2) Guangxi Key Laboratory of Marine Disaster in the Beibu Gulf, Qinzhou 535011, China;
3) Department of Chemistry, City University of Hong Kong, Hong Kong SAR, China;
4) State Key Laboratory of Marine Pollution, City University of Hong Kong, Hong Kong SAR, China;
5) Guangxi Key Laboratory of Beibu Gulf Marine Biodiversity Conservation, Qinzhou 535011, China
Marine plastic pollution and its effects on ecosystems are recently of particular concern due to its ubiquitous presence from polar to the equator regions (Amin et al., 2020; Sfriso et al., 2020). Microplastics (MPs), referred to plastic items with a diameter from 1 μm to 5 mm (Arthur et al., 2009), overlap with natural organic matter and plankton. Consequently, MPs are found to be ingested by myriad assortments of marine organisms with varying feeding modes (Wright et al., 2013), and transported along marine food webs via trophic interactions (Carbery et al., 2018). MP ingestion may represent physical and/or toxicological threats to marine organisms. Controlled laboratory studies reported MP-induced adverse effects on assimilation, development, regeneration, reproduction and behavior in various examples of marine species (Haegerbaeumer et al., 2019). Owing to the large surface area/volume ratio and hydrophobic properties, MPs are important resource of organic pollutant contaminations (Lo et al., 2019), and may result in the bioaccumulation and biomagnification in the aquatic food webs.
The occurrence of MPs in marine sediments has been broadly reported (Yao et al., 2019; Harris, 2020; Zhou et al., 2020; Sun et al., 2021), and the densities were much higher than those detected in the nearby waters (Haegerbaeumer et al., 2019). Therefore, benthic fauna should be more susceptible to the ecological risk caused by plastic pollution. Among marine sedimentary environments, estuaries contain higher concentrations of MPs due to their greater sediment trapping efficiency (Harris, 2020). However, the distribution pattern of MPs in benthic species in response to habitat type, feeding mode and trophic level is currently unclear. Field and laboratory studies demonstrated that filter feeders suffered the most from MP pollution (Setälä et al., 2015; Scherer et al., 2017; Bour et al., 2018b); nonetheless, in another study the count of MPs per individual in filter feeders was significantly lower than that in predators (Bour et al., 2018a). Xu et al. (2020b) found a significantly higher quantity of MPs in gastropods independent of their feeding mode, indicating that MP levels in benthic invertebrates may be species-specific because of their great variation in feeding selectivity, gut retention time and egestion rate.
To date, the occurrence of MPs in horseshoe crabs and their interactions with the sedimentary environment has not been determined. Horseshoe crabs are important prey and predators in estuarine and coastal food webs (Botton, 2009). Their eggs are also vital sources of lipids and proteins to support shorebird population along the Atlantic coast (Botton, 2009). Different from most other benthic invertebrates, horseshoe crabs utilize a wide variety of coastal habitats ranging from upper and mid-intertidal zones to shallow waters within 30 m deep (John et al., 2018; Laurie et al., 2019; Wang et al., 2020). For the juveniles, they have restricted dispersal capability and may spend nine years or longer time living in the intertidal areas (Hu et al., 2009, 2015; Xie et al., 2020). Their foraging activities follow the tidal cycle: burrowing in the sediment during the rising tide to avoid predators and emerging when the tide is receding. Horseshoe crabs forage on mixed diets of small benthic invertebrates, but predominantly assimilate nutrients from sedimentary organic matter and benthic plankton during their early growth stages (Gaines et al., 2002; Kwan et al., 2021). Such foraging behavior and life-history characteristic may render them exposed to a higher abundance of MPs in the estuarine environment.
The northern Beibu Gulf is perceived as one of the least disturbed semi-enclosed areas in the southern China, which supporting the largest population of tri-spine horseshoe crab, Tachypleus tridentatus in China (Liao and Li, 2001; Brockmann and Smith, 2009). Extensive historical harvest records of adult horseshoe crabs for bleeding to produce endotoxin tests in pharmaceutical products and for local food consumption have been well documented (Liao and Li, 2001; Fu et al., 2019; Liao et al., 2019). However, approximately 6.8 million coastal residents in the northern Beibu Gulf (Fu et al., 2019) and a lack of an efficient plastic recycling policy have made the widespread occurrence of MPs in coastal seawater, mangrove sediment and marine organisms. MP concentration in the Beibu Gulf coastal water ranged from 399 to 5531 items m-3 (Li et al., 2020b), 273 – 6360 items kg-1 in mangrove sediment (Li et al., 2019, 2020a, b; Zhang et al., 2020b), 1 – 8 items ind-1 in mangrove crab, 2 – 14 items ind-1 in mangrove fish (Koongolla et al., 2020; Zhang et al., 2021a), 3 – 9 items ind-1 in oysters (Zhu et al., 2019), and 7 – 53 items kg-1 in mangrove snail, Ellobium chinense (Li et al., 2020b). Aquaculture activities along the coast with widespread plastic ropes and foam buoys are suggested to be the important source of MPs in the region (Zhang et al., 2020a), which can be confirmed easily by the researchers' observations.
In this study, juvenile T. tridentatus was chosen as the representative benthic macroinvertebrate species in coastal and estuarine ecosystems. The objectives were to understand whether MP abundance and composition in juvenile horseshoe crabs varied among growth stages and sampling sites. The baseline information is fundamental to a better understanding of MP availability to benthic macroinvertebrates, and the potential to accumulate and transfer across the benthic food webs.
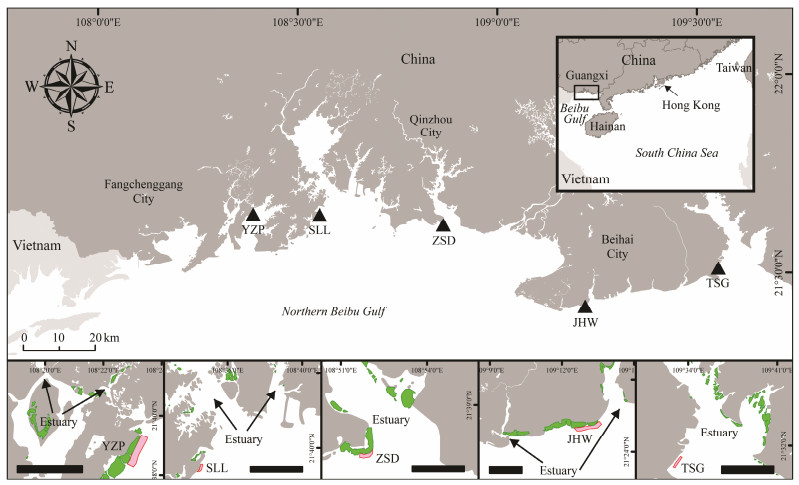
2 Materials and Methods 2.1 Study Locations and SamplingsSix important nursery habitats for T. tridentatus were visited in summer (August – September 2018) according to the previous population studies along the coast of the northern Beibu Gulf, China (Xie et al., 2020) (Fig. 1). Yuzhouping (YZP), Tieshangang (TSG) and Shatiangang (STG) are located in regions with fast-growing industrial ports in Southwest China. Shaluoliao (SLL) is positioned along the coast of Qinzhou Bay and next to a new power station under construction. Zhongsandun (ZSD) is located within the estuary of Dafengjiang River and near the Sanniang Bay, a famous ecotourism spot for dolphin watching (Wu et al., 2020). Xiacun (XC) is situated within the Beihai National Wetland Park, nonetheless, small-scale intertidal beachcombing activities are commonly found (Wang et al., 2020). All these sampling sites are sandy-mudflats (grain size: 0.22 – 0.44 mm) with sediment total organic carbon in the range of 0.14% – 0.34% (Xie et al., 2020).
At each study site, two transects were set horizontal to the coastline at 1.6 m and 1.3 m above chart datum, i.e., the lowest astronomical tide, to include only the intertidal areas with the high-density juvenile horseshoe crab populations (Kwan et al., 2016; Xie et al., 2020). The first transect was located along the outer edges of the mangroves or the saltmarsh cordgrass, except that SLL was in front of the seawall (Fig. 1). Lengths of each transect ranged 1.0 – 2.1 km, according to the shore length or the determined core feeding area of the juveniles. Along each transect, five equaldistance quadrats (0.33 × 0.33 m2) were placed. For each site, juvenile horseshoe crabs (n = 5 – 39; prosomal width, PW = 33.1 – 77.3 mm) found within the 10 quadrats were collected. Approximately 1 – 2 kg of wet sediment was also sampled within each quadrat at the depth of 2 – 3 cm during low tides with an aid of stainless-steel shovel. All samples, including juvenile horseshoe crabs and sediment, were stored in separate cotton bags and transported to the laboratory for microplastic analysis.
|
Fig. 1 Locations of study sites and sampling areas (highlighted in red) along the northern Beibu Gulf shoreline. The relative position of mangrove patches is denoted in green. YZP, Yuzhouping; SLL, Shaluoliao; ZSD, Zhongsandun; XC, Xiacun; TSG, Tieshangang; STG, Shatiangang. |
Sediment samples were dried naturally in the laboratory for two months, sieved through 125 μm and 5 mm sieves, and weighed. The separation between MPs and sediment particles followed the protocol of Lo et al. (2018). In brief, 3 L of ZnCl2 solution (density of 1.6 – 1.7 g mL-1) was added into a 5 L glass beaker and stirred for 5 min using a stainless-steel spatula. The mixture was covered with aluminum foil, settled overnight and the overflow supernatant was filtered through a 5 μm glass microfiber filter membrane (GF/C grade, binder-free, Millipore, UK). The separation process of each sediment sample was repeated thrice to enhance the recovery rate. The surface of the beaker was rinsed thoroughly with distilled water to ensure all remaining plastic items were collected. Those on the filters were dried at 65℃, and stored in covered glass Petri dishes before microscopic examination. To avoid any possible MP contamination, all laboratory apparatus were repeatedly rinsed with distilled water prior to use.
For juvenile T. tridentatus, they were frozen to death prior to dissection in the laboratory. The animals were thawed, and their PW and body weight were measured. Each individual was classified following their instar stages as described by Hu et al. (2015). The juveniles were rinsed with distilled water to eliminate any possible particles adhered to the body surface, then their gastrointestinal tracts (GITs) and gills were isolated and stored in glass beakers covered with aluminum foil at -20℃. Several early-instar juveniles were found at YZP, ZSD and STG, and their GITs were too fragile and not successfully isolated during dissection. The GIT samples obtained from larger-instar juveniles were digested using 10% KOH solution at 40℃ for 48 h (Li et al., 2019). For gill samples, however, their surface was covered with chitin and could not be digested by the alkaline solution, even the samples had been cut into small pieces. The use of strong acids for digestion probably lead to the fragmentation or eventually the loss of microplastics such as polyamide (nylon) and polyoxymethylene (Bürkle GmbH, 2021), which may end up with inaccurate or biased results. Therefore, the gill samples were not included in the experiment. The digested liquids of GIT samples were then filtered through 5 μm glass microfiber membranes (GF/C, Whatman, France), and stored in clean Petri dishes with lids for further examination.
2.3 Qualification and Quantification of MicroplasticThe filters were visually inspected and counted under a stereomicroscope (Olympus, Japan) at a magnification of 40× to 120×. All suspected items were recorded in images using CapStudio software (SC600 digital CMOS camera, Nanjing Lookout Photoelectric, China), and categorized based on their shapes and colors as described previously (Lo et al., 2018; Zhu et al., 2019). The length of items was quantified using ImageJ program (https://imagej.net/). To reduce the tremendous amount of work, only characteristics (i.e., length, shape and color) of suspected MPs in GITs of juvenile T. tridentatus were quantified.
For the composition quantification, approximately 20% of the items on each filter paper were chosen at random (Xu et al., 2020b) and verified using micro-Fourier Transform Infrared Microscope (μFTIR; Thermo Fisher Nicolet iN5, USA) under attenuated total reflection mode. All suspected MPs would be subjected to the composition identification if there were less than five items on a filter paper. In total, 440 out of 1301 suspected items in GIT and sediment samples were tested.
A matching between the suspected MP spectrum and the OMNIC standard spectra library of Hummel Polymer Sample Library was conducted. Any item with a matching score greater than 70% would be counted as a MP (Lo et al., 2018). The final abundance of MPs was revised by the ratio of the suspected plastics confirmed as MPs with 78.01% in GITs and 100% in sediments. A procedural control was implemented weekly with the inclusion of all the digestion procedures, except for the sediment and biological samples, to assess possible air contamination during tissue digestion. No MP was detected during the control experiments (n = 12).
2.4 Statistical AnalysisPrior to the analyses, all data were subjected to the tests of normality and homogeneity of variance. Abundance data of MPs in juvenile GITs and sediments did not meet the normality requirements even though all possible arithmetic transformations had been attempted. As a result, the difference in sediments among sampling sites was tested using non-parametric Kruskal-Wallis test. If a significant difference was detected, multiple pair-wise comparisons with Mann-Whitney U tests would be performed. Non-parametric Scheirer-Ray-Hare extension of Kruskal-Wallis test (site [fixed] × instar [fixed]) was used to examine MP level differences in juvenile GITs. Spearman rank-order correlation was employed to determine the possible relationship of MP abundance as well as length in GIT samples with the changes in juvenile prosomal width. The above calculations were performed by SPSS statistics software (IBM version 22, New York, USA), and Scheirer-Ray-Hare extension of the Kruskal-Wallis test was conducted following the modified SPSS protocol described by Shen et al. (2013).
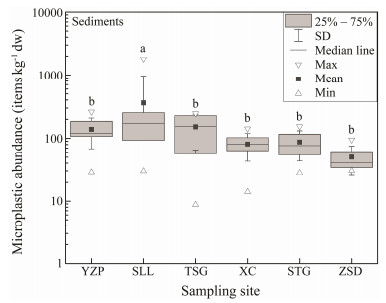
3 ResultsMicroplastic items were found in all sediment samples obtained from the six study sites. The concentrations ranged from 8.8 to 1818.2 items kg-1 dry weight (dw) with the mean value of (130.8 ± 594.8) items kg-1 dw. SLL had the highest ((361 ± 594.8) items kg-1 dw), in which the concentration of MPs was significantly higher than those of the other sampling sites, i.e., TSG ((152.1 ± 87.1) items kg-1 dw) > YZP ((138.7 ± 70.4) items kg-1 dw) > STG ((87.6 ± 44.0) items kg-1 dw) > XC ((81.4 ± 38.1) items kg-1 dw) > ZSD ((50.4 ± 24.6) items kg-1 dw) (Fig. 2).
|
Fig. 2 Microplastic abundance in sediments among different sampling sites. Significant statistical difference among sampling sites (P < 0.05) are represented by different lowercase letters. Site abbreviations are mentioned in Fig. 1 caption. |
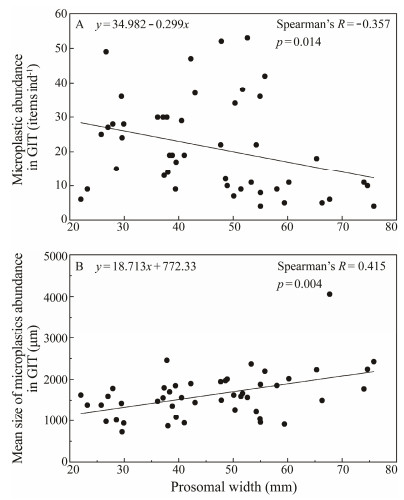
MPs were also detected in all extracted GIT samples of juvenile horseshoe crabs. The abundance of GIT samples varied from 4 to 53 items ind-1 (0 – 28.7 items g-1, wet weight, ww). The mean value was (21.1 ± 13.4) items ind-1 (3.2 ± 5.4 items g-1 ww). Neither the sampling site nor instar stage of juveniles was found to influence the MP abundances in GITs (site: H = 0.154, p = 0.930; instar: H = 9.426, p = 0.090; site × instar: H = 0.160, p = 1.000). MP abundance in GITs was found to be negatively correlated with prosomal width (size) of juvenile T. tridentatus (Fig. 3A, Spearman's R = -0.357, p = 0.014). However, a positive relationship was found between MP size and the juvenile body size (Fig. 3B; Spearman's R = 0.415, p = 0.004).
|
Fig. 3 Relationships of (A) abundance and (B) mean length (size) of microplastics in gastrointestinal tract (GIT) with prosomal width of juvenile T. tridentatus. |
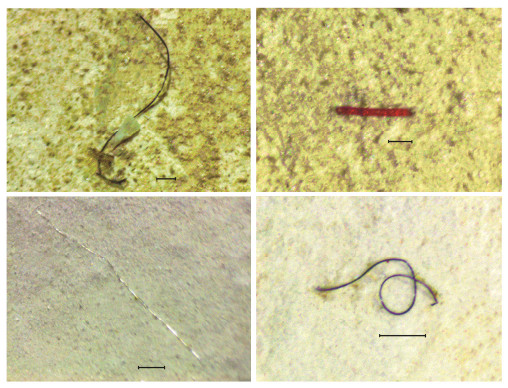
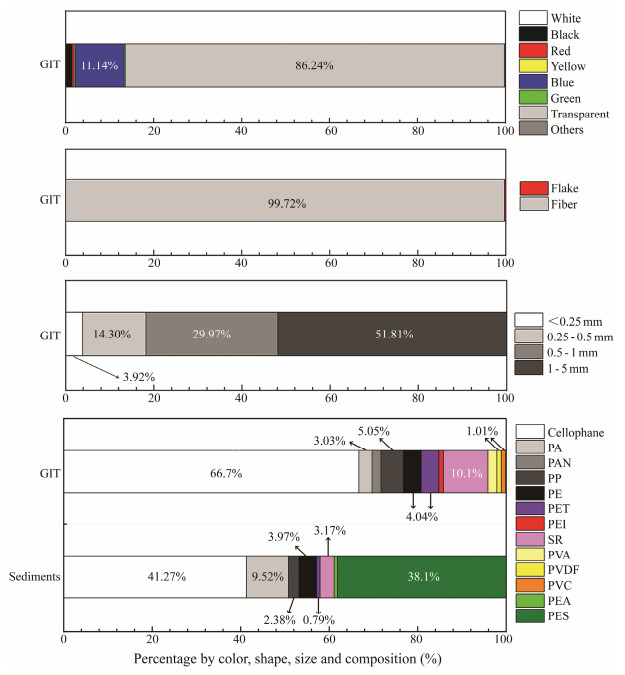
Transparent MPs were the most commonly encountered (86.2%) in GIT of T. tridentatus (Figs. 4 – 5). In terms of MP shape, only fiber and flake items were noted in all juvenile samples, and fiber contributed greater than 99% of all the samples (Figs. 4 – 5). MPs that were greater than 1 mm in length contributed about half in GIT samples (51.81%). Thirteen polymers were noted in sediments and GITs of juvenile T. tridentatus, which included cellophane, polyamide (PA), polyacrylonitrile (PAN), polypropylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polyethylenimine (PEI), synthetic rubber (SR), polyvinyl acetate (PVA), polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC), polyethylacrylate acrylamide (PEA) and polyester (PES). Cellophane was the most dominant polymer in both sediments (41.3%) and juvenile horseshoe crab GITs (66.7%) (Fig. 5). In addition to cellophane, PES (38.1%) and PA (9.5%) were the second and third most abundance. These three polymers accounted for > 88.9% of total MPs in sediments. However, the composition of remaining MPs in GIT samples greatly varied from PVDF (1.0%) to SR (10.1%) (Fig. 5).
|
Fig. 4 Microfibers found in gastrointestinal tracts of juvenile T. tridentatus with varying colors. The scale bars represent 200 μm. |
|
Fig. 5 Characteristics (color, shape and size) and compositions of microplastics in the sediments and juvenile T. tridentatus. PA, polyamide; PAN, polyacrylonitrile; PP polypropylene; PE, polyethylene; PET, polyethylene terephthalate; PEI, polyethylenimine; SR, synthetic rubber; PVA, polyvinyl acetate; PVDF, polyvinylidene fluoride; PVC, polyvinyl chloride; PEA, polyethylacrylate acrylamide; PES, polyester; GIT, gastrointestinal tracts. |
The northern Beibu Gulf is regarded as one of the undisturbed wilderness areas retaining its relatively pristine state compared to other coastal regions in China (Zhang et al., 2020b). Nevertheless, there are recently increasing studies confirming that the gulf has been polluted by various MPs, although their abundances in coastal water and sediment samples were highly variable even in the same or adjacent area. For example, Qiu et al. (2015) reported that the MP abundance of mangrove sediment on a beach (site name: Beihai) close to XC in this study was 6080 items kg-1 dw. In another adjacent sampling site (site name: Daguan Sha) within 1 km from XC, however, Zhang et al. (2020b) found that the concentration was only approximately one-seventh ((873 ± 63) items kg-1 dw) of that reported in Qiu et al. (2015). Similar cases were found in the MP abundance in mangrove sediments at Yong An (> 500 items kg-1 dw; Zhang et al., 2020b) and Guangxi station 7 (87.8 items kg-1 dw; Zhou et al., 2020), in which both sites are in the vicinity of STG in this study ((138.7 ± 70.4) items kg-1 dw). Overall, the abundance of MPs in sediments from the important nursery habitats of T. tridentatus in this study was relatively low < (400 items kg-1 dw per site). These inconsistencies may be due to the currently limited environmental baseline data regarding MP occurrence in the Beibu Gulf region.
For MPs in juvenile horseshoe crabs, the abundance (4 – 53 items ind-1 or 0 – 28.7 items g-1 ww) was higher than that in most marine benthic invertebrates reported in Chinese waters (Table 1). In the coastal and offshore areas of Beibu Gulf, the count of MPs in oyster Crassostrea hongkongensis and various mangrove crabs were recorded in the range of 2 – 9 items ind-1 (Table 1, Zhu et al., 2019; Zhang et al., 2021a). In other coastal regions, those in bivalves, gastropods and crustaceans were reported at 2 – 15 items ind-1 (0.2 – 7.1 items g-1 ww), 1.5 – 5.4 items g-1 ww and 0 – 10 items ind-1 (0 – 22.7 items g-1 ww), respectively, except for the considerably high MP levels occurred in clam Scapharca subcrenata (about 49 items ind-1) and scallop Patinopecten yessoensis (57.2 items ind-1) obtained from a fishery market in Shanghai (Li et al., 2015). The relatively higher MP abundance in juvenile T. tridentatus was believed to be related to their life-history characteristics with limited dispersal capability, spending at least nine years feeding in the intertidal mangrove areas at low tides (Hu et al., 2015; Kwan et al., 2021; Xie et al., 2020).
|
|
Table 1 Microplastic (MP) contamination in marine benthic invertebrates in Chinese waters |
Consistent with the previous reports on other marine invertebrates, the relationship between the MP levels in sediments and organisms was not evident (Hipfner et al., 2018; Li et al., 2020b; Xu et al., 2020b). Despite the fact that a higher level of MPs in habitats may increase the probability of passive intake by the organisms, recent studies demonstrated that marine organisms such as juvenile palm ruff, Seriolella violacea (Ory et al., 2018), and blue mussel, Mytilus edulis (Woods et al., 2018; Chae and An, 2020) can effectively excrete most of the MPs in the form of feces or psedofeces. Zhu et al. (2018) also documented the avoidance behavior against MP ingestion in the collembolans. It is intuitive that the older an organism, the higher the abundance of MPs in the body. The present findings, however, found a weak but significant negative relationship between the quantity of MPs and the body size of T. tridentatus. Such correlation is possibly related to the apparent ontogenetic changes in juvenile horseshoe crab, while their diets change from primarily sedimentary organic matter to small-sized invertebrates with aging (Gaines et al., 2002; Kwan et al., 2021). The marked difference in MP compositions between sediments and the juvenile GITs also suggest that the MPs in GIT samples were mainly derived from other organisms and transferred through benthic food webs.
Coastal anthropogenic activities, including tourism, aquaculture and industrial production, have been identified as the primary contributors to the distribution of MPs in marine sediments (e.g., Nor et al., 2014; Zhang et al., 2020b; Zhang et al., 2021a). The pattern is consistent with the present results that, the sampling sites near the industrial ports (TSG, STG and YZP) had a relatively higher MP abundance (88 – 152 items kg-1 dw) in sediments compared to those located within the wetland park and/or away from intensive human activities (XC and ZSD, 50 – 81 items kg-1 dw). The presence of circulating water pipelines outside the power station near SLL may have lowered the exchange rate of coastal water, consequently enhanced the sedimentation and MP accumulation (Willemsen et al., 2016; Zhang et al., 2020b).
Apart from industrial activities, the direct sources of MP pollution at these sampling sites were possibly due to traditional intertidal aquaculture (Zhu et al., 2019), smallscale seafood harvesting activities (Wang et al., 2020) and/ or domestic wastewater inputs from the coastal populations in this region (Zhang et al., 2020a). The ubiquitous presence of MP fibers (> 99%) in both previous and present studies could also explain the major impacts of aquaculture and fishing activities, which involved a tremendous amount of plastic waste discarded or lost in the marine environment (Andrady, 2011). Microfibers derived from clothing were also suspected to be a primary source of MPs in the coastal and estuarine environment. Consequently, a great number of studies reported the presence of MP fibers in marine fishes (Jabeen et al., 2017; Garcés-Ordóñez et al., 2020; Koongolla et al., 2020) and benthic invertebrates (Xu et al., 2020a, b; Zhang et al., 2021). Laboratory studies also suggested that MPs in the form of fibers were more prone to ingestion by marine living organisms (Hämer et al., 2014; Rochman et al., 2015).
In terms of MP compositions, the occurrence of polymers such as PP, PE, PET and PS were frequently reported in the Beibu Gulf region (Qiu et al., 2015; Li et al., 2018; Zhang et al., 2020a). Our current findings, however, indicated the considerably higher percentage of cellophane found in the sediments and juvenile horseshoe crabs (Fig. 4). Cellophane is an organic cellulose-based polymer, and has been classified as microplastic (Woodall et al., 2014). As a typical raw material for food packaging as well as a releasing agent in fiberglass and rubber production, cellophane was documented as one of the most commonly available polymers in the sediments of the Yellow Sea, the East China Sea and Ma'an Archipelago marine ranching area of China (Zhang et al., 2020a, b), in surface water from Qin River within the Beibu Gulf, as well as in marine fishes (Jabeen et al., 2017) and edible oysters (Zhu et al., 2020). The abundant cellophane in the nursery habitats of horseshoe crabs in the northern Beibu Gulf may originate from the weathering of fiberglass products and/ or cellophane wrappers, which suggest the primary contribution of land-derived MPs, particularly the beaches near the river mouth.
To conclude, MPs can be widely detected in both sediment and juvenile T. tridentatus from the northern Beibu Gulf shores. Most identified microplastics in the juveniles were transparent microfibers with length > 1 mm, and at relatively higher concentrations compared to those in other marine benthic invertebrates found in Chinese waters. The MP abundance decreased with horseshoe crab ages, but the MP size increased with their growth. These results demonstrated that the influence of MP abundance in marine benthic invertebrates can be species-specific, which may be governed by their feeding behavior and selectivity, ingestion and egestion rates, as well as life-history characteristics. As a result, baseline studies in marine invertebrates are necessary to obtain a clearer picture regarding the fate and trophic interactions of MPs in benthic sedimentary environments. Owing to the limitations in this study, which included 1) MP occurrence in juvenile GIT samples was not completely determined, and 2) only about 34% of the suspected MPs were analyzed by μ-FTIR, the level of MPs in juvenile horseshoe crabs could be underestimated. Given the high ecological importance of conserving T. tridentatus population, future studies to enhance our knowledge regarding the source and sink of MPs in the region are therefore necessary. It is also dire to determine the MP egestion rate of juvenile T. tridentatus, and their potential physical and/or toxicological impacts on the organisms.
AcknowledgementsWe thank Dr. Paul Shin and many other researchers who provided feedback and information which considerably enhanced the quality of this manuscript. The assistance from Dr. Chun-Chieh Wang from Guangxi Academy of Sciences for preparing the maps is much appreciated. This research was supported by the National Natural Science Foundation of China (No. 41907320), the Natural Science Foundation of Guangxi Region (No. 2019 JJA150043), the Guangxi BaGui Youth Scholars Programme, and Guangxi Recruitment Program of 100 Global Experts.
Amin R. M., Sohaimi E. S., Anuar S. T., Bachok Z.. 2020. Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea. Marine Pollution Bulletin, 150: 110616. DOI:10.1016/j.marpolbul.2019.110616 (  0) 0) |
Andrady A. L.. 2011. Microplastics in the marine environment. Marine Pollution Bulletin, 62: 1596-1605. DOI:10.1016/j.marpolbul.2011.05.030 (  0) 0) |
Arthur, C., Baker, J., and Bamford, H., 2009. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. NOAA Technical Memorandum NOS-OR & R-30, 528pp.
(  0) 0) |
Botton, M. L., 2009. The ecological importance of horseshoe crabs in estuarine and coastal communities: A review and speculative summary. In: Biology and Conservation of Horseshoe Crabs. Tanacredi, J. T., et al., eds., Springer, Massachusetts, Boston, 45-63.
(  0) 0) |
Bour A., Avio C. G., Gorbi S., Regoli F., Hylland K.. 2018a. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environmental Pollution, 243: 1217-1225. DOI:10.1016/j.envpol.2018.09.115 (  0) 0) |
Bour A., Haarr A., Keiter S., Hylland K.. 2018b. Environmentally relevant microplastic exposure affects sediment-dwelling bivalves. Environmental Pollution, 236: 652-660. DOI:10.1016/j.envpol.2018.02.006 (  0) 0) |
Brockmann, H. J., and Smith, M. D., 2009. Reproductive competition and sexual selection in horseshoe crabs. In: Biology and Conservation of Horseshoe Crabs. Tanacredi, J. T., et al., eds., Springer, Massachusetts, Boston, 199-221.
(  0) 0) |
Bürkle GmbH, 2021. Chemical resistance of plastics. https://www.buerkle.de/en/chemical-resistance. Accessed on 13 August 2021.
(  0) 0) |
Carbery M., O'Connor W., Palanisami T.. 2018. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environmental International, 115: 400-409. DOI:10.1016/j.envint.2018.03.007 (  0) 0) |
Chae Y., An Y. J.. 2020. Effects of food presence on microplastic ingestion and egestion in Mytilus galloprovincialis. Chemosphere, 240: 124855. DOI:10.1016/j.chemosphere.2019.124855 (  0) 0) |
Fu Y., Huang S., Wu Z., Wang C. C., Su M., Wang X., et al. 2019. Socio-demographic drivers and public perceptions of consumption and conservation of Asian horseshoe crabs in northern Beibu Gulf, China. Aquatic Conservation: Marine and Freshwater Ecosystems, 29: 1268-1277. DOI:10.1002/aqc.3125 (  0) 0) |
Gaines E. F., Carmichael R. H., Grady S. P., Valiela I.. 2002. Stable isotopic evidence for changing nutritional sources of juvenile horseshoe crabs. Biological Bulletin, 203: 228-230. DOI:10.2307/1543412 (  0) 0) |
Garcés-Ordóñez O., Mejía-Esquivia K. A., Sierra-Labastidas T., Patiño A., Blandón L. M., Díaz L. F. E.. 2020. Prevalence of microplastic contamination in the digestive tract of fishes from mangrove ecosystem in Cispata, Colombian Caribbean. Marine Pollution Bulletin, 154: 111085. DOI:10.1016/j.marpolbul.2020.111085 (  0) 0) |
Haegerbaeumer A., Mueller M. T., Fueser H., Traunspurger W.. 2019. Impacts of micro-and nano-sized plastic particles on benthic invertebrates: A literature review and gap analysis. Frontiers in Environmental Science, 7: 17. DOI:10.3389/fenvs.2019.00017 (  0) 0) |
Hämer J., Gutow L., Köhler A., Saborowski R.. 2014. Fate of microplastics in the marine isopod Idotea emarginata. Environmental Science and Technology, 48: 13451-13458. DOI:10.1021/es501385y (  0) 0) |
Harris P. T.. 2020. The fate of microplastic in marine sedimentary environments: A review and synthesis. Marine Pollution Bulletin, 158: 111398. DOI:10.1016/j.marpolbul.2020.111398 (  0) 0) |
Hipfner J. M., Galbraith M., Tucker S., Studholme K. R., Domalik A. D., Pearson S. F., et al. 2018. Two forage fishes as potential conduits for the vertical transfer of microfibres in northeastern Pacific Ocean food webs. Environmental Pollution, 239: 215-222. DOI:10.1016/j.envpol.2018.04.009 (  0) 0) |
Hu, M., Kwan, B. K. Y., Wang, Y., Cheung, S. G., and Shin, P. K. S., 2015. Population structure and growth of juvenile horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicauda (Xiphosura) in southern China. In: Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management. Carmichael, R. H., et al., eds., Springer, Zug, Cham, 167-180.
(  0) 0) |
Hu M., Wang Y., Chen Y., Cheung S. G., Shin P. K. S., Li Q.. 2009. Summer distribution and abundance of juvenile Chinese horseshoe crabs Tachypleus tridentatus along an intertidal zone in southern China. Aquatic Biology, 7: 107-112. DOI:10.3354/ab00194 (  0) 0) |
Jabeen K., Su L., Li J., Yang D., Tong C., Mu J., et al. 2017. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environmental Pollution, 221: 141-149. DOI:10.1016/j.envpol.2016.11.055 (  0) 0) |
John B. A., Nelson B. R., Sheikh H. I., Cheung S. G., Wardiatno Y., Dash B. P., et al. 2018. A review on fisheries and conservation status of Asian horseshoe crabs. Biodiversity and Conservation, 27: 3573-3598. DOI:10.1007/s10531-018-1633-8 (  0) 0) |
Koongolla J. B., Lin L., Pan Y. F., Yang C. P., Sun D. R., Liu S., et al. 2020. Occurrence of microplastics in gastrointestinal tracts and gills of fish from Beibu Gulf, South China Sea. Environmental Pollution, 258: 113734. DOI:10.1016/j.envpol.2019.113734 (  0) 0) |
Kwan B. K. Y., Hsieh H. L., Cheung S. G., Shin P. K. S.. 2016. Present population and habitat status of potentially threatened Asian horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicauda in Hong Kong: A proposal for marine protected areas. Biodiversity and Conservation, 25: 673-692. DOI:10.1007/s10531-016-1084-z (  0) 0) |
Kwan K. Y., Bopp J., Huang S., Chen Q., Wang C. C., Wang X., et al. 2021. Ontogenetic resource use and trophic dynamics of endangered juvenile Tachypleus tridentatus among diversified nursery habitats in the northern Beibu Gulf, China. Integrative Zoology, 16(6): 908-928. DOI:10.1111/1749-4877.12495 (  0) 0) |
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H. L., John, A., et al., 2019. Tachypleus tridentatus (Errata Version Published in 2019). e. T21309A149768986. IUCN, Gland, Switzerland, 60pp.
(  0) 0) |
Li J., Lusher A. L., Rotchell J. M., Deudero S., Turra A., Bråte I. L. N., et al. 2019. Using mussel as a global bioindicator of coastal microplastic pollution. Environmental Pollution, 244: 522-533. DOI:10.1016/j.envpol.2018.10.032 (  0) 0) |
Li J., Yang D., Li L., Jabeen K., Shi H.. 2015. Microplastics in commercial bivalves from China. Environmental Pollution, 207: 190-195. DOI:10.1016/j.envpol.2015.09.018 (  0) 0) |
Li J., Zhang H., Zhang K., Yang R., Li R., Li Y.. 2018. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Marine Pollution Bulletin, 136: 401-406. DOI:10.1016/j.marpolbul.2018.09.025 (  0) 0) |
Li J. N., Qu X. Y., Su L., Zhang W. W., Yang D. Q., Kolandhasamy P., et al. 2016. Microplastics in mussels along the coastal waters of China. Environmental Pollution, 214: 177-184. DOI:10.1016/j.envpol.2016.04.012 (  0) 0) |
Li R., Yu L., Chai M., Wu H., Zhu X.. 2020a. The distribution, characteristics and ecological risks of microplastics in the mangroves of Southern China. Science of the Total Environment, 708: 135025. DOI:10.1016/j.scitotenv.2019.135025 (  0) 0) |
Li R., Zhang S., Zhang L., Yu K., Wang S., Wang Y.. 2020b. Field study of the microplastic pollution in sea snails (Ellobium chinense) from mangrove forest and their relationships with microplastics in water/sediment located on the north of Beibu Gulf. Environmental Pollution, 263: 114368. DOI:10.1016/j.envpol.2020.114368 (  0) 0) |
Liao Y., Hsieh H. L., Xu S., Zhong Q., Lei J., Liang M., et al. 2019. Wisdom of Crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China.. Oryx, 53: 222-229. DOI:10.1017/S003060531700117X (  0) 0) |
Liao Y. Y., Li X. M.. 2001. Present situation of horseshoe crab resources in the sea area of China and tactics of preservation. Resource Science, 23: 53-57 (in Chinese with English abstract). (  0) 0) |
Lo H. S., Wong C. Y., Tam N. F. Y., Cheung S. G.. 2019. Spatial distribution and source identification of hydrophobic organic compounds (HOCs) on sedimentary microplastic in Hong Kong. Chemosphere, 219: 418-426. DOI:10.1016/j.chemosphere.2018.12.032 (  0) 0) |
Lo H. S., Xu X., Wong C. Y., Cheung S. G.. 2018. Comparisons of microplastic pollution between mudflats and sandy beaches in Hong Kong. Environmental Pollution, 236: 208-217. DOI:10.1016/j.envpol.2018.01.031 (  0) 0) |
Nor N. H. M., Obbard J. P.. 2014. Microplastics in Singapore's coastal mangrove ecosystems. Marine Pollution Bulletin, 79: 278-283. DOI:10.1016/j.marpolbul.2013.11.025 (  0) 0) |
Ory N. C., Gallardo C., Lenz M., Thiel M.. 2018. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environmental Pollution, 240: 566-573. DOI:10.1016/j.envpol.2018.04.093 (  0) 0) |
Qiu Q., Peng J., Yu X., Chen F., Wang J., Dong F.. 2015. Occurrence of microplastics in the coastal marine environment: First observation on sediment of China. Marine Pollution Bulletin, 98: 274-280. DOI:10.1016/j.marpolbul.2015.07.028 (  0) 0) |
Rochman C. M., Tahir A., Williams S. L., Baxa D. V., Lam R., Miller J. T., et al. 2015. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Scientific Reports, 5: 14340. DOI:10.1038/srep14340 (  0) 0) |
Scherer C., Brennholt N., Reifferscheid G., Wagner M.. 2017. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Scientific Reports, 7: 17006. DOI:10.1038/s41598-017-17191-7 (  0) 0) |
Setälä O., Norkko J., Lehtiniemi M.. 2015. Feeding type affects microplastic ingestion in a coastal invertebrate community. Marine Pollution Bulletin, 102: 95-101. (  0) 0) |
Sfriso A. A., Tomio Y., Rosso B., Gambaro A., Sfriso A., Corami F., et al. 2020. Microplastic accumulation in benthic invertebrates in Terra Nova Bay (Ross Sea, Antarctica). Environmental International, 137: 105587. DOI:10.1016/j.envint.2020.105587 (  0) 0) |
Shen X., Qi H., Liu X., Ren X., Li J.. 2013. Two-way non-parametric ANOVA in SPSS. Chinese Journal of Health Statistics, 30: 913-914 (in Chinese with English abstract). (  0) 0) |
Sun X., Wang T., Chen B., Booth A. M., Liu S., Wang R., et al. 2021. Factors influencing the occurrence and distribution of microplastics in coastal sediments: From source to sink. Journal of Hazardous Materials, 410: 124982. DOI:10.1016/j.jhazmat.2020.124982 (  0) 0) |
Wang C. C., Kwan K. Y., Shin P. K. S., Cheung S. G., Itaya S., Iwasaki Y., et al. 2020. Future of Asian horseshoe crab conservation under explicit baseline gaps: A global perspective. Global Ecology and Conservation, 24: e01373. DOI:10.1016/j.gecco.2020.e01373 (  0) 0) |
Weng Z. H., Xie Y. J., Xiao Z. Q., Huang L. M., Li J., Wang S. H., et al. 2012. Survey on resource distribution of Chinese horseshoe crab (Tachypleus tridentatus) in Fujian and other coast water of China.. Chinese Journal of Zoology, 47: 40-48 (in Chinese with English abstract). (  0) 0) |
Willemsen P. W. J. M., Horstman E. M., Borsje B. W., Friess D. A., Dohmen-Janssen C. M.. 2016. Sensitivity of the sediment trapping capacity of an estuarine mangrove forest. Geomorphology, 273: 189-201. DOI:10.1016/j.geomorph.2016.07.038 (  0) 0) |
Woodall L. C., Sanchez-Vidal A., Canals M., Paterson G. L. J., Coppock R., Sleight V., et al. 2014. The deep sea is a major sink for microplastic debris. Royal Society Open Science, 1: 140317. DOI:10.1098/rsos.140317 (  0) 0) |
Woods M. N., Stack M. E., Fields D. M., Shaw S. D., Matrai P. A.. 2018. Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Marine Pollution Bulletin, 137: 638-645. DOI:10.1016/j.marpolbul.2018.10.061 (  0) 0) |
Wright S. L., Thompson R. C., Galloway T. S.. 2013. The physical impacts of microplastics on marine organisms: A review. Environmental Pollution, 178: 483-492. DOI:10.1016/j.envpol.2013.02.031 (  0) 0) |
Wu H., Peng C., Huang H., Jefferson T. A., Huang S. L., Chen M., et al. 2020. Dolphin-watching tourism and Indo-Pacific humpback dolphins (Sousa chinensis) in Sanniang Bay, China: Impacts and solutions. European Journal of Wildlife Research, 66: 1-9. DOI:10.1007/s10344-019-1327-x (  0) 0) |
Xie X., Wu Z., Wang C. C., Fu Y., Wang X., Xu P., et al. 2020. Nursery habitat for Asian horseshoe crabs along the northern Beibu Gulf, China: Implications for conservation management under baseline gaps. Aquatic Conservation: Marine and Freshwater Ecosystems, 30: 260-272. DOI:10.1002/aqc.3259 (  0) 0) |
Xu X., Wong C. Y., Tam N. F. Y., Liu H. M., Cheung S. G.. 2020a. Barnacles as potential bioindicator of microplastic pollution in Hong Kong. Marine Pollution Bulletin, 154: 111 081. DOI:10.1016/j.marpolbul.2020.111081 (  0) 0) |
Xu X., Wong C. Y., Tam N. F. Y., Lo H. S., Cheung S. G.. 2020b. Microplastics in invertebrates on soft shores in Hong Kong: Influence of habitat, taxa and feeding mode. Science of the Total Environment, 715: 136999. DOI:10.1016/j.scitotenv.2020.136999 (  0) 0) |
Yao P., Zhou B., Lu Y., Yin Y., Zong Y., Chen M. T., et al. 2019. A review of microplastics in sediments: Spatial and temporal occurrences, biological effects, and analytic methods. Quaternary International, 519: 274-281. DOI:10.1016/j.quaint.2019.03.028 (  0) 0) |
Zhang L., Liu J., Xie Y., Zhong S., Yang B., Lu D., et al. 2020a. Distribution of microplastics in surface water and sediments of Qin River in Beibu Gulf, China. Science of the Total Environment, 708: 135176. DOI:10.1016/j.scitotenv.2019.135176 (  0) 0) |
Zhang L., Zhang S., Guo J., Yu K., Wang Y., Li R.. 2020b. Dynamic distribution of microplastics in mangrove sediments in Beibu Gulf, South China: Implications of tidal current velocity and tidal range. Journal of Hazardous Materials, 399: 122849. DOI:10.1016/j.jhazmat.2020.122849 (  0) 0) |
Zhang S., Sun Y., Liu B., Li R.. 2021a. Full size microplastics in crab and fish collected from the mangrove wetland of Beibu Gulf: Evidences from Raman Tweezers (1 – 20 μm) and spectroscopy (20 – 5000 μm). Science of the Total Environment, 759: 143504. DOI:10.1016/j.scitotenv.2020.143504 (  0) 0) |
Zhang T., Sun Y., Song K., Du W., Huang W., Gu Z., et al. 2021b. Microplastics in different tissues of wild crabs at three important fishing grounds in China. Chemosphere, 271: 129479. DOI:10.1016/j.chemosphere.2020.129479 (  0) 0) |
Zhao S., Zhu L., Wang T., Li D.. 2014. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Marine Pollution Bulletin, 86: 562-568. DOI:10.1016/j.marpolbul.2014.06.032 (  0) 0) |
Zhou Q., Tu C., Fu C., Li Y., Zhang H., Xiong K., et al. 2020. Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Science of the Total Environment, 703: 134807. DOI:10.1016/j.scitotenv.2019.134807 (  0) 0) |
Zhu D., Bi Q. F., Xiang Q., Chen Q. L., Christie P., Ke X., et al. 2018. Trophic predator-prey relationships promote transport of microplastics compared with the single Hypoaspis aculeifer and Folsomia candida. Environmental Pollution, 235: 150-154. DOI:10.1016/j.envpol.2017.12.058 (  0) 0) |
Zhu J., Zhang Q., Li Y., Tan S., Kang Z., Yu X., et al. 2019. Microplastic pollution in the Maowei Sea, a typical mariculture bay of China. Science of the Total Environment, 658: 62-68. (  0) 0) |
Zhu X., Qiang L., Shi H., Cheng J.. 2020. Bioaccumulation of microplastics and its in vivo interactions with trace metals in edible oysters. Marine Pollution Bulletin, 154: 111079. DOI:10.1016/j.marpolbul.2020.111079 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


