Chinese seabass (Lateolabrax maculatus) can adapt to the salinity of 0 – 35 and is widely distributed along the coast of China. The rapid growth and delectable meat make it highly favored by farmers and consumers (Zhang et al., 2017). However, traditional aquaculture method of Chinese seabass can create a stressful environment, decrease their ability to resist pathogens, cause water quality to deteriorate, lead to the outbreak of various diseases, and ultimately result in a drastic decrease in the yield (Tian et al., 2019). Vibrio anguillarum, one of the main pathogens in the aquaculture, can infect aquaculture animals through their gills, skin, intestine and lateral line, leading to hemorrhagic septicemia and vibriosis (Frans et al., 2011; Hou et al., 2021). The pathogenicity of V. anguillarum to L. maculatus was different under different conditions, and the pathogenicity of V. anguillarum decreased slightly under multiple passages at room temperature. A strain of V. anguillarum W-1, which was isolated from the infected L. japonicus fingerlings, demonstrated strong pathogenicity in the L. japonicus, causing redness and ulcers on their fins, an enlarged spleen, intestinal congestion, and other signs of infection (Guo et al., 2011; Wang et al., 2021). A recent study discovered that the intestine of fish could serve as a gateway for V. anguillarum, suggesting that V. anguillarum can adhere and proliferate in the intestine. This highlights the vital role of the intestine in maintaining fish health by aiding the immune system (Engelsen et al., 2008).
The gut microbiota play crucial roles in development, growth and immune responses in different organisms (Jin et al., 2017). Several researches have demonstrated that gut microbiota and the host can exchange metabolites that can influence host health (Gentile and Weir, 2018; Li et al., 2018). Infections by pathogenic microorganisms can disturb the balance of gut microbiota, leading to health issues and increasing susceptibility to diseases (Jin et al., 2023). For example, the composition and diversity of gut microbiota decreased markedly in Chinese mitten crab (Eriocheir sinensis) after hepatopancreas necrosis disease (HPND) infection (Zhan et al., 2022). In addition, the relative abundance of Proteobacteria and Firmicutes phyla changed in the gut microbiota of Ctenopharyngodon idella after A. hydrophila infection (Zhu et al., 2023). V. anguillarum infection severely disrupts the composition and interspecific interactions of the intestinal bacterial community in ayu (Plecoglossus altivelis), thereby altering the gut microbiota-mediated function and inducing host immune responses (Nie et al., 2017).
Pathogenic bacteria can penetrate the epithelial cells of intestine via endocytosis. Subsequently, the bacteria access to the bloodstream and causing the internal organs infection such as the spleen and kidneys (Engelsen et al., 2008). Blood homeostasis is crucial for understanding the health status of fish (Liu et al., 2020). Recent studies found that the AKP activity in serum significantly decreased after A. hydrophila infection in common carp. After being exposed to paraquat, the levels of AST and ALT increased, while the AKP activity notably decreased in serum of common carp. The concentrations of ALT, ALB, GLU and TP changed significantly in serum of Chinese seabass infected with V. harveyi (Ma et al., 2018; Chen et al., 2020; Nawaz et al., 2023). In general, the blood biochemical indexes are important parameters for evaluating the health status of fish.
V. anguillarum utilizes flagellum-mediated motility to infect and form colonies on the surface of fish intestine, resulting in the inflammation response activation through TLR5 signaling pathway (Tsujita et al., 2004; Hung et al., 2016; Senevirathne et al., 2020). In the Oncorhynchus mykiss, the expression of IL-1β and TNFα increased, and the signaling pathways of cytokine-cytokine receptor interaction and focal adhesion were activated after V. anguillarum infection. After infected with A. hydrophila, the TLR, NOD and insulin signaling pathway were activated in the endoplasmic reticulum of Ctenopharyngodon idellus (Song et al., 2017). With poly(I: C) and LPS challenge, the hematopoietic cell lineage, MAPK signaling pathway and cytokine-cytokine receptor interaction were activated in the Micropterus salmoides (Qi et al., 2023). To explore the immune response in L. maculatus after V. anguillarum infection, the blood homeostasis, intestinal microbiota and transcriptomic profiling of L. maculatus were investigated in this study. It was found that the blood homeostasis and intestinal microbiota imbalance and immune and pro-flammatory responses was activated in the L. maculatus when infected with V. anguillarum.
2 Materials and Methods 2.1 Experimental FishThe Chinese seabass (L. maculatus) was collected from a farm in Dongying City, Shandong Province, China. The animals were acclimated in a flow-through system with 12 h/12 h (light/dark) cycle for one week, and the water temperature was 25℃ ± 2℃, salinity was 15 – 20.
2.2 Bacterial Challenge and Sample CollectionThe V. anguillarum was isolated from diseased Chinese seabass and stored at −80℃ in our laboratory. A total of 56 L. maculatus (20 g ± 1 g) were divided into two main groups: Va group (V. anguillarum stimulated) and PBS group (control). Two hundred (200) μL of sterile PBS (PBS group) and V. anguillarum (1 × 108 CFU mL−1, Va group) were injected into the abdominal cavity of L. maculatus, respectively. Subsequently, the blood, intestines, spleen and kidney were collected at 6, 12, 24 and 48 hpi (hours post injured) and frozen immediately by liquid nitrogen. The samples were transferred to −80℃ freezer to store. Three replicate samples were collected from PBS group or Va group at the time set above, respectively. Prior to dissection, 100 mg L−1 tricaine methane sulfonate (MS-222) was used for euthanizing animals.
2.3 Blood Enzyms Activities AnalysesThe sterile disposable syringe was used to extract the blood sample through inserting into the caudal vein of immobilizing L. maculatus. The collected blood samples were analyzed using Bio-Tek testing methods and the automatic animal blood analyzer TEK-Ⅱ mini to determine the levels of total protein (TP), blood glucose (BG), albumin (ALB), total bilirubin (TBIL), superoxide dismutase (SOD), alkaline phosphatase (AKP), glutamic pyruvic transaminase (ALT) and glutamic oxalacetic transaminase (AST). The experiment was performed in triplicates.
2.4 Extracting Genomic DNA and Amplifying 16S rDNA Through PCRThe intestine genome DNA was extracted after V. anguillarum challenged for 12 and 24 hpi using the commercial kit (Tiangen Biotech, China) following the manufacturer's instructions. The V3-V4 region of the bacterial 16S rRNA gene were amplified with primer pairs 338F (5'-ACTCCT ACGGGAGGCAGCA-3') and 806R (5'-GGACTACHVG GGTWTCTAAT-3'). Subsequently, the PCR products were validated by gel electrophoresis and purified through DNA purification kit (Omega, USA). The purified DNA was sequenced using paired-end sequencing method with 2 × 250 bp reads length on the Illumina Novaseq 6000 platform by Biomarker Technologies Co., Ltd. (Beijing, China).
2.5 Bioinformatic AnalysisSequences that had more than 97% similarity were grouped into one operational taxonomic unit (OTU) using USEARCH 10.0 software. Classify and annotate OTUs using the naive Bayes classifier of QIIME 2 with the SILVA database, applying a 70% confidence threshold. Alpha diversity and species diversity were evaluated through beta diversity analysis using PCoA (Principal Coordinates Analysis) in QIIME 2. The data were analyzed on the online platform BMK Cloud.
2.6 Extraction of Total RNA and Library PreparationThe total RNA of the spleen and kidney was extracted using the commercial kit (Tiangen, China) following the manufacturer's instructions. The purity and concentration of RNA were assessed using NanoDrop 2000 (Thermo Fisher Scientific, USA). The RNA integrity was evaluated using the RNA Nano 6000 Assay Kit on the Agilent Bioanalyzer 2100 system (Agilent Technologies, USA). Subsequently, sequencing libraries were prepared with the Hieff NGS Ultima Dual-mode mRNA Library Prep Kit for Illumina (Yeasen Biotechnology, China) according to the manufacturer's protocol, with index codes assigned to each sample for tracking purposes.
2.7 Quality Control and Reads Mapping to the Reference GenomeThe clean data were obtained by removing adapters, lowquality reads and reads with poly-N sequences from the raw data. Additionally, metrics like sequence duplication level, GC-content, Q30 and Q20, were computed for the clean data. The clean reads were then aligned to the L. maculatus reference genome sequence (available at http://gigadb.org/dataset/view/id/100458) using the Hisat 2 tools.
2.8 Differentially Expressed Genes (DEGs) and KEGG Pathway Enrichment AnalysisDifferentially expressed genes (DEGs) from spleen and kidney of PBS and Va groups were analyzed at 12 and 24 hpi (designed as PBS-S-12, Va-S-12, PBS-K-12, Va-K-12, PBS-S-24, Va-S-24, PBS-K-24, and Va-K-24) using the DESeq software. Herein, the |Fold change (FC)| ≥ 1.5 and P value ≤ 0.05 was used for detecting the DEGs. Functional annotations were carried out using the GO database, while KEGG enrichment analysis was performed by comparing sequences with public databases. The function and pathway analyses of differentially expressed genes (DEGs) were described through GO database and KEGG database, respectively.
2.9 Validation of DEGs with Quantitative Real-Time PCR (qRT-PCR)To validate the accuracy of RNA-seq data, the DEGs of specific genes involved in metabolism and immune response were picked out for qRT-PCR analysis. Gene-specific primers were designed using Beacon Designer 8.0 (Table 1), with 18S gene serving as the internal reference gene. Then the Transcript Uni One-Step method was utilized to synthesize first-stranded cDNA and remove gDNA. According to the instructions, 1000 ng of RNA was reversely transcribed into cDNA. For the qRT-PCR reaction, 10 μL SYBR Green I fluorescent dye, 1 μL cDNA template, 0.4 μL primers, and 8.2 μL double distilled water (ddH2O) were mixed. The qRT-PCR amplification process involved an initial denaturation at 95℃ for 30 s, followed by 40 cycles of denaturation at 95℃ for 5 s, annealing at 60℃ for 10 s, and extension at 72℃ for 10 s. The relative expression levels of target genes were calculated and analyzed using the 2−ΔΔCT method, with experimental data presented as means ± standard error (SE).
|
|
Table 1 The specific primers used for qRT-PCR |
All experiments were conducted in triplicate and bacterial relative abundance was compared using one-way analysis of variance (ANOVA). The data were reported as means ±SE.
3 Results 3.1 Blood Homeostasis of L. maculatus Was Unbalance After V. anguillarum ChallengedThe levels of TP, BG, ALB, TBIL, SOD, AKP, ALT, and AST of blood were measured in Chinese seabass following V. anguillarum challenged. The results indicated that the level of ALB decreased at 6 and 24 hpi, while the levels of TP and BG decreased at 24 and 48 hpi following V. anguillarum challenge (Figs.1A – C). However, the level of SOD increased at 24 hpi, the level of TBIL increased at 6 and 24 hpi, the activity of ALT increased at 24 and 48 hpi following V. anguillarum challenge (Figs.1D – F). In addition, the activities of AKP and AST did not change significantly following V. anguillarum challenge (Figs.1G and H).
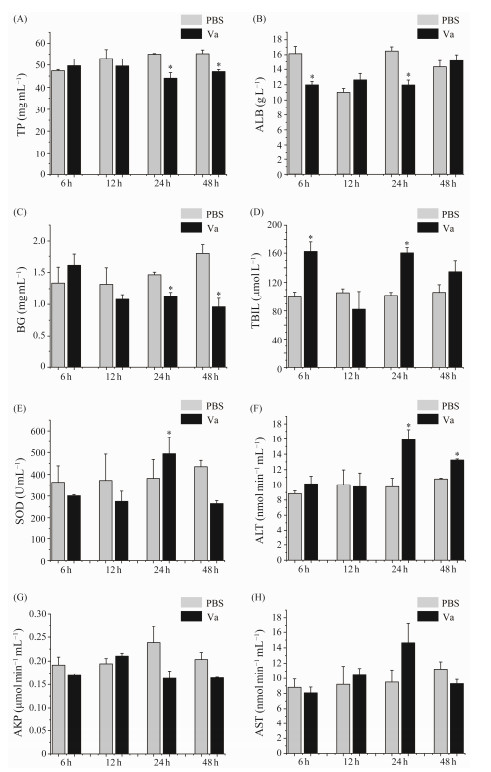
|
Fig. 1 Blood homeostasis of L. maculatus was unbalance after V. anguillarum challenge. (A), TP (total protein, g L−1); (B), ALB (albumin, g L−1); (C), BG (blood glucose, mmol L−1); (D), TBIL (total bilirubin, μmol L−1); (E), SOD (superoxide dismutase, U mg−1 protein); (F), ALT (alanine aminotransferase, nmol min−1 mL−1); (G), AKP (alkaline phosphatase, μmol min−1 mL−1); (H), AST (aspartate aminotransferase, nmol min−1 mL−1) in the blood of V. anguillarum infected with L. maculatus at 6 h, 12 h, 24 h, and 48 h. Each column represents the mean ± SE (n = 3); PBS group was as control; Va group was V. anguillarum infection group. * indicates significance at P < 0.05. |
Firstly, the composition of dominant microbes of gut microbiome in L. maculatus in phyla level were identified after V. anguillarum challenge. The results showed that the dominant phyla of gut microbiome were phylum Proteobacteria (60.68% and 41.75%) and phylum Firmicutes (23.47% and 43.06%) in PBS group at 12 and 24 hpi, respectively, while the dominant phyla of gut microbiome were phylum Proteobacteria (58.56% and 75.38%) and phylum Firmicutes (35.13% and 15.29%) in Va group at 12 and 24 hpi, respectively (Fig.2A). Interestingly, the amount of phylum Proteobacteria of gut microbiome of L. maculatus increased, whereas the amount of phylum Firmicutes of gut microbiome of L. maculatus decreased after V. anguillarum challenge (Figs.2C and D). Additionally, the abundance of Vibrio at the genus level increased after infected by V. anguillarum (Figs.2B and E).
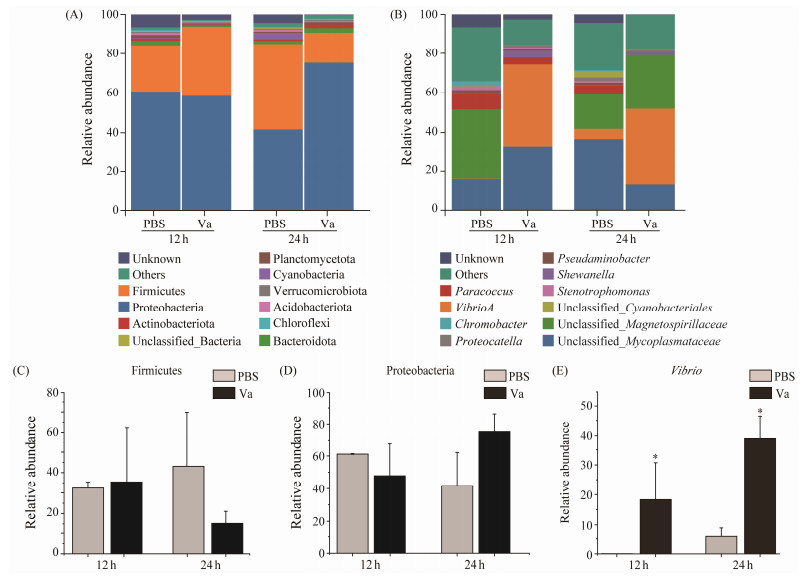
|
Fig. 2 Intestinal microbiota structure variation in L. maculatus after V. anguillarum challenge. (A), the relative abundance of intestinal microbiota at the phylum level; (B), the relative abundance of intestinal microbiota at the genus level; (C), the relative abundance of phylum Firmicutes; (D), the relative abundance of phylum Proteobacteria; (E), the relative abundance of genus Vibrio after V. anguillarum challenge. * indicates significance at P < 0.05. |
Secondly, the diversity analysis of gut microbiome was performed to detect the difference of gut microbiome after V. anguillarum challenge. The results indicated that the indexes of ACE, Chao1, Simpson and Shannon showed no significant difference after V. anguillarum challenge (Table 2). The result of PCoA analysis showed that though the diversity of gut microbiota overlapped at 12 hpi after V. anguillarum infection, the microbial community composition was significantly different after V. anguillarum infection at 24 hpi, suggesting that the composition and diversity of gut microbiota of L. maculatus can be affected by V. anguillarum (Fig.3).
|
|
Table 2 Influence of intestinal microbiota alpha diversity |
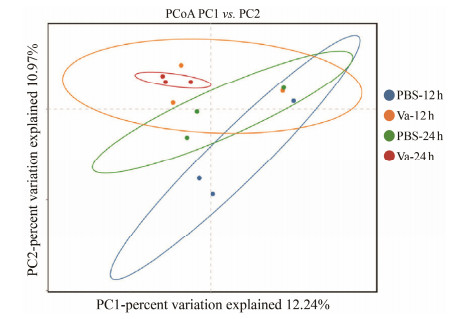
|
Fig. 3 PCoA analysis. |
From Table 2, total of 163.18 GB clean data were obtained. The quality of Q20 was 98.11% ± 0.07%, Q30 was 94.73% ± 0.17%, GC content was 48.38% ± 0.61% and mapping rate was 81.35% ± 0.33% (Table 2).
The results of DEGs analysis indicated that a total of 1026 genes had different expression profiles in spleen of L. maculatus at 12 hpi after V. anguillarum challenge, including 677 up-regulated genes and 349 down-regulated genes. In addition, a total of 1055 genes had different expression profiles in spleen of L. maculatus at 24 hpi after V. anguillarum challenge, including 543 up-regulated genes and 512 downregulated genes (Figs.4A and B). In kidney, after V. anguillarum infection for 12 h, there were 505 DEGs, including 54 down-regulated genes and 451 up-regulated genes. After V. anguillarum infection for 24 h, there were 356 DEGs, including 129 down-regulated genes and 227 up-regulated genes (Figs.4C and D). Interestingly, the number of DEGs increased in spleen while decreased in kidney after V. anguillarum challenge.
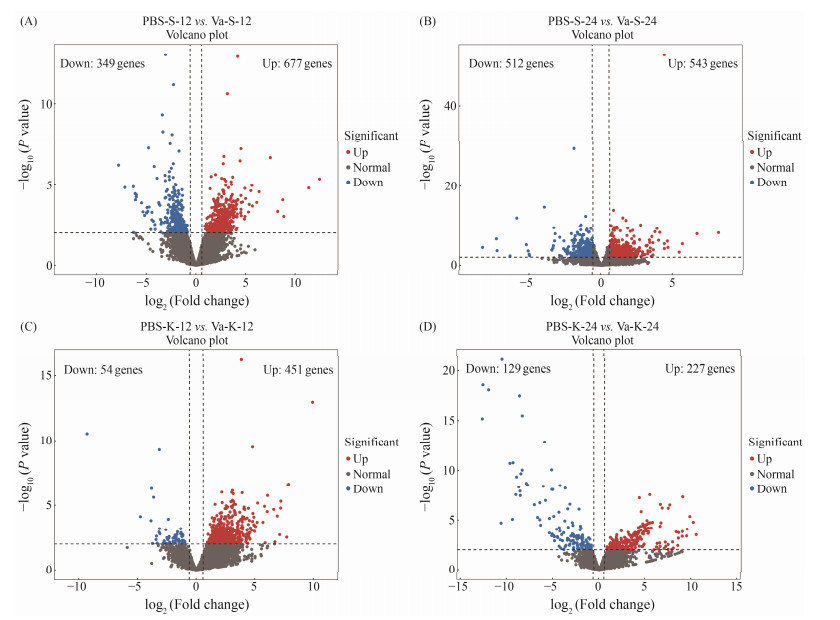
|
Fig. 4 Volcano plot of DEGs distribution trends. (A), PBS-S-12 vs. Va-S-12; (B), PBS-S-24 vs. Va-S-24; (C), PBS-K-12 vs. Va-K-12; (D), PBS-K-24 vs. Va-K-24. |
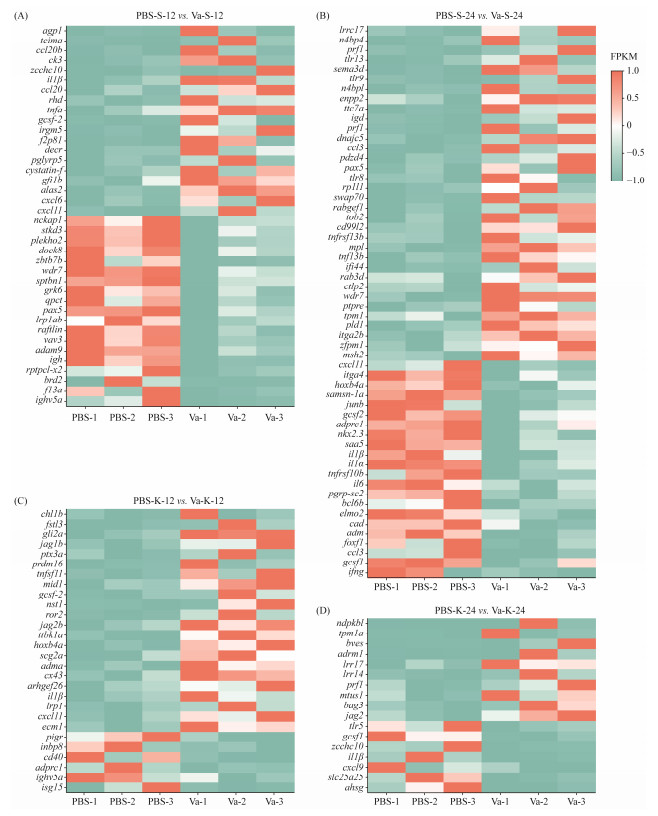
Some DEGs related to immunity such as TLR5, TLR8, TLR9, IL-1β, CCL3, IFNγ, CXCL11 and TNFα were analyzed after V. anguillarum challenge in L. maculatus (Fig.5). Specifically, the mRNA level of IL-1β was up-regulated at 12 hpi in the spleen and kidney, while the mRNA level of CXCL11 and TNFα was up-regulated at 12 hpi in the spleen. Interestingly, the mRNA level of IL-1β was down-regulated in the kidney and spleen at 24 hpi and the mRNA levels of CXCL11 and TNFα were down-regulated in the spleen at 24 hpi. Additionally, TLR8 and TLR9 were upregulated in the spleen at 24 hpi, CCL3 and IFNγ was downregulated in the spleen at 24 hpi and TLR5 was down-regulated at 24 hpi in the kidney.
|
Fig. 5 Analysis of heat map of the immune-related DEGs in the L. maculatus after V. anguillarum challenge. (A), PBS-S-12 vs. Va-S-12; (B), PBS-S-24 vs. Va-S-24; (C), PBS-K-12 vs. Va-K-12; (D), PBS-K-24 vs. Va-K-24. |
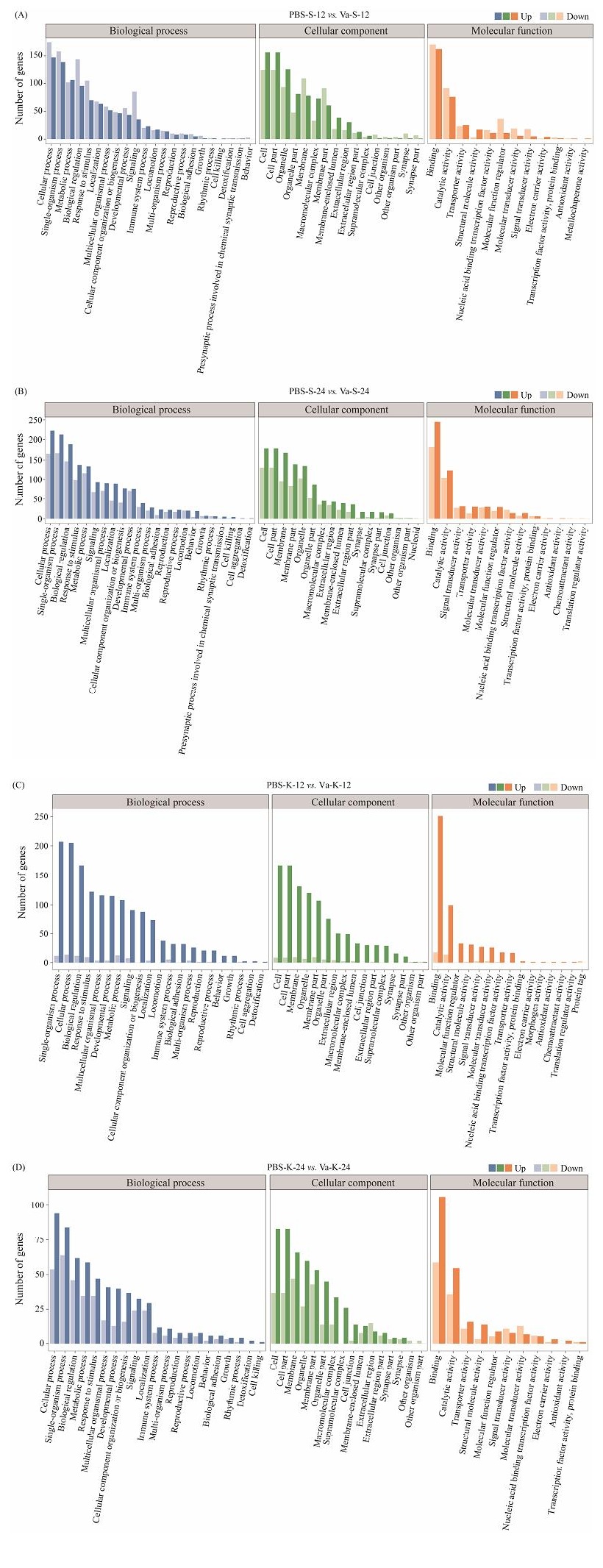
The GO annotation distribution indicated that the differentially expressed genes (DEGs) were classified into three main groups: CC (cellular component), BP (biological process) and MF (molecular function). In terms of biological processes, a significant proportion of DEGs were enriched in cellular processes and single-organism processes. Within the cellular component category, the majority of DEGs exhibited enrichment in cell and cell part terms. In the molecular function category, most DEGs were enriched during cell binding and catalytic activity (Fig.6).
|
Fig. 6 Gene function classification in GO. (A), PBS-S-12 vs. Va-S-12; (B), PBS-S-24 vs. Va-S-24. |
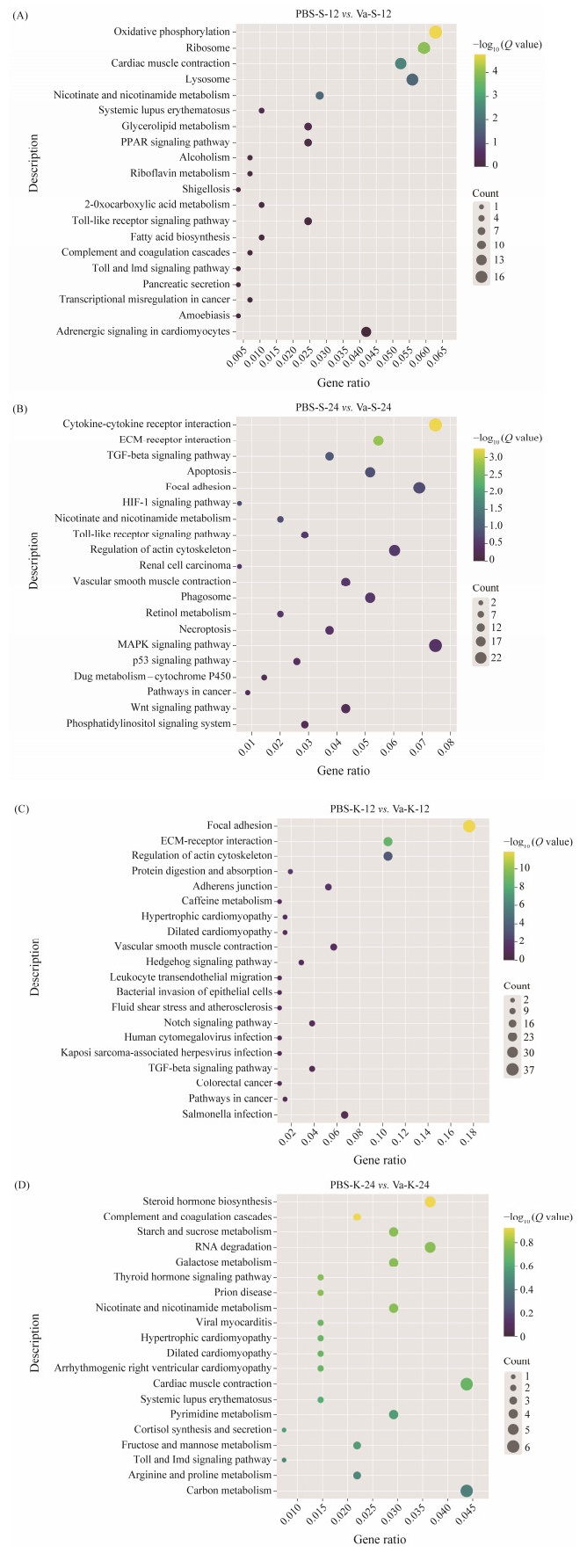
The KEGG pathway enrichment analysis results indicated that the DEGs were enriched to 144 and 144 pathways in spleen following V. anguillarum challenge at 12 and 24 hpi, respectively. And the DEGs were enriched to 111 and 139 pathways in kidney at 12 and 24 hpi, respectively. The top 20 KEGG enrichment pathways in spleen and kidney were shown in Fig.7. The KEGG enrichment pathways of DEGs were mostly related to immune response, proinflammatory, cellular processes and metabolism, such as cytokinecytokine receptor interaction, toll-like receptor signaling pathway, NOD-like receptor signaling pathway, MAPK signaling pathway, focal adhesion, carbon metabolism and purine metabolism.
|
Fig. 7 Distribution of KEGG pathway enrichment. (A), PBS-S-12 vs. Va-S-12; (B), PBS-S-24 vs. Va-S-24. |
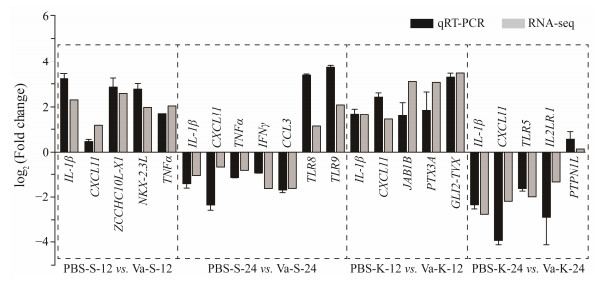
The qRT-PCR was employed to randomly assess the expression levels of immune response genes across four groups. The results of the qRT-PCR analysis demonstrated a strong correlation with the RNA-seq findings (Pearson correlation coefficient: r = 0.897, P < 0.001; Spearman's rank correlation: ρ = 0.801, P < 0.001), suggesting that both approaches yielded consistent trends (Fig.8).
|
Fig. 8 Validation of DEGs with qRT-PCR. |
The changes of blood homeostasis, expression of immune-related genes and gut microbiota can reflect the physiological state of aquaculture animals after pathogenic bacteria infection (Adams et al., 1993; Fan and Pedersen, 2021). Fish have a greater reliance on the non-specific immune system than other higher vertebrates to protect the host from pathogens infection (Chen et al., 2017). The enzymes' activities in the blood can be used to assess the health status of the fish (Adams et al., 1993). Recent studies showed that the activity of SOD increased in the blood of Cyprinus carpio following A. hydrophila challenge (Song et al., 2017). The ALB content in the blood of L. maculatus decreased after V. harveyi infection and the contents of TP and ALB in blood decreased in the Salmo salar after vibriosis infection (Waagbø et al., 1988; Mao et al., 2020). ALT is a biomarker used to evaluate liver damage (Banaee, 2013). In addition, the level of ALT increased in spotted seabass after V. harveyi infection (Mao et al., 2020). In this research, we found that the expression levels of ALB, TP and BG decreased, while the expression levels of SOD, TBIL and ALT increased after V. anguillarum challenge, suggesting that it can affect the blood homeostasis and induce liver damage in L. maculatus after V. anguillarum challenge.
The gut microbiota plays a crucial role in host metabolism, development, immune defense, and disease susceptibility, encompassing both infectious and noninfectious conditions (Nayak, 2010). Plenty of investigations showed that Vibrio infections in aquatic animals caused significant changes in the intestinal microflora, reduced the immune response of host and increased bacterial presence, thereby leading to the occurrence of diseases (Rungrassamee et al., 2016; He et al., 2017). The composition of gut microbiota and their inter-species interactions can be affected in the Siniperca robust after V. anguillarum infection, altering the functional and host immune response mediated through the intestinal microbiota (Nie et al., 2017). Therefore, the host immune response caused by pathogenic bacteria infection may alter the gut microbiota composition and microbiota-mediated functions.
TLRs (Toll-like receptors) signaling pathway is essential for the immune response in organisms, involving both innate and adaptive immunity (Muzio et al., 1997). Recent studies indicate that the expression of TLRs was induced by bacterial infection. TLR5 can recognize flagellin protein to activate the TLR5 signaling pathway and induce inflammation response in mammals and zebrafish. For example, the ectodomain of TLR5 in Ctenopharyngodon idella is essential for IFN signaling triggered by dsRNA, connecting viral and bacterial immune responses (Tsujita et al., 2004; Kanwal et al., 2014; Liao et al., 2022). The expression of Danio rerio TLR8 increased in the blood and lymph following Mycobacterium marinum infection (Meijer et al., 2004). TLR8 can induce the production of other TLRs to modulate the immune response triggered by bacteria (Cervantes et al., 2012). Interestingly, CiTLR8 engages in antiviral innate immune defense in Ctenopharyngodon idella (Chen et al., 2013). Furthermore, the mRNA level of TLR9 increased in the blood, kidney, gills and spleen of Paralichthys olivaceu following Edwardsiella tarda challenge (Takano et al., 2007). The expression levels of TLR9A and TLR9B increased in the blood and spleen of Larimichthys crocea infected with Vibrio parahaemolyticus (Yao et al., 2008). Similar to previous studies, our results indicated that the expressions of TLR5, TLR8 and TLR9 increased in the spleen of L. maculatus following V. anguillarum challenge, suggesting that TLRs of L. maculatus can recognize the flagellin protein of V. anguillarum to induce innate immune response.
Bacterial infection can induce TLR signaling pathway to activate the NF-κB pathway, and then release to various cytokines, such as IL-1β and TNFα, for triggering immune and inflammatory responses (Bogdan et al., 2000; McGuinness, 2005; Grivennikov et al., 2010). In Cynoglossus semilaevis, the expression of IL-1β gene was significantly upregulated in the spleen and head kidney following V. anguillarum challenge (Yu et al., 2012). They play important roles in inflammatory reactions, pathogen infection, pathogen clearance, cell and organ development (Tu et al., 2016). CCL3 is an inflammatory chemokine, which increased during bacterial infection or damage to promote the migration of inflammatory white blood cells and regulate immune response (Palomino and Marti, 2015). Recent study found that CCL3 can mediate the transportation of immune cells in inflammation although the mechanism remains unclear (Jin et al., 2003). Furthermore, high expression levels of specific CC chemokines are linked to susceptibility to diseases and intolerance to hypoxia (Fu et al., 2017a, 2017b). Activated T cells and natural killer cells produce IFN-γ, which can activate macrophages, facilitate antigen presentation, and coordinate interactions between leukocytes and endothelial cells to regulate theimmune cells to fight against pathogens (Schroder et al., 2004). In human, the expression of CXCL11 is primarily in the thymic tissue, and can be induced by IFN-γ (Mold, 1999). However, the expression pattern of CXCL11 in fish remains unclear. Our study demonstrated that immune genes, such as GCSF2, TNFα, CXCL11, IL-1β and PEX12 in the spleen and IL-1β, LSP1a, RO60, TNS1a, and CX43 in the kidney, were upregulated at 12 hpi but downregulated at 24 hpi following V. anguillarum challenge. We speculate that these genes play a crucial role in the resistance of the Chinese seabass to V. anguillarum infection and exhibit an early positive immune response.
Recent studies have shown that MAPK and focal adhesion signaling pathways are linked to inflammation, cell proliferation, heart and liver development, angiogenesis, and neurogenesis (Lu et al., 2011; Layden et al., 2016). MAPK signaling pathway can be activated by various inflammatory stimuli and plays an important regulatory role in the occurrence and development of inflammation (Pizarro-Cerda et al., 1998). Extracellular stimuli activate MAPK by a multi-step kinase cascade acting on cells. Activated MAPK regulates various physiological processes of cells through the phosphorylation of transcription factors, cytoskeletal proteins, enzymes, and other substrates (Lu et al., 2011; Layden et al., 2016). Focal adhesion is a specialized structure that forms at the interface between cells and the extracellular matrix (Petit and Thiery, 2000). The components of focal adhesion are involved in connecting the membrane receptors to the actin cytoskeleton, coordinating cell structure and movement. Pathogens often target and manipulate host cells by interacting with the cytoskeleton, a crucial structural component (Murphy and Brinkworth, 2021). In this study, we found that lots of DEGs were enriched in MAPK and focal adhesion signaling pathway, suggesting that the MAPK and focal adhesion signaling pathway can rapidly and effectively activate the innate immune and inflammatory responses to combat V. anguillarum infection.
5 ConclusionsThe biological changes caused by V. anguillarum infection in L. maculatus were investigated in this study. We observed the shifts in blood homeostasis, alterations in the L. maculatus were microbiome composition and diversity, and identified differentially expressed genes. The DEGs are mainly enriched in biological functions, cellular processes, and biological adhesion. Activation of key pathways like the Toll-like receptor signaling and cytokine-cytokine receptor interactions is essential. These discoveries offer valuable insights for investigating disease mechanisms, progression, and host health protection.
AcknowledgementsThis study received funding from the Natural Science Foundation of Shandong Province (No. ZR2023MC141) and the Innovation and Entrepreneurship Training Program for College Students (No. 202210435003). Additionally, financial support was also provided by the 'First Class Fishery Discipline' Program and the Special Talent Program 'One Thing One Decision (Yishi Yiyi)' in Shandong Province, China. The funders were not involved in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author Contributions
Jiagen Ming and Xiaojun Song designed, conducted, and analyzed the experiments, contributed to the study design, and prepared the manuscript. Dongyan Su, Xiufeng Han, Xiangyang Wu, and Bo Li were involved in the study design and result discussions. Jiagen Ming, Jiabo Tan, and Xiaojun Song conceived the study, coordinated the experiments, and assisted in manuscript writing. All authors contributed to and approved the final version of the article.
Data Availability
The authors will make the raw data supporting the conclusions of this article available without any restrictions.
Declarations
Ethics Approval and Consent to Participate
All experimental procedures involving animals were conducted in accordance with the guidelines established by the School of Marine Science and Engineering, Qingdao Agricultural University (QAU-SMSE-202201), adhering to national standards for the care and use of laboratory animals.
Consent for Publication
All authors agree to publish the paper in the Journal of China Ocean University.
Conflict of Interests
The authors declare that they have no conflict of interests.
Adams, S. M., Brown, A. M., and Goede, R. W., 1993. A quantitative health assessment index for rapid evaluation of fish condition in the field. Transactions of the American Fisheries Society, 122(1): 63-73. DOI:10.1577/1548-8659(1993)122<0063:AQHAIF>2.3.CO;2 (  0) 0) |
Banaee, M., 2013. Physiological dysfunction in fish after insecticides exposure. Insecticides – Development of Safer and More Effective Technologies, 30: 103-143. DOI:10.5772/54742 (  0) 0) |
Bogdan, C., Röllinghoff, M., and Diefenbach, A., 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Current Opinion in Immunology, 12(1): 64-76. DOI:10.1016/S0952-7915(99)00052-7 (  0) 0) |
Cervantes, J. L., Weinerman, B., Basole, C., and Salazar, J. C., 2012. TLR8: The forgotten relative revindicated. Cellular & Molecular Immunology, 9(6): 434-438. DOI:10.1038/cmi.2012.38 (  0) 0) |
Chen, J., Liu, N., Zhang, H., Zhao, Y., and Cao, X., 2020. The effects of Aeromonas hydrophila infection on oxidative stress, nonspecific immunity, autophagy, and apoptosis in the common carp. Developmental & Comparative Immunology, 105: 103587. DOI:10.1016/j.dci.2019.103587 (  0) 0) |
Chen, X., Wang, Q., Yang, C., Rao, Y., Li, Q., Wan, Q., et al., 2013. Identification, expression profiling of a grass carp TLR8 and its inhibition leading to the resistance to reovirus in CIK cells. Developmental and Comparative Immunology, 41(1): 82-93. DOI:10.1016/j.dci.2013.04.015 (  0) 0) |
Chen, Y., Huang, X., Wang, J., and Li, C., 2017. Effect of pure microcystin-LR on activity and transcript level of immunerelated enzymes in the white shrimp (Litopenaeus vannamei). Ecotoxicology, 26: 702-710. DOI:10.1007/s10646-017-1802-7 (  0) 0) |
Engelsen, A. R., Sandlund, N., Fiksdal, I. U., and Bergh, O., 2008. Immunohistochemistry of Atlantic cod larvae Gadus morhua experimentally challenged with Vibrio anguillarum. Diseases of Aquatic Organisms, 80(1): 13-20. DOI:10.3354/dao01926 (  0) 0) |
Fan, Y., and Pedersen, O., 2021. Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology, 19(1): 55-71. DOI:10.1038/s41579-020-0433-9 (  0) 0) |
Frans, I., Michiels, C. W., Bossier, P., Willems, K. A., Lievens, B., and Rediers, H., 2011. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. Journal of Fish Diseases, 34(9): 643-661. DOI:10.1111/j.1365-2761.2011.01279.x (  0) 0) |
Fu, Q., Yang, Y., Li, C., Zeng, Q., Zhou, T., Li, N., et al., 2017a. The chemokinome superfamily: Ⅱ. The 64 CC chemokines in channel catfish and their involvement in disease and hypoxia responses. Developmental & Comparative Immunology, 73: 97-108. DOI:10.1016/j.dci.2017.03.012 (  0) 0) |
Fu, Q., Zeng, Q., Li, Y., Yang, Y., Li, C., Liu, S., et al., 2017b. The chemokinome superfamily in channel catfish: I. CXC subfamily and their involvement in disease defense and hypoxia responses. Fish & Shellfish Immunology, 60: 380-390. DOI:10.1016/j.fsi.2016.12.004 (  0) 0) |
Gentile, C. L., and Weir, T. L., 2018. The gut microbiota at the intersection of diet and human health. Science, 362(6416): 776-780. DOI:10.1126/science.aau5812 (  0) 0) |
Guo, W., Liu, L., Zhang, Z., and Lin, S., 2011. The impact of Vibrio anguillarm challenging on non-specific immune responses of Lateolabrax japonicus. Journal of Shanghai Ocean University, 20(1): 89-95 (in Chinese with English abstract). (  0) 0) |
Grivennikov, S. I., Greten, F. R., and Karin, M., 2010. Immunity, inflammation, and cancer. Cell, 140(6): 883-899. DOI:10.1016/j.cell.2010.01.025 (  0) 0) |
He, W., Rahimnejad, S., Wang, L., Song, K., Lu, K., and Zhang, C., 2017. Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish & Shellfish Immunology, 70: 164-173. DOI:10.1016/j.fsi.2017.09.007 (  0) 0) |
Hou, Z., Xin, Y., Yang, X., Zeng, C., Zhao, H., Liu, M., et al., 2021. Transcriptional profiles of genes related to stress and immune response in rainbow trout (Oncorhynchus mykiss) symptomatically or asymptomatically infected with Vibrio anguillarum. Frontiers in Immunology, 12: 639489. DOI:10.3389/fimmu.2021.639489 (  0) 0) |
Huang, N. B., Ramkumar, G., Bhattacharyya, D., and Lee, Y., H., 2016. Elucidation of the functional role of flagella in virulence and ecological traits of Pseudomonas cichorii using flagella absence (ΔfliJ) and deficiency (ΔfliI) mutants. Research in Microbiology, 167(4): 262-271. DOI:10.1016/j.resmic.2016.01.006 (  0) 0) |
Jin, S., Mazzacurati, L., Zhu, X., Tong, T., Song, Y., and Shao, S., et al., 2003. Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene, 22(52): 8536-8540. DOI:10.1038/sj.onc.1206907 (  0) 0) |
Jin, W., Jiang, L., Hu, S., and Zhu, A., 2023. Metabolite features of serum and intestinal microbiota response of largemouth bass (Micropterus salmoides) after Aeromonas hydrophila challenge. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 263: 109496. DOI:10.1016/j.cbpc.2022.109496 (  0) 0) |
Jin, Y., Wu, S., Zeng, Z., and Fu, Z., 2017. Effects of environmental pollutants on gut microbiota. Environmental Pollution, 222: 1-9. DOI:10.1016/j.envpol.2016.11.045 (  0) 0) |
Kanwal, Z., Wiegerthes, G. F., Veneman, W. J., Merher, A. H., and Spaink, H. P., 2014. Comparative studies of Toll-like receptor signalling using zebrafish. Developmental & Comparative Immunology, 46(1): 35-52. DOI:10.1016/j.dci.2014.02.003 (  0) 0) |
Layden, M. J., Johnston, H., Amiel, A. R., Havrilak, J., Steinworth, B., Chock, T., et al., 2016. MAPK signaling is necessary for neurogenesis in Nematostella vectensis. BMC Biology, 14(1): 61. DOI:10.1186/s12915-016-0282-1 (  0) 0) |
Li, E., Xu, C., Wang, X., Wang, S., Zhao, Q., Zhang, M., et al., 2018. Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Reviews in Fisheries Science & Aquaculture, 26(3): 381-399. DOI:10.1080/23308249.2018.1440530 (  0) 0) |
Liu, H., Yang, J., Dong, X., Tan, B., Zhang, S., Chi, S., et al., 2020. Effects of different dietary carbohydrate-to-lipid ratios on growth, plasma biochemical indexes, digestive and immune enzymes activities of juvenile orange-spotted grouper Epinephelus coioides. Aquaculture Research, 51(10): 4152-4164. DOI:10.1111/are.14757 (  0) 0) |
Lu, J. W., Hsia, Y., Tu, H. C., Hsiao, Y. C., Yang, W. Y., Wang, H. D., et al., 2011. Liver development and cancer formation in zebrafish. Birth Defects Research Part C: Embryo Today: Reviews, 93(2): 157-172. DOI:10.1002/bdrc.20205 (  0) 0) |
Ma, J., Li, Y., Wu, M., Zhang, C., Che, Y., Li, W., et al., 2018. Serum immune responses in common carp (Cyprinus carpio) to paraquat exposure: The traditional parameters and circulating microRNAs. Fish & Shellfish Immunology, 76: 133-142. DOI:10.1016/j.fsi.2018.02.046 (  0) 0) |
Mao, X., Tian, Y., Wen, H., Liu, Y., Sun, Y., Yanglang, A., et al., 2020. Effects of Vibrio harveyi infection on serum biochemical parameters and expression profiles of interleukin-17 (IL-17)/interleukin-17 receptor (IL-17R) genes in spotted sea bass. Developmental & Comparative Immunology, 110: 103731. DOI:10.1016/j.dci.2020.103731 (  0) 0) |
Mcguinness, O. P., 2005. Defective glucose homeostasis during infection. Annual Review of Nutrition, 25: 9-35. DOI:10.1146/annurev.nutr.24.012003.132159 (  0) 0) |
Meiger, A. H., Krens, S. F. G., Rodriguez, I. A. M., He, S., Bitter, W., Snaar-Jagalska, B. E., et al., 2004. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Molecular Immunology, 40(11): 773-783. DOI:10.1016/j.molimm.2003.10.003 (  0) 0) |
Mold, C., 1999. Role of complement in host defense against bacterial infection. Microbes and Infection, 1(8): 633-638. DOI:10.1016/S1286-4579(99)80063-X (  0) 0) |
Murpiiy, K. N., and Brinkworth, A. J., 2021. Manipulation of focal adhesion signaling by pathogenic microbes. International Journal of Molecular Sciences, 22(3): 1358. DOI:10.3390/ijms22031358 (  0) 0) |
Muzio, M., Ni, J., Feng, P., and Dixit, V. M., 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science, 278(5343): 1612-1615. DOI:10.1126/science.278.5343.1612 (  0) 0) |
Nawaz, M., Gouife, M., Zhu, S., Yue, X., Huang, K., Ma, R., et al., 2023. Transcriptome profiling and differential expression analysis of altered immune-related genes in goldfish (Carassius auratus) infected with Aeromonas hydrophila. Fish & Shellfish Immunology, 137: 108789. DOI:10.1016/j.fsi.2023.108789 (  0) 0) |
Nayak, S. K., 2010. Role of gastrointestinal microbiota in fish. Aquaculture Research, 41(11): 1553-1573. DOI:10.1111/j.1365-2109.2010.02546.x (  0) 0) |
Nie, L., Zhou, Q. J., Qiao, Y., and Chen, J., 2017. Interplay between the gut microbiota and immune responses of ayu (Plecoglossus altivelis) during Vibrio anguillarum infection. Fish & Shellfish Immunology, 68: 479-487. DOI:10.1016/j.fsi.2017.07.054 (  0) 0) |
Palomino, D. C. T., and Marti, L. C., 2015. Chemokines and immunity. Einstein (sã o paulo), 13: 469-473. DOI:10.1590/S1679-45082015RB3438 (  0) 0) |
Petit, V., and Thiery, J. P., 2000. Focal adhesions: Structure and dynamics. Biology of the Cell, 92(7): 477-494. DOI:10.1016/S0248-4900(00)01101-1 (  0) 0) |
Pizrro-Cerda, J., Meresse, S., Parton, R. G., Goot, G. V. D., Sola-Landa, A., Lopez-Goni, I., et al., 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infection and Immunity, 66(12): 5711-5724. DOI:10.1128/IAI.66.12.5711-5724.1998 (  0) 0) |
Qi, Z., Xu, Y., Liu, Y., Zhang, Q., Wang, Z., Mei, J., et al., 2023. Transcriptome analysis of largemouth bass (Micropterus salmoides) challenged with LPS and polyI: C. Fish & Shellfish Immunology, 133: 108534. DOI:10.1016/j.fsi.2023.108534 (  0) 0) |
Rungrassamee, W., Klanchui, A., Maibunkaew, S., and Karoonuthaisiri, N., 2016. Bacterial dynamics in intestines of the black tiger shrimp and the Pacific white shrimp during Vibrio harveyi exposure. Journal of Invertebrate Pathology, 133: 12-19. DOI:10.1016/j.jip.2015.11.004 (  0) 0) |
Schroder, K., Hertzog, P. J., Ravasi, T., and Hume, D. A., 2004. Interferon-γ: An overview of signals, mechanisms and functions. Journal of Leucocyte Biology, 75(2): 163-189. DOI:10.1189/jlb.0603252 (  0) 0) |
Senevirathne, A., Hewawaduge, C., Park, J. Y., Park, S., and Lee J. H., 2020. Parenteral immunization of Salmonella Typhimurium ghosts with surface-displayed Escherichia coli flagellin enhances TLR-5 mediated activation of immune responses that protect the chicken against Salmonella infection. Microbial Pathogenesis, 147: 104252. DOI:10.1016/j.micpath.2020.104252 (  0) 0) |
Song, X., Hu, X., Sun, B., Bo, Y., Wu, K., Xiao, L., et al., 2017. A transcriptome analysis focusing on inflammation-related genes of grass carp intestines following infection with Aeromonas hydrophila. Scientific Reports, 7(1): 40777. DOI:10.1038/srep40777 (  0) 0) |
Tajano, T., Kondo, H., Hirono, I., Endo, M., Saito-Taki, T., and Aoki, T., 2007. Molecular cloning and characterization of Tolllike receptor 9 in Japanese flounder, Paralichthys olivaceus. Molecular Immunology, 44(8): 1845-1853. DOI:10.1016/j.molimm.2006.10.018 (  0) 0) |
Tian, Y., Wen, H., Qi, X., Mao, X., Shi, Z., Li, Z., et al., 2019. Analysis of apolipoprotein multigene family in spotted sea bass (Lateolabrax maculatus) and their expression profiles in response to Vibrio harveyi infection. Fish & Shellfish Immunology, 92: 111-118. DOI:10.1016/j.fsi.2019.06.005 (  0) 0) |
Tsujita, T., Tsukada, H., Nakao, M., Oshiumi, H., Matsumoto, M., and Seya, T., 2004. Sensing bacterial flagellin by membrane and soluble orthologs of Toll-like receptor 5 in rainbow trout (Onchorhynchus mikiss). Journal of Biological Chemistry, 279(47): 48588-48597. DOI:10.1074/jbc.M407634200 (  0) 0) |
Tu, Z., Xiao, R., Xiong, J., Tembo, K. M., Deng, X., Xiong, M., et al., 2016. CCR9 in cancer: Oncogenic role and therapeutic targeting. Journal of Hematology & Oncology, 9(1): 1-9. DOI:10.1186/s13045-016-0236-7 (  0) 0) |
Waagbø, R., Sandnes, K., Espelid, S., and Lie, Ø., 1988. Haematological and biochemical analyses of Atlantic salmon, Salmo solar L., suffering from coldwater vibriosis ('Hitra disease'). Journal of Fish Diseases, 11(5): 417-423. DOI:10.1111/j.1365-2761.1988.tb00737.x (  0) 0) |
Wang, X., Li, J., Li, G., Tang, L., Yang, H., and Mo, Z., 2021. The effect of rpoS on Hcp expression and bactericidal activity in Vibrio anguillarum MHK3. Progress in Fishery Sciences, 42(6): 125-134 (in Chinese with English abstract). DOI:10.19663/j.issn2095-9869.20200504001 (  0) 0) |
Yao, C., Kong, P., Wang, Z., Ji, P., Cai, M., Liu, X., et al., 2008. Cloning and expression analysis of two alternative splicing toll-like receptor 9 isoforms A and B in large yellow croaker, Pseudosciaena crocea. Fish & Shellfish Immunology, 25(5): 648-656. DOI:10.1016/j.fsi.2008.07.006 (  0) 0) |
Yu, Y., Zhong, Q., Li, C., Jiang, L., Sun, Y., Wang, X., et al., 2012. Molecular cloning and characterization of interleukin-1β in halfsmooth tongue sole Cynoglossus semilaevis. Veterinary Immunology and Immunopathology, 146(3-4): 270-276. DOI:10.1016/j.vetimm.2012.02.011 (  0) 0) |
Zhan, M., Xi, C., Gong, J., Zhu, M., Shui, Y., Xu, Z., et al., 2022. 16S rRNA gene sequencing analysis reveals an imbalance in the intestinal flora of Eriocheir sinensis with hepatopancreatic necrosis disease. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 42: 100988. DOI:10.1016/j.cbd.2022.100988 (  0) 0) |
Zhang, X., Wen, H., Wang, H., Ren, Y., Zhao, J., and Li, Y., 2017. RNA-Seq analysis of salinity stress-responsive transcriptome in the liver of spotted sea bass (Lateolabrax maculatus). PLoS One, 12(3): e0173238. DOI:10.1371/journal.pone.0173238 (  0) 0) |
Zhu, Q., Chen, R., Yu, J., Ding, G., Seah, R. W. X., and Chen, J., 2023. Antimicrobial peptide hepcidin contributes to restoration of the intestinal flora after Aeromonas hydrophila infection in Acrossocheilus fasciatus. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 263: 109486. DOI:10.1016/j.cbpc.2022.109486 (  0) 0) |
Liao, Z., Yang, C., Jiang, R., Zhu, W., Zhang, Y., and Su, J., 2022. Cyprinid-specific duplicated membrane TLR5 senses dsRNA as functional homodimeric receptors. EMBO Reports, 23(8): e54281. DOI:10.15252/EMBR.202154281 (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


