhang Y A, Salinas I, Li J, et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity[J]. Nature Immunology, 2010, 11(9): 827-835. DOI:10.1038/ni.1913
(  0) 0) |
| [8] |
Tadiso T M, Sharma A, Hordvik I. Analysis of polymeric immunoglobulin receptor-and CD300-like molecules from Atlantic salmon[J]. Molecular Immunology, 2012, 49(3): 462-473.
(  0) 0) |
| [9] |
Xu G J, Zhan W B, Ding B J, et al. Molecular cloning and expression analysis of polymeric immunoglobulin receptor in flounder (Paralichthys olivaceus)[J]. Fish & Shellfish Immunology, 2013, 35(3): 653-660.
(  0) 0) |
| [10] |
丁冰洁, 绳秀珍, 唐小千, 等. 大菱鲆多聚免疫球蛋白受体基因的克隆及表达分析[J]. 中国水产科学, 2013, 178(4): 792-801. Ding B J, Sheng X Z, Tang X Q, et al. Molecular cloning and expression analysis of the pIgR gene in Scophthalmus maximus[J]. Journal of Fishery Sciences of China, 2013, 178(4): 792-801. (  0) 0) |
| [11] |
Kortum A N, Rodriguez-Nunez I, Yang J, et al. Differential expression and ligand binding indicate alternative functions for zebrafish polymeric immunoglobulin receptor (pIgR) and a family of pIgR-like (PIGRL) proteins[J]. Immunogenetics, 2014, 66(4): 267-279. DOI:10.1007/s00251-014-0759-4
(  0) 0) |
| [12] |
Rombout J H W M, Yang G, Kiron V. Adaptive immune responses at mucosal surfaces of teleost fish[J]. Fish & Shellfish Immunology, 2014, 40(2): 634-643.
(  0) 0) |
| [13] |
Yang S, Liu S, Qu B, et al. Identification of sea bass pIgR shows its interaction with vitellogenin inducing antibody-like activities in HEK 293T cells[J]. Fish & Shellfish Immunology, 2016, 63: 394-404.
(  0) 0) |
| [14] |
Wang L, Zhang J, Kong X, et al. Molecular characterization of polymeric immunoglobulin receptor and expression response to Aeromonas hydrophila challenge in Carassius auratus[J]. Fish & Shellfish Immunology, 2017, 70: 372-380.
(  0) 0) |
| [15] |
Yu Y, Liu Y, Li H, et al. Polymeric immunoglobulin receptor in dojo loach (Misgurnus anguillicaudatus): Molecular characterization and expression analysis in response to bacterial and parasitic challenge[J]. Fish & Shellfish Immunology, 2018, 73: 175-184.
(  0) 0) |
| [16] |
王占飞.牙鲆多聚免疫球蛋白受体与黏膜免疫球蛋白免疫应答动态分析[D].青岛: 中国海洋大学, 2015. Wang Z F. Immune Response of Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins in Flounder (Paralichthys olivaceus)[D]. Qingdao: Ocean University of China, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10423-1015719079.htm (  0) 0) |
| [17] |
Armelle P, Blaise C. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins[J]. Trends in Immunology, 2003, 24(2): 55-58. DOI:10.1016/S1471-4906(02)00031-5
(  0) 0) |
| [18] |
Holly T, Christiane E W. The role of the polymeric immunoglobulin receptorand secretory immunoglobulins during mucosal infection and immunity[J]. Viruses, 2018, 10(5): 237-252. DOI:10.3390/v10050237
(  0) 0) |
| [19] |
Goldman A S. The immune system of human milk: Antimicrobial, antiinflammatory and immunomodulating properties[J]. Pediatric Infectious Disease Journal, 1993, 12(8): 664-671. DOI:10.1097/00006454-199308000-00008
(  0) 0) |
| [20] |
Mostov K E. Transepithelial transport of immunoglobulins[J]. Annual Review of Immunology, 1994, 12(1): 63-84.
(  0) 0) |
| [21] |
Johansen F E, Pekna M, Norderhaug I N, et al. Absence of epithelial immunoglobulin a transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice[J]. Journal of Experimental Medicine, 1999, 190(7): 915-922. DOI:10.1084/jem.190.7.915
(  0) 0) |
| [22] |
Shimada S, Kawaguchi-Miyashita M, Kushiro A, et al. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA[J]. Journal of Immunology, 1999, 163(10): 5367-5373.
(  0) 0) |
| [23] |
Brandtzaeg P, Carlsen H S, Halstensen T S. The B-cell system in inflammatory bowel disease[J]. Advances in Experimental Medicine & Biology, 2006, 579(579): 149-167.
(  0) 0) |
| [24] |
Dallas S D, Rolfe R D. Binding of Clostridium difficile toxin A to human milk secretory component[J]. Journal of Medical Microbiology, 1998, 47(10): 879-888. DOI:10.1099/00222615-47-10-879
(  0) 0) |
| [25] |
Sunyer J O. Fishing for mammalian paradigms in the teleost immune system[J]. Nature Immunology, 2013, 14(4): 320-326.
(  0) 0) |
| [26] |
Xu Z, Parra D, GóMez D, et al. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(32): 13097-13102. DOI:10.1073/pnas.1304319110
(  0) 0) |
| [27] |
Xu Z, Takizawa F, Parra D, et al. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods[J]. Nature Communications, 2016, 7: 10728-10742. DOI:10.1038/ncomms10728
(  0) 0) |
| [28] |
Robinson J K, Blanchard T G, Levine A D, et al. A mucosal IgA-mediated excretory immune system in vivo[J]. Journal of Immunology, 2001, 166(6): 3688-3692. DOI:10.4049/jimmunol.166.6.3688
(  0) 0) |
| [29] |
Gurevich P, Zusman I, Moldavsky M, et al. Secretory immune system in human intrauterine development: Immunopathomorphological analysis of the role of secretory component (pIgR/SC) in immunoglobulin transport (review)[J]. International Journal of Molecular Medicine, 2003, 12(3): 289-297.
(  0) 0) |
| [30] |
Kaetzel C S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces[J]. Immunological Reviews, 2005, 206(1): 83-99.
(  0) 0) |
| [31] |
Braathen R, Hohman V S, Brandtzaeg P, et al. Secretory antibody formation: Conserved binding interactions between J chain and polymeric Ig receptor from humans and amphibians[J]. Journal of Immunology, 2007, 178(3): 1589-1597. DOI:10.4049/jimmunol.178.3.1589
(  0) 0) |
| [32] |
唐庆娟, 戚欣, 耿美玉. 多聚免疫球蛋白受体(pIgR)在粘膜免疫中的重要功能[J]. 中国生物化学与分子生物学报, 2007, 23(9): 724-729. Tang Q J, Qi X, Geng M Y. Important role of polymeric immunoglobulin receptor in mucosal immunity[J]. Chinese Journal of Biochemistry and Molecular Biology, 2007, 23(9): 724-729. DOI:10.3969/j.issn.1007-7626.2007.09.005 (  0) 0) |
| [33] |
Schwartz-Cornil I, Benureau Y, Greenberg H, et al. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins[J]. Journal of Virology, 2002, 76(16): 8110-8117. DOI:10.1128/JVI.76.16.8110-8117.2002
(  0) 0) |
| [34] |
Kelly C, Takizawa F, Sunyer J O, et al. Rainbow trout (Oncorhynchus mykiss) secretory component binds to commensal bacteria and pathogens[J]. Scientific Reports, 2017, 7: 41753-41762. DOI:10.1038/srep41753
(  0) 0) |
| [35] |
Oliveira I R D, Araújo A N D, Bao S N, et al. Binding of lactoferrin and free secretory component to enterotoxigenic Escherichia coli[J]. FEMS Microbiology Letters, 2001, 203(1): 29-33. DOI:10.1111/fml.2001.203.issue-1
(  0) 0) |
| [36] |
Zhang J R, Mostov K E, Lamm M E, et al. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells[J]. Cell, 2000, 102(6): 827-837. DOI:10.1016/S0092-8674(00)00071-4
(  0) 0) |
| [37] |
Sollid L M, Kvale D, Brandtzaeg P, et al. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins[J]. Journal of Immunology, 1987, 138(12): 4303-4306.
(  0) 0) |
| [38] |
Blanch V J, Piskurich J F, Kaetzel C S. Cutting edge: coordinate regulation of IFN regulatory factor-1 and the polymeric Ig receptor by proinflammatory cytokines[J]. Journal of Immunology, 1999, 162(3): 1232-1235.
(  0) 0) |
| [39] |
Piskurich J F, Youngman K R, Phillips K M, et al. Transcriptional regulation of the human polymeric immunoglobulin receptor gene by interferon-gamma[J]. Molecular Immunology, 1997, 34(1): 75-91.
(  0) 0) |
| [40] |
Ackermann L W, Wollenweber L A, Denning G M. IL-4 and IFN-gamma increase steady state levels of polymeric Ig receptor mRNA in human airway and intestinal epithelial cells[J]. Journal of Immunology, 1999, 162(9): 5112-5118.
(  0) 0) |
| [41] |
Kvale D, Løvhaug D, Sollid L M, et al. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig[J]. Journal of Immunology, 1988, 140(9): 3086-3089.
(  0) 0) |
| [42] |
Richard Pine. Convergence of TNFα and IFNγ signalling pathways through synergistic induction of IRF1/ISGF-2 is mediated by acomposite GAS/κBpromoter element[J]. Nucleic Acids Research, 1997, 25(21): 4346-4354. DOI:10.1093/nar/25.21.4346
(  0) 0) |
| [43] |
Bruno M E, Kaetzel C S. Long-term exposure of the HT-29 human intestinal epithelial cell line to TNF causes sustained up-regulation of the polymeric Ig receptor and proinflammatory genes through transcriptional and posttranscriptional mechanisms[J]. Journal of Immunology, 2005, 174(11): 7278-7284. DOI:10.4049/jimmunol.174.11.7278
(  0) 0) |
| [44] |
Hayashi M, Takenouchi N, Asano M, et al. The polymeric immunoglobulin receptor (secretory component) in a human intestinal epithelial cell line is up-regulated by interleukin-1[J]. Immunology, 1997, 92(2): 220-225. DOI:10.1046/j.1365-2567.1997.00341.x
(  0) 0) |
| [45] |
Dunne A, O'Neill L A. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense[J]. Science Signaling, 2003, 2003(171): 3. DOI:10.1126/stke.2003.171.re3
(  0) 0) |
| [46] |
Ackermann L W, Denning G M. Nuclear factor-κB contributes to interleukin-4-and interferon-dependent polymeric immunoglobulin receptor expression in human intestinal epithelial cells[J]. Immunology, 2010, 111(1): 75-85.
(  0) 0) |
| [47] |
Bjercke S, Brandtzaeg P. Glandular distribution of immunoglobulins, J chain, secretory component, and HLA-DR in the human endometrium throughout the menstrual cycle[J]. Human Reproduction, 1993, 8(9): 1420-1425. DOI:10.1093/oxfordjournals.humrep.a138271
(  0) 0) |
| [48] |
Kaushic C, Richardson J M, Wira C R. Regulation of polymeric immunoglobulin a receptor messenger ribonucleic acid expression in rodent uteri: Effect of sex hormones[J]. Endocrinology, 1995, 136(7): 2836-2844. DOI:10.1210/endo.136.7.7789308
(  0) 0) |
| [49] |
Claessens F, Verrijdt G, Schoenmakers E, et al. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation[J]. Journal of Steroid Biochemistry & Molecular Biology, 2001, 76(1): 23-30.
(  0) 0) |
| [50] |
Li T W, Wang J, Lam J T, et al. Transcriptional control of the murine polymeric IgA receptor promoter by glucocorticoids[J]. American Journal of Physiology, 1999, 276(1): 1425-1434.
(  0) 0) |
| [51] |
Zhao J, Yeong L H, Wong W S. Dexamethasone alters bronchoalveolar lavage fluid proteome in a mouse asthma model[J]. International Archives of Allergy & Immunology, 2007, 142(3): 219-229.
(  0) 0) |
| [52] |
Rinchevalarnold A, Belair L, Djiane J. Developmental expression of pIgR gene in sheep mammary gland and hormonal regulation[J]. Journal of Dairy Research, 2002, 69(1): 13-26.
(  0) 0) |
| [53] |
Takenouchi-Ohkubo N, Asano M, Chihaya H, et al. Retinoic acid enhances the gene expression of human polymeric immunoglobulin receptor (pIgR) by TNF-alpha[J]. Clinical & Experimental Immunology, 2010, 135(3): 448-454.
(  0) 0) |
| [54] |
Kvale D, Brandtzaeg P. Constitutive and cytokine induced expression of HLA molecules, secretory component, and intercellular adhesion molecule-1 is modulated by butyrate in the colonic epithelial cell line HT-29[J]. Gut, 1995, 36(5): 737-742. DOI:10.1136/gut.36.5.737
(  0) 0) |
| [55] |
Fernandez M I, Pedron T, Tournebize R, et al. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells[J]. Immunity, 2003, 18(6): 739-749. DOI:10.1016/S1074-7613(03)00122-5
(  0) 0) |
| [56] |
Hooper L V, Wong M H, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine[J]. Science, 2001, 291(5505): 881-884. DOI:10.1126/science.291.5505.881
(  0) 0) |
| [57] |
Pal K, Kaetzel C S, Brundage K, et al. Regulation of polymeric immunoglobulin receptor expression by reovirus[J]. Journal of General Virology, 2005, 86(8): 2347-2357. DOI:10.1099/vir.0.80690-0
(  0) 0) |
| [58] |
Buts J P, Bernasconi P, Vaerman J P, et al. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii[J]. Digestive Diseases & Sciences, 1990, 35(2): 251-256.
(  0) 0) |
| [59] |
Silva-Rossi-Aguiar V, Navarro-Rodriguez T R, Siqueira-De-Melo-Peres M, et al. Role of nuclear factor-κB in the expression by tumor necrosis factor-α of the human polymeric immunoglobulin receptor (pIgR) gene[J]. Immunogenetics, 2000, 51(4-5): 289-295. DOI:10.1007/s002510050622
(  0) 0) |
| [60] |
Piskurich J F, France J A, Tamer C M, et al. Interferon-γ induces polymeric immunoglobulin receptor mRNA in human intestinal epithelial cells by a protein synthesis dependent mechanism[J]. Molecular Immunology, 1993, 30(4): 413-421. DOI:10.1016/0161-5890(93)90071-I
(  0) 0) |
| [61] |
Denning G M. IL-4 and IFN-gamma synergistically increase total polymeric IgA receptor levels in human intestinal epithelial cells. Role of protein tyrosine kinases[J]. Journal of Immunology, 1996, 156(12): 4807-4814.
(  0) 0) |
| [62] |
Johansen F E, Kaetzel C. Regulation of the polymeric immunoglobulin receptor and IgA transport: New advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity[J]. Mucosal Immunol, 2011, 4(6): 598. DOI:10.1038/mi.2011.37
(  0) 0) |
| [63] |
Bonizzi G, Karin M. The two NF-B activation pathways and their role in innate and adaptive immunity[J]. Trends in Immunology, 2004, 25(6): 280-288. DOI:10.1016/j.it.2004.03.008
(  0) 0) |
| [64] |
Schjerven H, Tran T N, Brandtzaeg P, et al. De novo synthesized RelB mediates TNF-induced up-regulation of the human polymeric Ig receptor[J]. Journal of Immunology, 2004, 173(3): 1849-1857. DOI:10.4049/jimmunol.173.3.1849
(  0) 0) |
| [65] |
Spehlmann M E, Eckmann L. Nuclear factor-kappa B in intestinal protection and destruction[J]. Current Opinion in Gastroenterology, 2009, 25(2): 92-99. DOI:10.1097/MOG.0b013e328324f857
(  0) 0) |
| [66] |
Bren G D, Solan N J, Miyoshi H, et al. Transcription of the RelB gene is regulated by NF-κB[J]. Oncogene, 2001, 20(53): 7722-7733. DOI:10.1038/sj.onc.1204868
(  0) 0) |
| [67] |
Schneeman T A, Bruno M E, Schjerven H, et al. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses[J]. Journal of Immunology, 2005, 175(1): 376-384. DOI:10.4049/jimmunol.175.1.376
(  0) 0) |
 2018, Vol. 48
2018, Vol. 48