2) Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao 266003, China;
3) Key Laboratory of Glycoscience & Glycotechnology of Shandong Province, Qingdao 266003, China;
4) Key Laboratory of Marine Drugs, Ministry of Education of China, Qingdao 266003, China
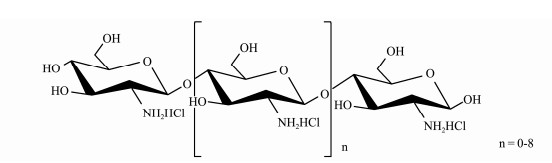
Chito-oligosaccharide (COS) were the hydrolyzed products of chitin or chitosan derived from abundant marine biological resource (shrimp and crab shells) and were an oligomer of β-(1-4)-linked D-glucosamine (Muanprasat and Chatsudthipong, 2017). Fig.1 shows the chemical structure of COS with complete deacetylation. Over the past decades, COS have been shown to exhibit remarkable antimicrobial (Rahman et al., 2014), anti-tumor (Park et al., 2014), antioxidant (Ngo et al., 2008), anti-inflammatory (Chung et al., 2012), immuno-stimulating (Zhang et al., 2014), anti-obesity (Huang et al., 2015), anti-diabetic (Zheng et al., 2018), anti-Alzheimer's disease (Pangestuti et al., 2011) effect. Overall, COS had drawn significant interest among scholars and researchers as bioactive molecules.
|
Fig. 1 The structure of the chito-oligosaccharide (COS). |
In contrast to the widely explored pharmacological actions, studies on the absorption mechanisms and the in vivo fate of COS were limited and the influence of DPs was also unknown. Several researchers had indicated only COS2 and COS3 could be absorbed from the gastrointestinal tract (Chen et al., 2005). On the contrary, COS6 protected against acetaminophen-induced hepatotoxicity in mice (Barman et al., 2016). Therefore, the pharmacokinetics and bioavailability of COS with other DPs remain to be addressed due to challenging aspects of quantitative analysis. To have a better understanding of the pharmacokinetics behavior of COS, a transport study is necessary to clarify its absorption mechanism. COS had been shown to enter cells by facilitated passive diffusion for the first time (Li et al., 2014) in previous studies. On the other hand, concentration and active transporter were capable of mediating the absorption of COS2 and COS5 (Chen et al., 2019), which was against the previous results. Meanwhile, FITC-COS were used for transport experiments instead of COS in these studies, so these results might not reflect its transport mechanism correctly. Thus, absorption mechanisms of COS should be further investigated. Caco-2 cells model is widely used as a stan-dard screening tool to evaluate the absorption mechanism of transport of drug candidates (Hidalgo et al., 1989). Thus, the Caco-2 monolayer model was chosen in this study.
Therefore, the present study aims to investigate the pharmacokinetics and bioavailability of COS in rats and to monitor absorption properties in Caco-2 cell models.
2 Materials and Methods 2.1 Chemicals and MaterialsCaco-2 cell lines were purchased from the cell resource center of the Shanghai Institutes for Biological Sciences (Shanghai, China). COS standards (purity > 95.0%) were provided by Qingdao BZ Oligo Biotech Co., Ltd (Qingdao, China). 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetra-zolium bromide (MTT), penicillin, streptomycin, propranolol, and atenolol were purchased from Sigma-Aldrich (St Louis, MO, USA). Phosphate buffer saline (PBS) and Hank's balanced salt solution (HBSS) were bought from Solarbio Life Science (Beijing, China). Fetal bovine serum (FBS), trypsin, and Iscove's Modified Dubecco's Medium (IMDM) were obtained from Gibco (Grand Island, NY). HPLC-grade ammonium hydroxide, ammonium acetate, phloretin, quercetin, melibiose (internal standard, IS), and sodium deoxycholate were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Transwell cell culture plate (0.4 µm pore size) and 96-well cell culture plates were purchased from Corning Inc. (New York, USA). Acetonitrile and water (LC-MS grade) were obtained from Merck technologies Co., Ltd. (Darmstadt, Germany).
2.2 UPLC-MS/MS Apparatuses and Operation ConditionsThe chromatography separation was performed using the UPLC System (UltiMate 3000, Thermo Fisher Scientific, MA, USA). The collected samples were separated by the XBridge Amide column (3.5 μm, 2.1 mm×150 mm). Optimized mobile phase A consisted of 10 mmol L-1 aqueous ammonium acetate (pH = 9) in water, whereas mobile phase B consisted of 10 mmol L-1 aqueous ammonium acetate (pH = 9) in acetonitrile run at a flow rate of 0.20 mL min-1 and the column temperature was 60℃. The proportion of organic phase used for elution of plasma samples and cell samples were 50% and 40%, respectively.
Quantitative analysis was conducted on TSQ QuantivaTM triple quadruple mass spectrometer (Thermo Fisher Scientific, MA, USA). The data were collected in the MRM mode, and the parameters were listed in Table 1.
|
|
Table 1 Optimized MS/MS parameters of analytes and internal standards in MRM mode |
The cells were grown in IMDM containing 20% FBS, 100 U mL-1 penicillin, and 100 μg mL-1 streptomycin and inoculated in polycarbonate at a density of 5 × 104 cells per well and cultured for 21 days. Caco-2 cells were cultured in an incubator at 37℃ in humidified air containing 5% CO2. The integrity of the cells monolayer was examined by calculating the apparent permeability coefficients (Papp) values of the markers atenolol and propranolol and measuring the transepithelial electrical resistance (TEER) across each well before and after transport experiments. When the TEER values of a consistent monolayer were no lower than 300 Ω cm2 during the experiment (Li et al., 2016) and atenolol (around 10-7cm s-1) and propranolol (around 10-5cm s-1) had appropriate Papp values, the Caco-2 monolayer cells can be used for transport experiments.
Before the study, the consistent cell monolayer was washed with HBSS (pH 7.4) twice times to remove the interfering substances on the cell surface and incubated in HBSS alone or HBSS containing phloretin (100 μmol L-1) or quercetin (50 μmol L-1) or sodium deoxycholate (1 mmol L-1). Following a 30 min incubation, for transfer in the AP-BL direction, 0.2 mL drug solution was added to the AP side as the supply pool, and 0.6 mL HBSS solution was added to the BL side as the receiving pool. In the BL-AP direction, 0.6 mL compound solution was added to the BL side as the supply pool, and 0.2 mL HBSS solution was added to the AP side as the receiving pool. To see whether transporters were involved in the absorption of COS2-7, the bidirectional transport assays with or without phloretin (Granchi et al., 2016) (100 μmol L-1, the inhibitor of GLUT1) or quercetin (Kwon et al., 2007) (50 μmol L-1, the inhibitor of GLUT2) were performed. Meanwhile, sodium deoxycholate (Chen et al., 2019) (1 mmol L-1) was used to explore whether the transport of COS was via the paracellular pathway. Samples (50 μL) were collected from the receiver chamber every half hour for three hours and replaced with an equal volume of HBSS alone or in HBSS containing phloretin (100 μmol L-1) or quercetin (50 μmol L-1) or sodium deoxycholate (1 mmol L-1). All samples were stored at -40℃ prior to testing. The absorption and transport characteristics of COS in the Caco-2 cell model were evaluated with the Papp (Grès et al., 1998), Efflux ratio (ER) (Ma et al., 2019).
2.4 Pharmacokinetics Study of COSThe male SD rats were purchased from Qingdao Daren Fortune Animal Technology Co., Ltd. (Qingdao, China, SCXK 20190003). The relevant animal experiment design was carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Qingdao. Animals were housed under controlled environmental conditions (12 h dark-light cycle, the temperature was 23 ± 2℃, and the humidity was 55 ± 5%). Male rats (body weight 200 ± 20 g) were fasted overnight with free access to water in preparation for the experiments. COS were dissolved in sterile saline and administered to rats via the caudal vein at doses of 3.5 mg kg-1. Blood samples (0.30 mL) were collected from the orbital cavity at 0, 0.083, 0.167, 0.25, 0.50, 0.75, 1.0, 1.5, 2.0, 4.0 and 8.0 h after drug administration. COS were dissolved in sterile saline and administered to rats by oral gavage at doses of 35 mg kg-1 (Chen et al., 2005). Blood samples were collected from the orbital cavity at 0, 0.083, 0.167, 0.25, 0.50, 1.0, 1.5, 2.0, 4.0, 8.0, 12.0, 24.0 h after intragastrical administration. All samples were stored in tubes moistened with heparin. After each sampling, an equal volume of heparinized normal saline was given to rats immediately for compensation of blood withdrawal. Plasma was obtained by centrifugation at 4000 r min-1 for 10 min. Rat plasma (100 µL) was extracted with 200 µL acetonitrile containing internal standard (IS, melibiose). Then the mixtures were vortexed and centrifuged at 14000 r min-1 for 10 min. The supernatant was evaporated and the residue was reconstituted in 50 µL of acetonitrile-water (1:1, v/v). The supernatant after vortex and centrifuged was used for LC-MS/MS detection (Elendran et al., 2019).
2.5 Statistical AnalysisAll data were expressed as the mean ± standard deviation (SD). The datum was processed with Microsoft Excel 2019 edited by Microsoft (Seattle, WA, USA). The pharmacokinetics data was analyzed using Phoenix WinNon-Lin 6.4 (Pharsight, CA) by non-compartmental analysis. The image was processed by GraphPad 7.0. The peak plasma concentration (Cmax) and time to reach maximum plasma concentration (Tmax) were obtained directly from the plasma values. Half-life (t1/2) - the time required for blood concentration to fall by 50%, is a way to express rate of drug elimination. Clearance (CL) is another pharmacokinetic parameter used to describe drug elimination. The AUC is quite literally the area under a concentration versus time graph. Apparent volume of distribution (Vd) refers to the ratio of drug dose in vivo to blood drug concentration after the drug has reached dynamic equilibrium in vivo. The bioavailability (F) of COS was the ratio of AUCig ×Div to AUCiv×Dig (iv, intravenous administration; ig, intragastrical administration).
3 Results and Discussion 3.1 Transport of COS Across Caco-2 CellsCaco-2 cell monolayer has been widely used to study the mechanism of drug absorption and transport (Volpe, 2011). The Caco-2 cell membrane resistance values had been more than 300 Ω cm2 during the experiment. In this study, the Papp value of propranolol and atenolol were (18.82 ± 1.90) × 10-6cm s-1 and (0.43 ± 0.09) × 10-6cm s-1 respectively, which was the same as previous studies (Madgula et al., 2008; Manda et al., 2013). These results showed that the Caco-2 cells model established in this study was complete and reliable. Thus, the Caco-2 cells model was successfully established, which could be applied to the next transport experiment. COS are non-toxic to Caco-2 cells at concentrations below 1000 μmol L-1. Only concentrations below this limit were used in subsequent experiments.
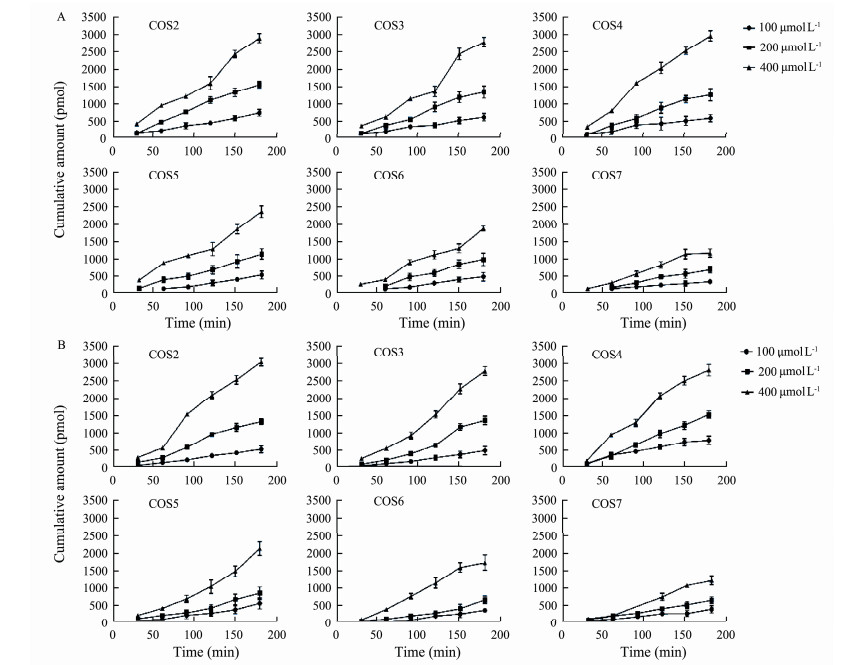
As shown in Figs.2A and 2B, regardless of the direction of transport, the amount of transported COS2-7 increased gradually with the increase of dosing concentration within 180 min. The results show that COS transport was clearly concentration-dependent and time-dependent and there was no saturation below 400 μmol L-1. In previous studies, model drugs exhibiting experimental Papp values > 3 × 10-6cm s-1 are highly permeable, whereas Papp values < 3 × 10-6cm s-1 are characteristic of low permeability model drugs (Artursson et al., 1991; Lau et al., 2004; Fossati et al., 2008). Based on these values (Table 2), it can be concluded that COS were lowly permeable.
|
Fig. 2 Cumulative amount of COS2-7 in different concentration across Caco-2 monolayers (A) from AP to BL; and (B) from BL to AP (n = 3). |
|
|
Table 2 Values of Papp and ER of COS2-7 (n = 3) |
The relationship between structure and permeability was analyzed by comparing Papp (AP-BL) values of COS2-7. As shown in Table 2, there is a decrease in the value of Papp (AP-BL) with increasing DPs. These results showed that the DPs could affect the absorption and transport of COS. No significant difference in Papp values for COS2-7 were observed in both the apical-to-basolateral and the basolateral-to-apical directions and the values of ER (Papp (BL-AP)/Papp (AP-BL)) of COS2-7 were closed to 1.0 (Elendran et al., 2019), suggesting that COS appear to be transported across the monolayers at a low rate via a direction-independent passive diffusion mechanism.
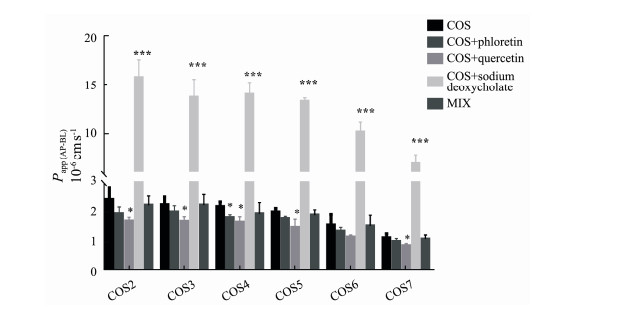
From Fig.3, the values of Papp (AP-BL) of COS decreased significantly in the presence of quercetin indicated that GLUT2 might be involved in the transport of COS. After adding phloretin, only the value of Papp (AP-BL) of COS4 reduced significantly showed that GLUT1 played almost no role in the transport of COS. When sodium deoxycholate was added, the value of Papp (AP-BL) increased significantly showed that COS might be absorbed through the paracellular pathway. There were no significant differences between the values of Papp (AP-BL) for a single COS and the mixture indicating that six oligosaccharides did not inhibit or promote each other during the transport process.
|
Fig. 3 The values of Papp (AP-BL) in the absence and presence of phloretin, quercetin, sodium deoxycholate and the mixture of COS2-7. *P < 0.05, ***P < 0.001 compared with the control (the corresponding COS, 200 μmol L-1) (n = 3). |
In contrast to our findings, Chen et al. (2019) reported that the transport of COS5 involved SGLTs mediated active transport in addition to passive diffusion, evidenced by a significantly increased transport in the presence of phlorizin. Their study, however, was carried out using FITC-COS. It is evident that the absorption between pure compounds and that of derives can be different.
3.2 Pharmacokinetics and Bioavailability of COSAfter a single oral gavage of 35 mg kg-1 and a single intravenous injection of 3.5 mg kg-1 of COS2-7, the concentrations of COS2-7 in plasma were monitored up to 24 h after intragastrical administration and 8 h after intravenous injection.
After intravenous administration of COS through the caudal vein, mean plasma concentration-time curves were presented in Fig.4A and the pharmacokinetics parameters were calculated and summarized in Table 3. These data showed Cmax (from 8.38 ± 1.53 to 2.99 ± 0.72 μg mL-1) and AUC of COS (from 2.96 ± 0.07 to 1.31 ± 0.18 h μg mL-1) to decrease as DPs increased. CL (from 1.11 ± 0.06 to 2.57 ± 0.32 L h-1) and Vd of COS (from 0.95 ± 0.21 to 1.61 ± 0.33 L h-1) increased when DPs increased.

|
Fig. 4 Mean plasma concentrations-time profiles of COS2-7 after intravenous administration at dose of 3.5 mg kg-1 (A) and oral administration at dose of 35 mg kg-1 (B) to rats (n = 3). |
|
|
Table 3 Pharmacokinetic parameters of COS2-7 after intravenous administration to rats (n = 3) |
Fig.4B presented the mean plasma concentration-time profiles of COS2-7 after intragastrical administration. The pharmacokinetics parameters of COS2-7 after intragastrical administration were summarized in Table 4. COS2-6 were detected at 5 min in plasma after intragastrical administration to rats, indicating their rapid in vivo absorption. The changes of Cmax and AUC of COS after intragastrical administration were similar to changes after intravenous administration with the increase of DPs. After intragastrical administration, the absorption of drugs in the gastrointestinal tract was largely determined by permeability (Motty et al., 2018). The bioavailability (from 7.33% to 0.99%) of COS were inversely correlated with the DP, had a similar trend to that of previous study. Fig.5 showed the correlation analysis between bioavailability and Papp (AP-BL). The correlation coefficient was 0.89, indicating that a certain correlation could be obtained to some extent between the in vitro permeability and in vivo pharmacokinetics of COS.
|
|
Table 4 Pharmacokinetic parameters of COS2-7 after oral administration to rats (n = 3) |

|
Fig. 5 Correlation between bioavailability and the values of Papp (AP-BL). |
In this study, COS could be rapidly absorbed by the Caco-2 cell model and the gastrointestinal tract through facilitation diffusion and paracellular absorption. But poor permeability leads to low oral bioavailability of COS. In addition, the DPs of COS had an effect on the pharmacokinetics and transport of COS. The pharmacokinetics of COS in vivo were a certain correlated with the permeability in vitro. These results provided meritorious information for the further investigate of COS absorption characteristics.
AcknowledgementsThis work was supported by the Shandong Provincial Natural Science Foundation, China (No. ZR2019BC025), and the Fundamental Research Funds for the Central Universities (Nos. 201912008, 201964019).
Artursson, P., and Karlsson, J.. 1991. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochemical & Biophysical Research Communications, 175(3): 880-885. (  0) 0) |
Barman, P. K., Mukherjee, R., Prusty, B. K., Suklabaidya, S., Senapati, S., and Ravindran, B.. 2016. Chitohexaose protects against acetaminophen-induced hepatotoxicity in mice. Cell Death & Disease, 7: e2224. (  0) 0) |
Chen, A. S., Taguchi, T., Okamoto, H., Danjo, K., Sakai, K., Matahira, Y., et al.. 2005. Pharmacokinetics of chitobiose and chitotriose administered intravenously or orally to rats. Biological & Pharmaceutical Bulletin, 28(3): 545-548. (  0) 0) |
Chen, P., Zhao, M., Chen, Q., Fan, L., Gao, F., and Zhao, L.. 2019. Absorption characteristics of chitobiose and chitopentaose in the human intestinal cell line Caco-2 and everted gut sacs. Journal of Agricultural and Food Chemistry Family, 67(16): 4513-4523. DOI:10.1021/acs.jafc.9b01355 (  0) 0) |
Chen, Z., Tang, J., Wang, P., Zhu, J., and Liu, Y.. 2019. GYY 4137 Attenuates sodium deoxycholate-induced intestinal barrier injury both in vitro and in vivo. Biomed Research International, 2019: 5752323. (  0) 0) |
Chung, M. J., Park, J. K., and Park, Y. I.. 2012. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. International Immunopharmacol, 12(2): 453-459. DOI:10.1016/j.intimp.2011.12.027 (  0) 0) |
Elendran, S., Muniyandy, S., Lee, W. W., and Palanisamy, U. D.. 2019. Permeability of the ellagitannin geraniin and its metabolites in a human colon adenocarcinoma Caco-2 cell culture model. Food & Function, 10(2): 602-615. (  0) 0) |
Fossati, L., Dechaume, R., Hardillier, E., Chevillon, D., Prevost, C., Bolze, S., et al.. 2008. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. International Journal of Pharmaceutics, 360(1-2): 148-55. DOI:10.1016/j.ijpharm.2008.04.034 (  0) 0) |
Granchi, C., Fortunato, S., and Minutolo, F.. 2016. Anticancer agents interacting with membrane glucose transporters. Medchemcomm, 7(9): 1716-1729. DOI:10.1039/C6MD00287K (  0) 0) |
Grès, M. C., Julian, B., Bourrié, M., Meunier, V., Roques, C., Berger, M., et al.. 1998. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: Comparison with the parental Caco-2 cell line. Pharmaceutical Research, 15(5): 726-733. DOI:10.1023/A:1011919003030 (  0) 0) |
Hidalgo, I. J., Raub, T. J., and Borchardt, R. T.. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology, 96(3): 736-749. DOI:10.1016/0016-5085(89)90897-4 (  0) 0) |
Huang, L., Chen, J., Cao, P., Pan, H., Ding, C., Xiao, T., et al.. 2015. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Marine Drugs, 13(5): 2732-2756. DOI:10.3390/md13052732 (  0) 0) |
Kwon, O., Eck, P., Chen, S., Corpe, C. P., Lee, J. H., Kruhlak, M., et al.. 2007. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB Journal, 21(2): 366-377. DOI:10.1096/fj.06-6620com (  0) 0) |
Lau, Y. Y., Chen, Y. H., Liu, T. T., Li, C., and Cheng, K. C.. 2004. Evaluation of a novel in vitro Caco-2 hepatocyte hybrid system for predicting in vivo oral bioavailability. Drug Metabolism & Disposition, 32(9): 937-942. (  0) 0) |
Li, S., Wang, Y., Jiang, T., Wang, H., Yang, S., and Lv, Z.. 2016. Absorption and transport of sea cucumber saponins from Apostichopus japonicus. Marine Drugs, 14(6): 114-121. DOI:10.3390/md14060114 (  0) 0) |
Li, X., Zhou, C., Chen, X., and Zhao, M.. 2014. Subcellular localization of chitosan oligosaccharides in living cells. Chinese Science Bulletin, 59(20): 2449-2454. DOI:10.1007/s11434-014-0304-8 (  0) 0) |
Ma, Z., Guo, R., Elango, J., Bao, B., and Wu, W.. 2019. Evaluation of marine diindolinonepyrane in vitro and in vivo: Permeability characterization in Caco-2 cells monolayer and pharmacokinetic properties in beagle dogs. Marine Drugs, 17(12): 651-665. DOI:10.3390/md17120651 (  0) 0) |
Madgula, V. L., Avula, B., Choi, Y. W., Pullela, S. V., Khan, I. A., Walker, L. A., et al.. 2008. Transport of Schisandra chinensis extract and its biologically-active constituents across Caco-2 cell monolayers - An in-vitro model of intestinal transport. Journal of Pharmacy and Pharmacology, 60(3): 363-370. (  0) 0) |
Manda, V. K., Avula, B., Ali, Z., Wong, Y. H., Smillie, T. J., Khan, I. A., et al.. 2013. Characterization of in vitro ADME properties of diosgenin and dioscin from Dioscorea villosa. Planta Medica, 79(15): 1421-1428. DOI:10.1055/s-0033-1350699 (  0) 0) |
Motty, S.. 2018. Drug-like properties: Concepts, structure design and methods from ADME to toxicity optimization. Chemistry in Australia, 7: 28-29. (  0) 0) |
Muanprasat, C., and Chatsudthipong, V.. 2017. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacology & Therapeutics, 170: 80-97. (  0) 0) |
Ngo, D. N., Kim, M. M., and Kim, S. K.. 2008. Chitin oligosaccharides inhibit oxidative stress in live cells. Carbohydrate Polymers, 74(2): 228-234. DOI:10.1016/j.carbpol.2008.02.005 (  0) 0) |
Pangestuti, R., Bak, S. S., and Kim, S. K.. 2011. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. International Journal of Biological Macromolecules, 49(4): 599-606. DOI:10.1016/j.ijbiomac.2011.06.014 (  0) 0) |
Park, J. K., Chung, M. J., Choi, H. N., and Park, Y. I.. 2011. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. International Journal of Molecular Sciences, 12(1): 266-277. DOI:10.3390/ijms12010266 (  0) 0) |
Rahman, M. H., Hjeljord, L. G., Aam, B. B., Sørlie, M., and Tronsmo, A.. 2014. Antifungal effect of chito-oligosaccharides with different degrees of polymerization. European Journal of Plant Pathology, 141(1): 147-158. (  0) 0) |
Volpe, D. A.. 2011. Drug-permeability and transporter assays in Caco-2 and MDCK cell lines. Future Medicinal Chemistry, 3(16): 2063-2077. DOI:10.4155/fmc.11.149 (  0) 0) |
Zhang, P., Liu, W., Peng, Y., Han, B., and Yang, Y.. 2014. Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. International Immunopharmacol, 23(1): 254-261. DOI:10.1016/j.intimp.2014.09.007 (  0) 0) |
Zheng, J., Yuan, X., Cheng, G., Jiao, S., Feng, C., Zhao, X., et al.. 2018. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydrate Polymers, 190: 77-86. DOI:10.1016/j.carbpol.2018.02.058 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


