2. 中国海洋大学海洋化学理论与工程技术教育部重点实验室,山东 青岛 266100
抗生素是一类天然产生、人工合成或半合成的,具有抑制细菌生长或杀灭细菌功能的化学物质[1],它们被广泛用于预防和治疗人类和动物的细菌感染,在畜牧业和水产养殖业中被用作生长促进剂[2]。目前全球抗生素的使用量还在持续增加[3],据估计,2013年中国抗生素的总使用量约为16.2万t,人类消耗约占48%,其余均用于动物体,其中磺胺类、四环素类、喹诺酮类和大环内酯类抗生素是中国使用较普遍的四类抗生素,在2013年分别占总使用量的5%、7%、17%和26%[4],2020年中国兽用抗生素中四环素类、磺胺类、大环内酯类及喹诺酮类抗生素占比分别为30%、13%、10%和3%[5]。
抗生素在人体和动物体内仅有30%~90%被吸收和代谢,最终会以母体或代谢产物的形式通过尿液、粪便等排泄方式排出体外[6]。含抗生素的生活污水和医疗废水进入污水处理厂后,利用传统的废水处理工艺只能去除约36%~79%[7]。此外,养殖废水排放、垃圾填埋、化粪池系统、下水管道浸出等都是抗生素的污染源[8]。抗生素通过排污、地表渗流等方式进入地下水、河流,最终被输送到河口、海湾和沿海地区的地表水和沉积物中,并在自然环境中广泛分布[9-12]。抗生素进入水生生态系统后,会影响水生生物幼体发育和基因表达[13-14],还会通过食物链在动物体和人体中富集[15],对人类健康造成潜在的威胁。环境中的抗生素还可以诱导产生抗性细菌 (antibiotics resistance bacteria, ARBs)和抗性基因 (antibiotics resistance genes, ARGs),ARGs可以稳定存在于细菌的染色体和质粒中[16-17],微生物的繁殖以及基因的水平转移会导致ARGs大量产生和存在,这将使抗生素对人类和动物体的治疗效果下降从而影响生态安全。目前,抗生素已经作为一类新污染物被广泛关注。
抗生素在自然环境中的消除是多过程共同作用的结果,包括生物过程 (真菌和细菌的代谢、降解)以及非生物过程 (吸附、水解、光解、氧化和还原)。这两类降解的程度取决于温度、环境介质的理化性质以及纬度等条件[18]。由于抗生素具有抗菌性,光降解成为天然水环境中抗生素降解的重要途径[19-21]。因磺胺类、四环素类、喹诺酮类、大环内酯类抗生素使用量较大,在中国主要水环境中检出频繁且浓度较高[22-23]。本文将对这四大类典型抗生素的光降解规律进行总结归纳,并提出今后水环境中抗生素光降解研究的建议。
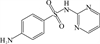
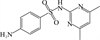
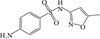
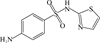
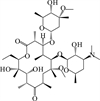
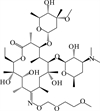
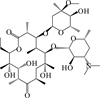
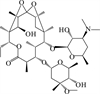
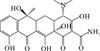
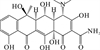
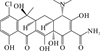
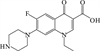
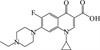
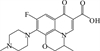
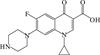
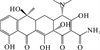
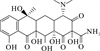
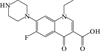
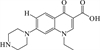
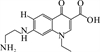
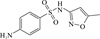
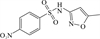
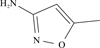
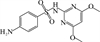
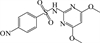
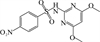
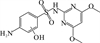
1 抗生素的光降解动力学及其影响因素本文涉及的四大类16种抗生素的结构如表 1所示,其中磺胺类抗生素以氨基苯磺酰胺为基本结构,磺胺基上的氢可被不同杂环取代;四环素类抗生素是由羟基、甲基、酮以及二甲氨基构成的四环体系;喹诺酮类抗生素的基本结构为氮 (杂)双并环;大环内酯类抗生素通常具有14—16元的内酯环,并有羟基、烷基和酮基取代,且红霉脱氧糖胺和红霉支糖通过醚键与大环相连[24]。
|
|
表 1 四大类16种典型抗生素的结构 Table 1 Structures of 16 typical antibiotics in four groups |
水体中抗生素光降解途径包括直接光降解、自敏化光降解和间接光降解[25-26]。表层水体中低浓度污染物的光降解一般都遵循一级反应动力学,其中光降解速率常数k是物质在某波长下的摩尔吸光系数与光量子产率的函数[27]。
1.1 直接光降解和自敏化光降解直接光降解是在光照条件下化合物直接吸收光子并引发的连续反应,而自敏化光降解是抗生素自身通过光转化形成激发三重态,并诱导产生活性氧 (reactive oxygen species,ROS)介导的反应,ROS包括羟基自由基·OH、单线态氧1O2、超氧阴离子O2·-等[28]。
抗生素的初始浓度会影响光降解的自敏化降解速率,如土霉素、恩诺沙星、诺氟沙星、氧氟沙星和磺胺二甲基嘧啶在纯水中的准一级反应速率常数随着初始浓度的升高而降低,这是因为抗生素浓度升高导致单位分子捕获的光子数和诱导产生的ROS浓度降低[29-32]。不同的ROS在自敏化反应中的贡献也不相同,如磺胺甲恶唑在pH为7.0的去离子水中进行光降解,利用异丙醇、对苯醌和NaN3作为相应的ROS清除剂,发现·OH、1O2和O2·-对磺胺甲恶唑自敏化光降解的贡献分别占24.6%、9.5%和38.9%,其中O2·-的贡献最大[33]。ROS清除实验还证实,pH为8.0时,1O2为诺氟沙星光降解过程中的主要贡献者[28]。然而,有研究表明抗生素在纯水中的光降解自敏性不强,主要以直接光降解为主[34-35]。Song等[36]发现,在纯水中四环素的水解和1O2对其光解的作用可以忽略,·OH对四环素的去除率约占22%,其余均由直接光降解引起,甚至在天然胶体颗粒 (natural colloidal particles, NCPs, 粒径1 nm~10 μm)存在下,土霉素、恩诺沙星和诺氟沙星主要也是直接光降解[21, 37]。
抗生素的降解速率还与其自身结构相关,不同结构类型的抗生素光量子产率差异较大,如磺胺类抗生素中的磺胺甲恶唑和喹诺酮类抗生素中的环丙沙星的降解在几小时内完成,而大环内酯类抗生素需要几天才能去除[38]。pH为6.8时,在310~410 nm的光辐照下环丙沙星、金霉素、磺胺甲恶唑和罗红霉素的光降解速率常数分别为7.47×10-3、2.84×10-4、6.62×10-5和1.33×10-6 s-1[34]。
1.2 间接光降解天然水体中存在的溶解性有机物 (dissolved organic matter, DOM)、无机离子 (卤离子、CO32-、HCO3-、NO3-、NO2-、Fe3+)等,很多都是光敏物质,间接光解就是由这些光敏物质产生的各种ROS与水体中的抗生素反应引起的降解,这些光敏物质的光反应过程如图 1所示。
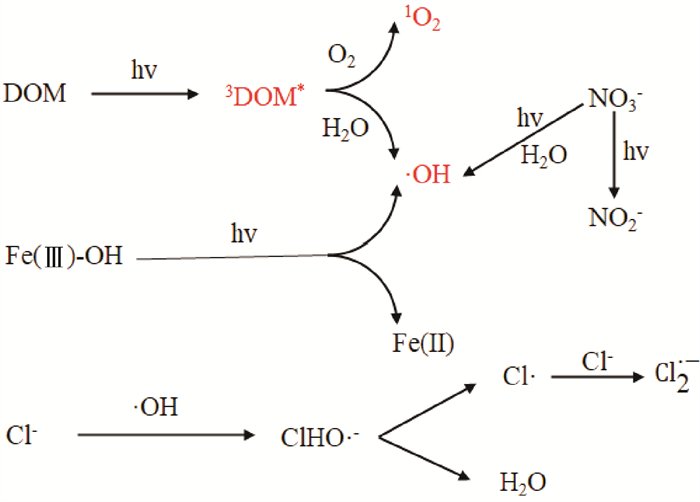
|
图 1 水体中光反应性可溶性物质的光化学过程[63] Fig. 1 Photochemical processes of photoreactive soluble substances in water[63] |
DOM广泛存在于自然水体中,是一个非常复杂的系统;腐殖质是其中的一类代表性物质,包括大小从几百到几千Da的胶体以及腐殖酸,具有相应的供电子能力和较强的光反应活性[33]。DOM对抗生素光降解具有双重作用:一方面,DOM吸收光子后生成激发三重态3DOM*,随后通过能量转移与溶解氧和水反应产生各种ROS,促进抗生素的光降解[39-40];3DOM*自身也是一种活性中间体,可以直接敏化降解抗生素;另一方面,DOM会通过与抗生素竞争吸收光子 (光屏蔽效应)或者猝灭ROS来抑制光降解[34]。DOM对光降解的作用机理复杂,与DOM的来源和性质有关[41],比如ROS的稳态浓度会随DOM的分子量和芳香性的增加而增加,DOM的芳香部分是3DOM*和1O2的敏化剂[42]。目前,许多研究对DOM存在下抗生素的光降解行为进行了探究,并对其作用机理进行了分析,表 2列出了一些主要的研究结果。
|
|
表 2 水体中溶解物质对抗生素光降解速率的影响 Table 2 Effect of dissolved substances in water on the photodegradation rate of various antibiotics |
还有研究者探究了实际水体中的天然溶解有机物对光降解的影响。Liu等[21]研究发现,与纯水相比,添加黄河三角洲潮间带表层水体中的NCPs后土霉素和四环素的光降解速率提高了约1~3倍。活性氧物种清除实验表明,三重激发态胶体有机质 (3COM*)对四环素的光降解起着最关键的作用[21];Cheng等[37]发现,白洋淀表层水中不同粒径的NCPs对恩诺沙星和诺氟沙星的光降解也以促进作用为主,且小颗粒的促进作用较差 (光屏蔽效应更强,且高有机质含量使ROS的猝灭更明显)。
水体中NO3-/NO2-、HCO3-/CO32-对光降解也具有双重作用。水中的NO3-、NO2-在太阳光作用下可产生·OH敏化光降解,但NO3-在315~320 nm处对光的吸收速率增大,NO2-也会抢夺光子从而抑制光降解[32, 43]。有研究结果显示,水中HCO3-/CO32-会与·OH发生反应产生CO3·-,而CO3·-与磺胺嘧啶的准二级反应速率常数低于·OH (分别为2.76×108和2.08×109 mol-1·L·s-1),因此会对光降解产生抑制作用;且随着HCO3-/CO32-浓度增加,可能会使pH增加,这也会对抗生素的光降解速率产生影响[44-45]。Song等[46]在不同浓度的HCO3-/CO32-下用缓冲溶液调节pH至7.4,发现四环素的降解速率并没有明显变化,表明HCO3-/CO32-对光降解的影响主要来源于pH的改变。
与淡水不同,海水中富含卤素离子,它们的存在会改变水体的离子强度,并且不同卤离子还会具有特殊效应,因此也会影响抗生素在水体中的光降解。研究发现,在海水离子强度含有DOM的情况下,3DOM*衰变速率常数降低,使其稳态浓度约增加一倍[50],盐度增大还会导致·OH增多[51]。然而在高盐度水中,Cl-和Br-能与3DOM*反应降低其稳态浓度[44],卤素离子还可以清除·OH,如Br-可以清除93%的·OH[44],因此卤素离子对抗生素光降解的作用也需要具体分析。有研究用NaClO4 (具有光反应惰性)来调节体系离子强度,随着溶液中离子强度变大,三重激发态磺胺类抗生素 (3SAs*)的损失率降低导致其稳态浓度增加,三种磺胺类抗生素 (磺胺二甲基嘧啶、磺胺吡啶、磺胺甲恶唑)的光降解速率提高。而在不同Cl-浓度下,只有磺胺二甲基嘧啶的光降解速率随Cl-浓度升高而增大,其他两种磺胺类抗生素变化不明显,这是因为三重激发态磺胺二甲基嘧啶 (3SMZ*)具有较高的氧化电位,可以将Cl-氧化为Cl2·- (其余两种3SAs*不足以将Cl-氧化),而Cl2·-也是光降解的重要中间体,Br-也有类似作用[52]。
Fe3+在水中可以形成配合物 (水合物或小分子有机酸的配合物),在光照条件下,电子会从配体向Fe (Ⅲ)转移,生成Fe2+和·OH[53-55]。溶解氧的存在使得Fe2+和O2之间发生电子转移产生ROS和H2O2,并且促进Fe3+的再生[56]。ROS和H2O2则会促进抗生素的光降解。Batista等[56]发现,在pH为2.5,Fe3+浓度为0.2 mmol·L-1且有溶解氧的条件下,磺胺噻唑和磺胺嘧啶在太阳光下辐照65 min后降解率分别达到31%和33%;Fe3+浓度为0.6 mmol·L-1时降解率达到约70%。Fe3+几乎不与磺胺类抗生素发生络合[57],而对于四环素类抗生素,除了FeOH2+在水溶液中通过光氧化还原过程生成Fe2+和·OH促进降解外,Fe3+还易与抗生素发生络合,使其更有利于光的吸收[58]。还有研究表明,在无光照射时,Fe3+也可以与四环素类抗生素络合促进其氧化降解[59]。Fe3+的加入也促进了恩诺沙星和诺氟沙星在NCPs溶液中的光降解[37]。
除了水中的溶解物质,水体的pH会通过影响抗生素的质子化形态和ROS的稳态浓度来影响光降解速率[60]。土霉素在紫外灯254 nm照射下,pH从2.0增加到11.5时反应速率增加了一个数量级,这是因为不同pH条件下土霉素的解离状态不同,导致量子产率不同 (碱性条件下高的电荷密度更有利于ROS的亲电进攻,使光照利用率更高)[27]。在天然有机物存在下,pH对土霉素的光降解也存在相似规律[61]。Zhang等[31]发现,喹诺酮类抗生素的可见吸收光谱峰值随pH的升高发生蓝移,使得254 nm处量子产率增大。不同质子化形态下诺氟沙星和氧氟沙星的光降解速率依次为:两性离子>阴离子>阳离子,这说明pH对光降解的影响不能只用不同pH条件下的吸收光谱来解释,还可能是因为不同pH条件下官能团的反应性不同:哌嗪环上有两个叔胺可在溶液中质子化,其裂解和光氧化的敏感度可能与质子化状态有关。pH也会影响抗生素与ROS的二级反应速率,有研究对两种ROS与不同质子化形态的9种磺胺类抗生素的二级反应常数进行了测定,发现·OH与8种磺胺类抗生素的中性离子和阴离子的反应性更强,1O2与7种磺胺类抗生素的阴离子形式反应最快,这可能是因为pH升高增加了反应性苯胺部分的去质子化能力和电荷密度,使其更易受到ROS的攻击[62]。然而,在实际环境pH对抗生素光降解还受许多因素影响,其作用程度也应具体分析。
此外,水环境中光降解是所有影响因素综合作用的结果。DOM对光降解的作用受其他水体溶解性物质的影响[21, 33, 64-65]。在存在天然胶体颗粒的模拟海水中,Liu等[21]发现,低盐度条件下 (Cl-∶Br-=675∶1),由DOM产生的活性物种 (包括3DOM*和ROS等)与卤素离子整体上表现为协同作用,促进了四环素类抗生素的光降解;而高盐度条件下,高离子强度可能通过阻碍3DOM*的电子传递过程,使卤素离子与3DOM*形成活性卤素,清除活性氧 (·OH)的机制影响光降解,最终表现为高盐度条件下对其几乎不产生影响。Bai等[44]也发现,DOM存在下不同盐度对磺胺嘧啶光降解的影响也存在上述规律。还有文献表明,DOM的存在还会对水体中其他光敏剂,如NO3-/NO2-产生光屏蔽作用[65]。
水体pH对抗生素光解的影响也受其他物质影响。DOM对三种不同质子化形态下的诺氟沙星光降解的影响有显著差异,DOM对低浓度诺氟沙星的阴离子和两性离子形态均表现出明显的抑制作用,而对其阳离子形态则表现为促进作用[26]。磺胺嘧啶的光降解速率在DOM存在下随pH呈现先升高后降低的趋势,pH为7 (磺胺嘧啶主要以阴离子形态存在)时达到最大值。这可能是因为pH升高会显著提高3DOM*、·OH、1O2的稳态浓度,因此pH < 7时光降解速率随pH增大而增大, 而不同质子化形态的磺胺嘧啶与各种ROS的二级反应速率常数可能随pH的增大而变小 (在DOM存在下,磺胺嘧啶与3DOM*的二级反应速率在pH为4时较pH为9时更大)。另一方面,DOM的酚类抗氧化剂还可以将3DOM*诱导的磺胺嘧啶光降解产物还原为磺胺嘧啶 (阴离子形态更明显),因此pH>7时光降解速率又会下降[44, 66]。在纯水中,pH从2.0增加到11.0,四环素类抗生素的光降解速率常数增加,但NCPs的存在略微抑制了pH的作用[21]。
目前有研究对抗生素光降解的决定因素进行探究,如Oliveira等[67]提出pH、DOM和盐度是决定磺胺甲恶唑光降解的决定因素;Cheng等[20]研究发现,DOM存在下间接光降解占总降解量的50%以上,是两种喹诺酮类抗生素的主要降解途径,且3DOM*是氧氟沙星和环丙沙星间接光解途径中的主要反应活性物种。
2 水环境中抗生素的降解途径对于大部分抗生素,其结构的稳定性使它们在自然环境中很难完全矿化。如初始浓度为10.0 mg·L-1的恩诺沙星在模拟太阳光辐照90 min后,总有机碳去除率仅为13.1%[30];紫外光照射下,不同pH条件的四环素的最高矿化率低于10%,说明大部分抗生素仅被降解成为其他中间产物,只有少部分被分解为CO2和H2O[46]。然而光降解前后抗生素母体和降解产物的化学结构不同,甚至可能对环境生物存在更强的毒性。有研究利用ECOSAR (ecological structure-activity relationship)软件对已知结构的抗生素母体和中间产物的毒性进行估计[68-73],结果如表 3所示。此外,还有研究对抗生素及降解产物混合体系的毒性进行比较,Xu等[74]发现10 mg/L磺胺吡啶母体对费氏弧菌的发光抑制率为12.5%,随着照射时间的延长,其毒性先在10 min时略有下降至5%,然后在120 min时上升至最高的50.6%。Li等[75]发现在苏万尼河腐殖酸存在下,10 mg/L罗红霉素溶液对费氏弧菌无毒性,照射12 h后对费氏弧菌发光抑制率达到最高值58%,照射36 h后,抑制率降至16%。因此有必要对光降解途径和产物进行研究。
|
|
表 3 抗生素母体及部分光降解中间产物的毒性预测 Table 3 Prediction of the toxicity of antibiotic parent and some photodegradation intermediates |
四环素类抗生素在自然水环境中的光降解可能会经历羟基化、去甲基化、脱胺化和脱水的过程[21, 36, 47]。其中四环素可能的降解途径见图 2 (a)。Li等[47]在HA存在和不存在的情况下均鉴定出m/z 413、m/z 459和m/z 461三种四环素的降解产物,其中m/z 461是·OH攻击C11a和C12之间的双键,在C11a上发生羟基化,之后·OH攻击C5,并在C5和C5a之间脱氢形成双键产生了m/z 459。而m/z 413是C4上失去1个-CH2-,C5a和C6之间再脱水形成双键产生的。在TiO2存在的光催化下,除上述产物外,还鉴定出m/z 431和m/z 417分别是C4上的叔胺失去1个和2个-CH2-形成的,可能是因为在光催化的条件下,这两种产物浓度增大使其更容易被检测到。Song等[36]发现在紫外光照射下,DOM的存在并未使体系产生新的中间产物,其中m/z 432是·OH攻击C2和C11a并发生连续羟基化,再裂解C2上的酰胺基或C4上的-N (CH3)2形成的,m/z 370是脱去分子中的3个-CH3、羟胺上的-NH2和C3上的-OH形成的。Liu等[21]在存在和不存在NCPs的条件下对四环素的光降解产物分析,共发现了上述4个过程的7种光降解产物,其中m/z 430.15的脱胺化产物是NCPs存在时才被检出的。土霉素的光降解过程也类似,共发现上述4种途径产生的5种中间产物。
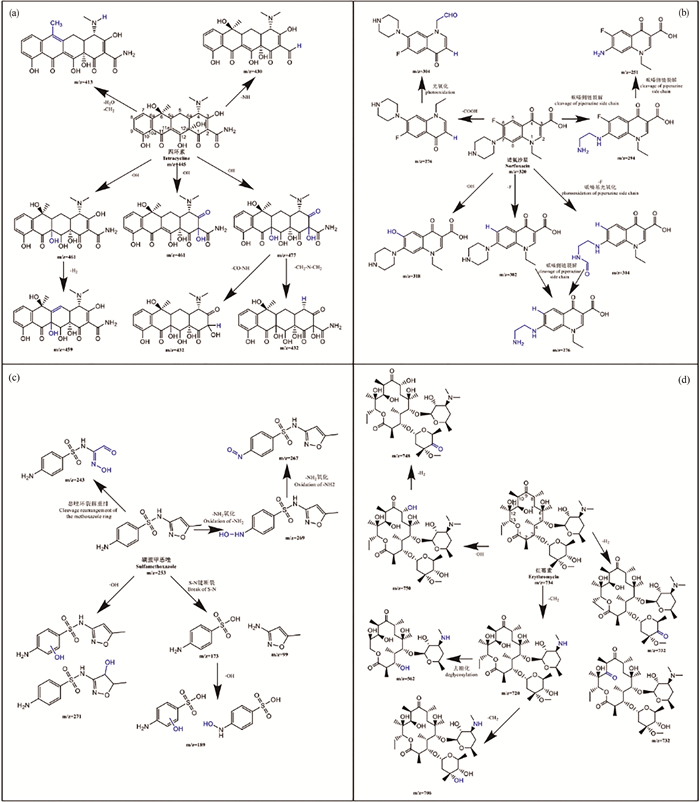
|
( (a):四环素[21, 36, 47], (b):诺氟沙星[26, 31, 76-77], (c): 磺胺甲恶唑[76, 80-83, 87], (d): 红霉素[48, 84-85]。(a): Tetracycline[21, 36, 47]; (b): Norfloxacin[26, 31, 76-77]; (c): Sulfamethoxazole[76, 80-83, 87]; (d): Erythromycin[48, 84-85]. ) 图 2 四种典型抗生素在自然水体中可能的降解途径 Fig. 2 Possible degradation pathways of four typical antibiotics in natural waters |
喹诺酮类抗生素在环境中的光降解途径主要包括羟基化、直接脱氟、直接裂解哌嗪基环和光氧化哌嗪基环、光诱导脱羧以及喹诺酮环N1上烷基的氧化和裂解[26, 31, 76-77]。Li等[30]测定了模拟太阳光下恩诺沙星在纯水中的光降解产物,其中包括脱羧产物 (m/z 316.3)、哌嗪基环N4-脱烷基产物 (m/z 332.1)、同时脱氟和脱环丙基的产物 (m/z 301.2)、哌嗪基环裂解产物 (m/z 263.2)以及哌嗪基环氧化产物 (m/z 374.1)。Liang等[26]发现DOM的存在对诺氟沙星光降解中间体的产物类型影响不大,但对中间体的浓度有影响,而不同pH会对光降解产物产生较大影响。还有研究表明,DOM可以作为电子供体提高诺氟沙星脱氟产物的生成速率[28]。Zhang等[31]在模拟太阳光照射下对3种不同质子形态喹诺酮类抗生素的光降解产物进行分析,发现诺氟沙星和氧氟沙星各有12和3种主要中间产物。在阳离子形态下这两种抗生素主要发生哌嗪基侧链的断裂,得到的主要降解产物分别为m/z 251 (哌嗪基裂解为-NH2)和m/z 348 (哌嗪基N4-脱烷基)。而阴离子和两性离子形态下诺氟沙星的主要降解产物分别为两种m/z 304 (脱羧、哌嗪基环和喹啉环N1上烷基的氧化;脱氟和哌嗪基环的氧化),而阴离子和两性离子形态氧氟沙星的降解产物为m/z 348的碎片 (脱羧和哌嗪基环氧化)。研究还发现,诺氟沙星两性离子和阴离子形态的脱氟作用比哌嗪基侧链的氧化更容易发生,而氧氟沙星由于C8上有强给电子基不易脱氟,因此脱羧和哌嗪基侧链氧化是两性离子和阴离子形态氧氟沙星最重要的光转化途径,且阴离子形态氧氟沙星哌嗪基侧链的光氧化作用略大于脱羧作用[31]。自然水体中 (两性离子形态)诺氟沙星可能的降解途径见图 2 (b)。
2.3 磺胺类抗生素的光降解途径磺胺类抗生素是以磺胺基团为特征,连接不同的杂环构成,其中五元和六元环取代的磺胺类抗生素光降解方式并不完全相同。Borren等[78-79]发现,含六元杂环取代基的磺胺类抗生素通过SO2的挤压发生直接光降解,而含有五元杂环取代基的磺胺类抗生素则易在不同位置发生裂解。磺胺甲恶唑可能的降解途径及产物见图 2 (c)。Niu等[80]发现,含五元杂环的磺胺甲恶唑在纯水中光降解的5种产物包括:羟基化产物 (m/z 271.1)、磺胺键 (S-N)断裂形成的产物 (m/z 99.1、173.1)、磺胺键断裂形成的氨基苯磺酸的羟基化产物 (m/z 189.2)和异恶唑环裂解重排的产物 (m/z 243.2)。Gmurek等[81-83]靶向分析了9种磺胺甲恶唑的光降解产物,发现除磺胺键裂解产物外,还有苯氨基发生羟基化及其连续氧化、乙酰化、-OH取代氨基和异恶唑环裂解产物等。磺胺甲恶唑还会形成不同位置的单羟基产物和多羟基化产物,并且中间产物也会继续发生羟基化[76, 82]。有研究在六元杂环取代的磺胺二甲基嘧啶和磺胺吡啶的光降解产物中检测到SO2挤压的产物,其他降解途径与五元杂环取代的磺胺类抗生素类似[62, 74]。
2.4 大环内酯类抗生素的光降解途径大环内酯类抗生素主要光反应途径为去甲基化 (N-去甲基和O-去甲基)、羟基化、脱水、脱氢以及去糖化[48, 84-85]。此外,得到的产物还可以进一步通过上述反应进行光降解。Jia等[86]对模拟阳光下一种天然溶解有机物SRNOM (苏万尼河天然有机质)存在时,包括克拉霉素和红霉素在内的4种大环内酯类的光降解产物进行了鉴定,其中红霉素的降解途径见图 2 (d)。在水溶液中,红霉素会形成一种红霉素的6, 9-半缩酮的异构体 (m/z 743),此外还检测到N-去甲基产物 (m/z 720)、羟基化产物 (m/z 750)、羰基化产物 (m/z 748)、脱氢产物- (m/z 732)以及同时去糖化和去甲基化的产物 (m/z 562)等。克拉霉素是红霉素C6的羟基被替换为甲氧基的结构,光降解途径也与红霉素类似[48]。
3 沉积物中抗生素的光解目前研究的重点集中在水或冰中抗生素光解动力学及光解机制的研究上,对于抗生素在固相表面的光降解行为的研究较少,对真实沉积物中抗生素光解行为的研究则更少。Xu等[88]将经离子交换 (Na+、K+和Ca2+)的两种蒙脱土吸附四环素后的悬浮液 (吸附率大于90%)与纯水中四环素的光降解速率进行对比发现,前者明显更快,归因于黏土表面特定的催化作用,且钠离子交换黏土的光催化最强,钠离子交换锂蒙脱石悬浮液中四环素的准一级速率常数为1.4×10-3 s-1,大于其在纯水中的降解速率 (2.2×10-4 s-1)。研究还表明,黏土吸附增强光转化的关键因素是促进了1O2的产生[88]。Lin等[89]发现,环丙沙星在高岭土层中均存在两步降解过程:第一步 (前1 h)光穿透表层进行快速降解 (厚度小于199 μm),符合准一级动力学,层厚14~199 μm, 其速率常数从0.015 4 min-1减小到0.001 6 min-1,均小于水相中的降解速率常数;第二步是环丙沙星从暗区扩散的过程,该过程较慢。直接光降解是环丙沙星在高岭土中的主要光降解机制,其降解产物与水中类似[89]。还有研究发现,金霉素在高岭土表面光降解约发生在约0.1 mm的深度内,且量子产率较水中小约一个数量级[90]。
土壤和沉积物是比以上使用的纯高岭土复杂得多的系统。有研究者对土壤中农药的光降解动力学开展了简单模拟,发现污染物在土壤中的光降解速率较水中更慢,也是因为光在土壤中不易穿透, 且与水中光降解类似,各种活性氧的产生也会使土壤中的污染物发生间接光降解[91]。污染物在土壤中的光降解受到多种环境因素的影响,如土壤类型、土壤湿度、微生物活性、光照强度以及透气性等。吡虫啉在潮湿土壤中的光降解符合一级反应动力学,而对于干燥土壤则不符合[92]。可能是因为对于潮湿土壤,污染物可以通过扩散作用进入光解区域。而在干燥土壤中,虽然光的穿透能力比潮湿土壤更深 (干燥土壤约0.5 mm),但在该深度以下不受直接光降解的影响,导致其降解速度在0~24 h内较快,之后就达到稳定,且湿度更大时光降解作用可能更显著。此外,Fe2+的存在也会促进土壤中污染物的光降解[92]。
4 结论与展望鉴于抗生素的广泛分布及潜在危害,其作为一类新污染物已被国内外学者广泛关注。光降解过程是天然水体中抗生素消除的重要途径,目前,对其光降解动力学及降解产物和途径已有较多的研究。但是,水环境中抗生素光降解的研究还存在一定的局限性,主要包括:
(1) 天然水环境中抗生素的光降解动力学和机制研究有待进一步深入:目前的研究初始浓度设置与抗生素的实际环境浓度相去甚远,后续应更侧重环境相关浓度 (ng·L-1、ng·g-1)下的研究;同时,许多研究提出的抗生素可能的降解途径都是在纯水中、单一因素 (pH、DOM等)或较少因素影响下,仅仅基于对光降解中间产物的检测而推测得到的,后续的研究还应结合多种技术手段 (如同位素标记和同位素分馏效应测定等),对多因素复合影响下的光降解途径进行研究。
(2) 抗生素的生态风险评估有待进一步完善:降解中间产物与母体具有不同的毒性效应,它们的环境分布及其生态毒性研究还很缺乏,抗生素母体及其主要中间产物的联合毒性评价也很不完善,需要进一步加强以更全面地了解抗生素对水生生态系统的实际生态效应。
(3) 固相介质中抗生素的光降解研究有待进一步加强:目前对土壤和沉积物中抗生素光降解动力学以及机制的研究极为少见,有必要对其中抗生素的光化学行为及光降解途径进行探究。
此外,中国作为抗生素消耗和使用大国,未来需要持续加强对各类抗生素药物使用和排放的监管力度,并努力开发出绿色且成本低廉的方法, 用于去除水体环境中的抗生素以维护生态安全。
| [1] |
Manyi-Loh C, Mamphweli S, Meyer E, et al. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications[J]. Molecules, 2018, 23(4): 795. (  0) 0) |
| [2] |
Lu S, Lin C Y, Lei K, et al. Profiling of the spatiotemporal distribution, risks, and prioritization of antibiotics in the waters of Laizhou Bay, northern China[J]. Journal of Hazardous Materials, 2022, 424(Pt B): 127487. (  0) 0) |
| [3] |
Liu C B, Li B L, Liu M, et al. Demand, status, and prospect of antibiotics detection in the environment[J]. Sensors and Actuators B: Chemical, 2022, 369: 132383. (  0) 0) |
| [4] |
Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance[J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. (  0) 0) |
| [5] |
中华人民共和国农业农村部. 2020年中国兽用抗菌药使用情况报告[N]. 北京: 中国畜牧兽医报, 2021-11-14 (003). Ministry of Agriculture and Rural Affairs of the People's Republic of China. Report on the Use of Veterinary Antimicrobial Drugs in China in 2020[N]. Beijing: Chinese Animal Husbandry and Veterinary News, 2021-11-14 (003). (  0) 0) |
| [6] |
Zhou L J, Ying G G, Zhao J L, et al. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China[J]. Environmental Pollution, 2011, 159(7): 1877-1885. (  0) 0) |
| [7] |
Aydin S, Aydin M E, Ulvi A, et al. Antibiotics in hospital effluents: Occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment[J]. Environmental Science and Pollution Research, 2018, 26(1): 544-558. (  0) 0) |
| [8] |
Kovalakova P, Cizmas L, Mcdonald T J, et al. Occurrence and toxicity of antibiotics in the aquatic environment: A review[J]. Chemosphere, 2020, 251: 126351. (  0) 0) |
| [9] |
Anh H Q, Le T P Q, Da Le N, et al. Antibiotics in surface water of east and southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives[J]. Science of the Total Environment, 2021, 764: 142865. (  0) 0) |
| [10] |
Lyu J, Yang L, Zhang L, et al. Antibiotics in soil and water in China-a systematic review and source analysis[J]. Environmental Pollution, 2020, 266(Pt 1): 115147. (  0) 0) |
| [11] |
Lu J, Wu J, Zhang C, et al. Occurrence, distribution, and ecological-health risks of selected antibiotics in coastal waters along the coastline of China[J]. Science of the Total Environment, 2018, 644: 1469-1476. (  0) 0) |
| [12] |
Chen H Y, Jing L J, Teng Y G, et al. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks[J]. Science of the Total Environment, 2018, 618: 409-418. (  0) 0) |
| [13] |
Gust M, Fortier M, Garric J, et al. Effects of short-term exposure to environmentally relevant concentrations of different pharmaceutical mixtures on the immune response of the pond snail Lymnaea stagnalis[J]. Science of the Total Environment, 2013, 445(445-446): 210-218. (  0) 0) |
| [14] |
Wang H L, Che B G, Duan A L, et al. Toxicity evaluation of β-diketone antibiotics on the development of embryo-larval zebrafish (Danio rerio)[J]. Environmental Toxicology, 2013, 29(10): 1134-1146. (  0) 0) |
| [15] |
Chao G X, Wang C, Wu T Q, et al. Molecular epidemiology and antibiotic resistance phenotypes and genotypes of salmonellae from food supply chains in China[J]. Food Control, 2017, 77: 32-40. DOI:10.1016/j.foodcont.2017.01.022 (  0) 0) |
| [16] |
Aminov R I. The role of antibiotics and antibiotic resistance in nature[J]. Environmental Microbiology, 2009, 11(12): 2970-2988. DOI:10.1111/j.1462-2920.2009.01972.x (  0) 0) |
| [17] |
Fan X T, Li H, Chen Q L, et al. Fate of antibiotic resistant Pseudomonas putida and broad host range plasmid in natural soil microcosms[J]. Frontiers in Microbiology, 2019, 10: 194-201. DOI:10.3389/fmicb.2019.00194 (  0) 0) |
| [18] |
Kümmerer K. Antibiotics in the aquatic environment: A review-Part Ⅰ[J]. Chemosphere, 2009, 75(4): 417-434. DOI:10.1016/j.chemosphere.2008.11.086 (  0) 0) |
| [19] |
Lu J X, Ji Y F, Chovelon J M, et al. Fluoroquinolone antibiotics sensitized photodegradation of isoproturon[J]. Water Research, 2021, 198: 117136. DOI:10.1016/j.watres.2021.117136 (  0) 0) |
| [20] |
Cheng D M, Liu H F, Yang E, et al. Effects of natural colloidal particles derived from a shallow lake on the photodegradation of ofloxacin and ciprofloxacin[J]. Science of the Total Environment, 2021, 773: 145102. DOI:10.1016/j.scitotenv.2021.145102 (  0) 0) |
| [21] |
Liu F, Liu X H, Zhao S N, et al. Photochemical transformations of tetracycline antibiotics influenced by natural colloidal particles: Kinetics, factor effects and mechanisms[J]. Chemosphere, 2019, 235: 867-875. DOI:10.1016/j.chemosphere.2019.06.201 (  0) 0) |
| [22] |
Yin Z Z. Distribution and ecological risk assessment of typical antibiotics in the surface waters of seven major rivers, China[J]. Environmental Science: Processes & Impacts, 2021, 23(8): 1088-1100. (  0) 0) |
| [23] |
Li S, Shi W Z, Liu W, et al. A duodecennial national synthesis of antibiotics in China's major rivers and seas (2005—2016)[J]. Science of the Total Environment, 2018, 615: 906-917. DOI:10.1016/j.scitotenv.2017.09.328 (  0) 0) |
| [24] |
杨妍, 王子宇, 葛林科, 等. 我国水体中大环内酯类抗生素的分布特征及环境光化学行为[J]. 环境化学, 2024, 43(3): 1-17. Yang Y, Wang Z Y, Ge L K, et al. Occurrence and photochemical behavior of macrolide antibiotics in the aquatic environment of China[J]. Environmental Chemistry, 2024, 43(3): 1-17. (  0) 0) |
| [25] |
Yan S W, Song W H. Photo-transformation of pharmaceutically active compounds in the aqueous environment: A review[J]. Environ Sci: Processes Impacts, 2014, 16(4): 697-720. (  0) 0) |
| [26] |
Liang C H, Zhao H M, Deng M J, et al. Impact of dissolved organic matter on the photolysis of the ionizable antibiotic norfloxacin[J]. Journal of Environmental Sciences, 2015, 27: 115-123. (  0) 0) |
| [27] |
张维玮, 孙贤波, 刘勇弟. 水中土霉素的紫外光解研究[J]. 水处理技术, 2018, 44(11): 33-37. Zhang W W, Sun X B, Liu Y D. Aqueous photolysis of oxytetracycline under UV irradiation[J]. Technology of Water Treatment, 2018, 44(11): 33-37. (  0) 0) |
| [28] |
Niu X Z, Busetti F, Langsa M, et al. Roles of singlet oxygen and dissolved organic matter in self-sensitized photo-oxidation of antibiotic norfloxacin under sunlight irradiation[J]. Water Research, 2016, 106: 214-222. DOI:10.1016/j.watres.2016.10.002 (  0) 0) |
| [29] |
张翠, 胡学锋, 骆永明. 模拟太阳光下水中土霉素的光化学降解[J]. 环境化学, 2016, 35(3): 430-438. Zhang C, Hu X F, Luo Y M. Aqueous photodegradation of oxytetracycline under simulated sunlight irradiation[J]. Environmental Chemistry, 2016, 35(3): 430-438. (  0) 0) |
| [30] |
Li Y, Niu J F, Wang W L. Photolysis of Enrofloxacin in aqueous systems under simulated sunlight irradiation: Kinetics, mechanism and toxicity of photolysis products[J]. Chemosphere, 2011, 85(5): 892-897. DOI:10.1016/j.chemosphere.2011.07.008 (  0) 0) |
| [31] |
Zhang Z C, Xie X D, Yu Z Q, et al. Influence of chemical speciation on photochemical transformation of three fluoroquinolones (FQs) in water: Kinetics, mechanism, and toxicity of photolysis products[J]. Water Research, 2019, 148: 19-29. DOI:10.1016/j.watres.2018.10.027 (  0) 0) |
| [32] |
黄春年, 李学德, 花日茂. 磺胺二甲嘧啶在水溶液中的光化学降解[J]. 环境污染与防治, 2011, 33(12): 59-64. Huang C N, Li X D, Hua R M. Photochemical degradation of sulfamethazine in aqueous solution[J]. Environmental Pollution & Control, 2011, 33(12): 59-64. (  0) 0) |
| [33] |
Zhang Y Y, Zhao F R, Wang F, et al. Molecular characteristics of leonardite humic acid and the effect of its fractionations on sulfamethoxazole photodegradation[J]. Chemosphere, 2020, 246: 125642. (  0) 0) |
| [34] |
Mangalgiri K P, Blaney L. Elucidating the stimulatory and inhibitory effects of dissolved organic matter from poultry litter on photodegradation of antibiotics[J]. Environmental Science & Technology, 2017, 51(21): 12310-12320. (  0) 0) |
| [35] |
Ge L K, Na G S, Zhang S Y, et al. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes[J]. Science of the Total Environment, 2015, 527. (  0) 0) |
| [36] |
Song C, Zhang K X, Wang X J, et al. Effects of natural organic matter on the photolysis of tetracycline in aquatic environment: Kinetics and mechanism[J]. Chemosphere, 2021, 263: 128338. DOI:10.1016/j.chemosphere.2020.128338 (  0) 0) |
| [37] |
Cheng D M, Liu X H, Li J P, et al. Effects of the natural colloidal particles from one freshwater lake on the photochemistry reaction kinetics of ofloxacin and enrofloxacin[J]. Environmental Pollution, 2018, 241: 692-700. DOI:10.1016/j.envpol.2018.06.017 (  0) 0) |
| [38] |
Batchu S R, Panditi V R, O'shea K E, et al. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate[J]. Science of the Total Environment, 2014, 470. (  0) 0) |
| [39] |
Santoke H, Cooper W J. Environmental photochemical fate of selected pharmaceutical compounds in natural and reconstituted Suwannee River water: Role of reactive species in indirect photolysis[J]. Science of the Total Environment, 2017, 580: 626-631. DOI:10.1016/j.scitotenv.2016.12.008 (  0) 0) |
| [40] |
Bodhipaksha L C, Sharpless C M, Chin Y P, et al. Triplet photochemistry of effluent and natural organic matter in whole water and isolates from effluent-receiving rivers[J]. Environmental Science & Technology, 2015, 49(6): 3453-3463. (  0) 0) |
| [41] |
Liu S K, Cui Z G, Ding D S, et al. Effect of the molecular weight of DOM on the indirect photodegradation of fluoroquinolone antibiotics[J]. Journal of Environmental Management, 2023, 348: 119192. (  0) 0) |
| [42] |
Zhou H X, Lian L S, Yan S W, et al. Insights into the photo-induced formation of reactive intermediates from effluent organic matter: The role of chemical constituents[J]. Water Research, 2017, 112: 120-128. (  0) 0) |
| [43] |
肖华花, 刘国光, 陈智明, 等. 水体中不同形态氮对兽药磺胺二甲基嘧啶溶液光降解的影响[J]. 环境化学, 2015, 34(5): 971-976. Xiao H H, Liu G G, Chen Z M, et al. The effect of different nitrogen forms on the photo-degradation of sulfamethazine in water[J]. Environmental Chemistry, 2015, 34(5): 971-976. (  0) 0) |
| [44] |
Bai Y, Zhou Y L, Che X W, et al. Indirect photodegradation of sulfadiazine in the presence of DOM: Effects of DOM components and main seawater constituents[J]. Environmental Pollution, 2021, 268(Pt B): 115689. (  0) 0) |
| [45] |
王鲁宁, 宋超. 碳酸根对四环素光降解的影响机理研究[J]. 工业水处理, 2018, 38(12): 81-84. Wang L N, Song C. Research on the mechanisms about the influences of carbonate on the photodegradation of tetracycline[J]. Industrial Water Treatment, 2018, 38(12): 81-84. (  0) 0) |
| [46] |
Song C, Liu H Y, Guo S, et al. Photolysis mechanisms of tetracycline under UV irradiation in simulated aquatic environment surrounding limestone[J]. Chemosphere, 2020, 244: 125582. DOI:10.1016/j.chemosphere.2019.125582 (  0) 0) |
| [47] |
Li S, Hu J Y. Photolytic and photocatalytic degradation of tetracycline: Effect of humic acid on degradation kinetics and mechanisms[J]. Journal of Hazardous Materials, 2016, 318: 134-144. DOI:10.1016/j.jhazmat.2016.05.100 (  0) 0) |
| [48] |
Jia X, Lian L S, Yan S W, et al. Comprehensive understanding of the phototransformation process of macrolide antibiotics in simulated natural waters[J]. ACS ES&T Water, 2021, 1(4): 938-948. (  0) 0) |
| [49] |
常海莎. 大环内酯类抗生素在水体中的光降解及毒性变化研究[D]. 石河子: 石河子大学, 2018: 39-45. Chang H S. Study on the Photodegradation and Change of Toxicity of Macrolide Antibiotics in Aqueous Environment[D]. Shihezi: Shihezi University, 2018: 39-45. (  0) 0) |
| [50] |
Parker K M, Pignatello J J, Mitch W A. Influence of ionic strength on triplet-state natural organic matter loss by energy transfer and electron transfer pathways[J]. Environmental Science & Technology, 2013, 47(19): 10987-10994. (  0) 0) |
| [51] |
Anastasio C, Newberg J T. Sources and sinks of hydroxyl radical in sea-salt particles[J]. Journal of Geophysical Research: Atmospheres, 2007, 112(D10): D10306. (  0) 0) |
| [52] |
Li Y, Qiao X, Zhang Y N, et al. Effects of halide ions on photodegradation of sulfonamide antibiotics: Formation of halogenated intermediates[J]. Water Research, 2016, 102: 405-412. (  0) 0) |
| [53] |
Faust B C, Hoigne J. Photolysis of Fe (Ⅲ)-hydroxy complexes as sources of OH radicals in clouds, fog and rain[J]. Atmospheric Environment Part a—General Topics, 1990, 24(1): 79-89. (  0) 0) |
| [54] |
Kawaguchi H, Inagaki A. Photochemical generation rates of hydroxyl radical in aqueous-solutions containing Fe (Ⅲ)-hydroxy complex[J]. Chemosphere, 1993, 27(12): 2381-2387. (  0) 0) |
| [55] |
Wu F, Deng N S. Photochemistry of hydrolytic iron (Ⅲ) species and photoinduced degradation of organic compounds: A minireview[J]. Chemosphere, 2000, 41(8): 1137-1147. (  0) 0) |
| [56] |
Batista A P S, Cottrell B A, Nogueira R F P. Photochemical transformation of antibiotics by excitation of Fe (Ⅲ)-complexes in aqueous medium[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2014, 274: 50-56. (  0) 0) |
| [57] |
Ouyang Z Z, Yang C, He J H, et al. Homogeneous photocatalytic degradation of sulfamethazine induced by Fe (Ⅲ)-carboxylate complexes: Kinetics, mechanism and products[J]. Chemical Engineering Journal, 2020, 402: 126122. (  0) 0) |
| [58] |
Chen Y, Hu C, Qu J H, et al. Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2008, 197(1): 81-87. (  0) 0) |
| [59] |
Wang H, Yao H, Sun P Z, et al. Oxidation of tetracycline antibiotics induced by Fe (Ⅲ) ions without light irradiation[J]. Chemosphere, 2015, 119: 1255-1261. (  0) 0) |
| [60] |
Bai Y, Cui Z G, Su R G, et al. Influence of DOM components, salinity, pH, nitrate, and bicarbonate on the indirect photodegradation of acetaminophen in simulated coastal waters[J]. Chemosphere, 2018, 205: 108-117. (  0) 0) |
| [61] |
Zhang Y N, Zhao J, Zhou Y, et al. Combined effects of dissolved organic matter, pH, ionic strength and halides on photodegradation of oxytetracycline in simulated estuarine waters[J]. Environmental Science: Processes & Impacts, 2019, 21(1): 155-162. (  0) 0) |
| [62] |
Ge L K, Zhang P, Halsall C, et al. The importance of reactive oxygen species on the aqueous phototransformation of sulfonamide antibiotics: Kinetics, pathways, and comparisons with direct photolysis[J]. Water Research, 2019, 149: 243-250. (  0) 0) |
| [63] |
Ge L K, Li X Y, Zhang S, et al. Comparing the photodegradation of typical antibiotics in ice and in water: Degradation kinetics, mechanisms, and effects of dissolved substances[J]. Chemosphere, 2024, 352: 141489. (  0) 0) |
| [64] |
Porras J, Bedoya C, Silva-Agredo J, et al. Role of humic substances in the degradation pathways and residual antibacterial activity during the photodecomposition of the antibiotic ciprofloxacin in water[J]. Water Research, 2016, 94: 1-9. (  0) 0) |
| [65] |
Guo Y H, Peng B, Liao J G, et al. Recent advances in the role of dissolved organic matter during antibiotics photodegradation in the aquatic environment[J]. Science of the Total Environment, 2024, 916: 170101. (  0) 0) |
| [66] |
Cui S Y, Qi Y W, Zhu Q, et al. A review of the influence of soil minerals and organic matter on the migration and transformation of sulfonamides[J]. Science of the Total Environment, 2023, 861: 160584. (  0) 0) |
| [67] |
Oliveira C, Lima D L D, Silva C P, et al. Photodegradation of sulfamethoxazole in environmental samples: The role of pH, organic matter and salinity[J]. Science of the Total Environment, 2019, 648: 1403-1410. (  0) 0) |
| [68] |
Liu H Y, Qu J, Zhang T T, et al. Insights into degradation pathways and toxicity changes during electro-catalytic degradation of tetracycline hydrochloride[J]. Environmental Pollution, 2020, 258: 113702. (  0) 0) |
| [69] |
吕逸寒. 光/电催化降解水中诺氟沙星及其毒性变化研究[D]. 长春: 东北师范大学, 2022. Lv Y H. Study on the Photo/Electrocatalytic Degradation and Toxicity Variety of Norfloxacin in Water[D]. Changchun: Northeast Normal University, 2022. (  0) 0) |
| [70] |
Kong D J, He L Y, Shen S T, et al. Unveiling the mechanisms of peracetic acid activation by iron-rich sludge biochar for sulfamethoxazole degradation with wide adaptability[J]. Journal of Environmental Management, 2023, 347: 119119. (  0) 0) |
| [71] |
Zhuang J G, Wang S Y, Tan Y, et al. Degradation of sulfadimethoxine by permanganate in aquatic environment: Influence factors, intermediate products and theoretical study[J]. Science of the Total Environment, 2019, 671: 705-713. (  0) 0) |
| [72] |
Li S, Yang Y, Zheng H, et al. Introduction of oxygen vacancy to manganese ferrite by Co substitution for enhanced peracetic acid activation and 1O2 dominated tetracycline hydrochloride degradation under microwave irradiation[J]. Water Research, 2022, 225: 119176. (  0) 0) |
| [73] |
Du Y Q, Cheng Q L, Qian M R, et al. Biodegradation of sulfametoxydiazine by Alcaligenes aquatillis FA: Performance, degradation pathways, and mechanisms[J]. Journal of Hazardous Materials, 2023, 452: 131186. (  0) 0) |
| [74] |
Xu J, Hao Z N, Guo C S, et al. Photodegradation of sulfapyridine under simulated sunlight irradiation: Kinetics, mechanism and toxicity evolvement[J]. Chemosphere, 2014, 99: 186-191. (  0) 0) |
| [75] |
Li W, Lyu B L, Li J P, et al. Phototransformation of roxithromycin in the presence of dissolved organic matter: Characteriazation of the degradation products and toxicity evaluation[J]. Science of the Total Environment, 2020, 733: 139348. (  0) 0) |
| [76] |
Baena-Nogueras R M, González-Mazo E, Lara-Martín P A. Photolysis of antibiotics under simulated sunlight irradiation: identification of photoproducts by high-resolution mass spectrometry[J]. Environmental Science & Technology, 2017, 51(6): 3148-3156. (  0) 0) |
| [77] |
Huang R S, Cao H Y, Huang T, et al. Effects of environmental factors on the fleroxacin photodegradation with the identification of reaction pathways[J]. Chemosphere, 2022, 308(Pt2): 136373. (  0) 0) |
| [78] |
Boreen A L, Arnold W A, Mcneill K. Triplet-sensitized photodegradation of sulfa drugs containing six-membered heterocyclic groups: Identification of an SO2 extrusion photoproduct[J]. Environmental Science & Technology, 2005, 39(10): 3630-3638. (  0) 0) |
| [79] |
Guerard J J, Chin Y P, Mash H, et al. Photochemical fate of sulfadimethoxine in aquaculture waters[J]. Environmental Science & Technology, 2009, 43(22): 8587-8592. (  0) 0) |
| [80] |
Niu J F, Zhang L L, Li Y, et al. Effects of environmental factors on sulfamethoxazole photodegradation under simulated sunlight irradiation: Kinetics and mechanism[J]. Journal of Environmental Sciences, 2013, 25(6): 1098-1106. (  0) 0) |
| [81] |
Trovó A G, Nogueira R F P, Agüera A, et al. Photodegradation of sulfamethoxazole in various aqueous media: Persistence, toxicity and photoproducts assessment[J]. Chemosphere, 2009, 77(10): 1292-1298. (  0) 0) |
| [82] |
Gmurek M, Horn H, Majewsky M. Phototransformation of sulfamethoxazole under simulated sunlight: Transformation products and their antibacterial activity toward Vibrio fischeri[J]. Science of the Total Environment, 2015, 538: 58-63. (  0) 0) |
| [83] |
Willach S, Lutze H V, Eckey K, et al. Direct photolysis of sulfamethoxazole using various irradiation sources and wavelength ranges: Insights from degradation product analysis and compound-specific stable isotope analysis[J]. Environmental Science & Technology, 2018, 52(3): 1225-1233. (  0) 0) |
| [84] |
Gozlan I, Koren I. Identification, Mechanisms and kinetics of macrolide degradation product formation under controlled environmental conditions[J]. Journal of Environmental Analytical Chemistry, 2016, 3(1): 1000171. (  0) 0) |
| [85] |
Li J P, Li W, Liu K, et al. Global review of macrolide antibiotics in the aquatic environment: Sources, occurrence, fate, ecotoxicity, and risk assessment[J]. Journal of Hazardous Materials, 2022, 439: 129628. (  0) 0) |
| [86] |
Li M, Yang X F, Wang D S, et al. Enhanced oxidation of erythromycin by persulfate activated iron powder-H2O2 system: Role of the surface Fe species and synergistic effect of hydroxyl and sulfate radicals[J]. Chemical Engineering Journal, 2017, 317: 103-111. (  0) 0) |
| [87] |
Marín-García M, De Luca M, Ragno G, et al. Coupling of spectrometric, chromatographic, and chemometric analysis in the investigation of the photodegradation of sulfamethoxazole[J]. Talanta, 2022, 239: 122953. (  0) 0) |
| [88] |
Xu L P, Li H, Mitch W A, et al. Enhanced phototransformation of tetracycline at smectite clay surfaces under simulated sunlight via a Lewis-Base catalyzed alkalization mechanism[J]. Environmental Science & Technology, 2018, 53(2): 710-718. (  0) 0) |
| [89] |
Lin Y C, Hsiao K W, Lin A Y C. Photolytic degradation of ciprofloxacin in solid and aqueous environments: kinetics, phototransformation pathways, and byproducts[J]. Environmental Science and Pollution Research, 2017, 25(3): 2303-2312. (  0) 0) |
| [90] |
Werner J J, Mcneill K, Arnold W A. Photolysis of chlortetracycline on a clay surface[J]. Journal of Agricultural and Food Chemistry, 2009, 57(15): 6932-6937. (  0) 0) |
| [91] |
Suzuki Y, Lopez A, Ponte M, et al. Photoinduced oxidation of the insecticide phenothrin on soil surfaces[J]. Journal of Agricultural and Food Chemistry, 2011, 59(18): 10182-10190. (  0) 0) |
| [92] |
Rafique N, Tariq S R, Abbas M. Effect of Fe2+ amendment on photodegradation kinetics of imidacloprid in moist soil[J]. Environmental Earth Sciences, 2013, 71(6): 2869-2874. (  0) 0) |
2. Key Laboratory of Marine Chemistry Theory and Engineering Technology, Ministry of Education, Ocean University of China, Qingdao 266100, China
 2025, Vol. 55
2025, Vol. 55