2) Tianjin Key Laboratory of Aqua-Ecology and Aquaculture, Department of Fishery Sciences, Tianjin Agricultural University, Tianjin 300384, China;
3) Tianjin Marine Ranching Technical Engineering Center, Tianjin 300457, China
Mass selection is a common and effective approach that has been widely used for genetic improvement of aquaculture species. Many studies have reported the positive effects of mass selection in many bivalve species during the past decades. For example, in many oyster species, all selection experiments conducted were effective and encouraging in altering the selected traits (e.g., growth rate, live weight, and survival) in the direction of selection (Newkirk and Haley, 1982, 1983; Sheridan, 1997; Nell et al., 1999; Langdon et al., 2003; Nell and Perkins, 2005, 2006; Li et al., 2011). Genetic gains in shell width and body weight were positive in the catarian scallop Argopecten circularis (Ibarra et al., 1999) and the bay scallop A. irradians (Stiles et al., 1997, 1998; Zheng et al., 2004, 2006), though negative responses to selection were found in the embryonic and larval stages of A. irradians (Heffernan et al., 1992). The positive selection response and realized heritability were reported in different geographical populations of the Manila clam Ruditapes philippinarum (Yan et al., 2010) and the Pacific oyster Crassostrea gigas (Li et al., 2011). However, Heffernan et al. (1991) reported negative larval responses to growth selection in the hard clam Marcenaria mercenaria.
The Manila clam R. philippinarum is mainly distributed along the coasts of the Pacific and Indian Oceans and is now cultured for commercial purposes in a number of Asian, American, and European countries (Huo et al., 2014, 2017; Nie et al., 2015a). The annual production of this species in China is as high as 3 million tones, accounting for about 73% of mudflat fishery production in the country (Nie et al., 2015b; Zhu et al., 2015). However, the production of this clam has decreased since the cultured stocks became slow growing with mass mortalities. A selective breeding program of this clam has been initiated since 2007, and a full-sib family with orange shell color was selected. This family features high survival rate but slow growth. Thereafter, two generations of mass selection for faster growth in shell length were carried out in this full-sib family. The positive responses to the selection were shown in the first two generations of mass selection (Zhao et al., 2012). The objective of this study was to evaluate the realized heritability (hR2), selection response (SR), and genetic gain (GG) for shell length and determine whether additional improvement in the growth can be achieved for this orange strain.
2 Materials and Methods 2.1 Base Population and Experimental DesignThe base population for this study was the second generation (S) of 10% upward selection for faster growth in the natural family with orange shell color in Dalian, Liaoning province. In April 2011, 600 clams were randomly selected from the S line, and their shell length was measured to determine the size-frequency distribution of this basic population. Sixty individuals were randomly selected as the parents of the selected-control (SC) line before. The clams constituting the largest 10% of the distribution were used as parents for the selected-selected line (SS). Sixty natural clams were served as parents for the control line (C) to estimate the inbreeding depression of the SS and SC lines. The selection intensity applied was +1.755 standard deviation units from the mean, corresponding to the maximum 10% of the size distribution (Falconer and Mackay, 1996). Overall, three lines were produced as listed below: selected-selected line (SS; subject to three generations of selection for growth in shell length), selected-control line (SC; selected for the second but not the third generation) and control line (C; randomly sampled individuals from the national population).
2.2 Establishment of the LinesAfter being dried for 4 h, the parents of each line were cultured in three 100 L buckets full of fresh seawater at 25℃ with salinity of 28 to induce spawning. The eggs from each line were then cultured in three different buckets. The initial density of the fertilized eggs for each line was controlled at 40–50 eggs mL−1. After 24 h, the fertilized eggs developed into D-larvae. The larvae and juveniles for all experimental lines were reared under the same condition described by Zhang and Yan (2006). The initial D-larvae density in each replicate bucket was 5–6 larvae mL−1. After metamorphosis, the density was adjusted gradually to 2–3 larvae mL−1. Seawater was replaced daily at 100%, and the temperature was maintained at 25–28℃. The salinity was kept at 28–29. The larvae were fed with Isochrysis galbana from day 1 to day 3 and a mixture of Chlorella spp. and I. galbana at a ratio of 1:2 after day 4. The feeding ration was increased with larval development, which were 2000–10000 cells mL−1 d−1 on days 1–3 and 30000–60000 cells mL−1 d−1 from day 4 to the juvenile stage. With the growth of the larvae, the sieves were changed to those with mesh size of 50–150 μm after day 10. After 60 d, the juveniles were transferred to the nursery ponds (775 m × 200 m × 1.2 m) and cultured in bags with a mesh size of 700 μm. The stocking densities were adjusted every 15 days, starting with 100–150 clams per bag and ending with 30–50 clams per bag. Every 10 bags were strung together using a rope, and one end of the rope was tied to the float and the other end was tied to the falling stone. The bags were then placed in a pond of Dalian for culturing.
2.3 Sampling and MeasurementOn days 3, 6, and 9 for larvae and on day 30 for juveniles, 30 individuals per replicate were randomly sampled and killed using 4% formaldehyde. Shell length was measured using a microscope with an ocular micrometer (Olympus, Japan). For juveniles on days 60, 90, 120, 270, and 360, 30 individuals per replicate were randomly sampled and measured by using a digital caliper (Guanglu, China, 0.01 mm accuracy).
2.4 Estimation of Genetic ParametersSelection response (SR) was measured as the difference of the selected trait in progeny between the selected-selected line (xss) and the selected-control line (xsc) (Hadley et al., 1991). The difference calculated can be divided by the standard deviation (SD) of the selected-control line (ssc) and subsequently converted into a standard SR value by using the following equation (Falconer and Mackay, 1996):
| $SR = \frac{{{x_{{\rm{ss}}}}-{x_{{\rm{sc}}}}}}{{{s_{{\rm{sc}}}}}}$. |
hR2 was measured as the ratio of the SR and the selection intensity (i) by using the following equation (Ibarra et al., 1999; Zheng et al., 2004):
| $h_R^2 = \frac{{{x_{{\rm{ss}}}}-{x_{{\rm{sc}}}}}}{{i{s_{{\rm{sc}}}}}}$, |
Genetic gain (GG) was measured as the proportional increment in the selected trait achieved by selection (Zheng et al., 2004):
| $GG = \frac{{{x_{{\rm{ss}}}}-{x_{{\rm{sc}}}}}}{{{x_{{\rm{sc}}}}}} \times 100\% $, |
where xss and xsc are the mean shell lengths of the selected-selected line and the selected-control line, respectively.
The magnitude of inbreeding depression (ID) was measured as the proportional decrement in the mean phenotypic value by using the equation proposed by Zheng et al. (2008):
| $ID = (1-\frac{{{x_x}}}{{{x_{\rm{c}}}}}) \times 100\% $, |
where xx is the mean shell length of the selected-selected line or selected-control line; and xc is the mean shell length of the control line from the national population.
2.5 Statistical AnalysisDifferences in the shell length of parents and their offspring between the selected and control lines were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's test in SPSS for windows (Version 16.0; SPSS, Chicago, IL, USA). Significance level for all analysis was set at P < 0.05.
3 Results 3.1 Base Population and Selection IntensityAs shown in Fig.1, the shell length distribution of the base population of 600 clams was normally distributed (one-sample Kolmogorov-Smirnov test). The shell length of the cut-off point was 21.93 mm for fast direction of the orange family. The mean shell length of the top 10% largest individuals was 23.22 mm. The intensity of selection was 1.755.
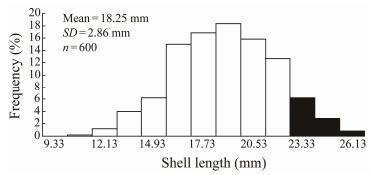
|
Fig. 1 Size distribution of the clam population after two generations of selection. Black areas indicate parents selected for truncation selection of the third generation. |
Table 1 shows the growth comparison among SS, SC, and C. The larval shell length was significantly different between SS and C, and the SS line was significantly larger than the SC except for day 6 (P < 0.05). For juvenile, the shell length of the SS line was significantly longer than those of the SC and C lines, except for day 30 (P < 0.05). However, no significant difference was found between the SC and C lines (P > 0.05). At the adult stage, the SS line showed the largest mean shell length. However, at the juvenile stage, no significant difference was found between SC and C lines (P > 0.05).
|
|
Table 1 Shell length of SS, SC, and C of R. philippinarum at different ages |
Table 2 shows the SR, hR2, and GG values after the thirdgeneration selection for shell length. SR varied from 0.15 to 1.29, with the average value of 0.73 for larvae, 0.69 for juveniles, and 0.83 for adult. The hR2 values after the third-generation selection were 0.42 for larvae, 0.40 for juveniles, and 0.48 for adult. On average, the GG values were 6.66 for larvae, 21.76 for juvenile, and 18.22 for adult. The larval and adult genetic gains were significantly higher than those observed at the juvenile stage. No significant differences of SR, hR2 and GG estimates were found among larval, juvenile, and adult stages.
|
|
Table 2 Genetic parameters after the third-generationselection for the shell length of R. philippinarum |
The inbreeding depression of the shell length for the SS and SC lines was not observed (Table 2) because their shell length was larger than that of the control line (C). The one-way ANOVA showed that the differences of inbreeding depression were not significant between SS and SC at larvae, juvenile, and adult stages.
4 DiscussionThe SR values of the shell length of R. philippinarum were 0.73 for larvae, 0.69 for juvenile, and 0.83 for adult after three successive generation selections. The progeny from the SS and SC lines was derived from the same parental stock and grown under the same environmental conditions at all stages. Therefore, the SR of the shell length in the selected line can be attributed to genetic differences among the parents. Most studies have reported significant and positive selection responses in shellfish species. Newkirk and Haley (1982) reported a large response to selection in the European oyster Ostrea edulis, and the average weight of high-selected groups was 23% higher than the control. The second generation was also heavier than the control (Newkirk and Haley, 1983). After crossing G0-selected lines in seven trials, the average weight of offspring (G1) was 9.5% greater than that of the control lines in the pacific oyster Crassostrea gigas (Langdon et al., 2003).
The hR2 of the shell length of R. philippinarum was estimated from the third-generation selection in the current study, and the values were 0.42 for larvae, 0.40 for juvenile, and 0.48 for adult. Nguyen et al. (2014) showed that a positive selection response (up to 10%) from the selection decisions in the first generation of the breeding program could indicate that further genetic gains can be achieved by applying selective breeding program in the blue mussel. The results indicated the potential of the SS line in selective breeding. Heritability for growth has been estimated in some shellfish species. In the Argopecten irradians concentricus, the realized heritability of the shell length was between 0.32 and 0.42 for the upward selection (Wang et al., 2014). In the pearl oyster Pinctada fucata martensii, the average heritability of the shell height was 0.713 at 3–15 months (He et al., 2008). Wang et al. (2011) estimated that the average realized heritability was 0.41 after the third-generation selection for superior growth in the pearl oyster P. martensii. The realized heritability of the shell width in the Japanese pearl oyster Pinctada fucata martensii was 0.467 after three generations of selections (Wada, 1986). In the bay scallop A. irradians irradians, Zheng et al. (2004) estimated that the realized heritability of Stock B (introduced to China in 1999) was significantly bigger than that of Stock A (introduced to China in 1982) at larvae, spat, and grow-out stages after selection for shell length. The realized heritability 0.434 at the larval stage after only two generations of selection is encouraging (Zheng et al., 2006). Our results indicated that continuous selective breeding for shell length in this strain will lead to further improvement in growth.
In this study, although the genetic gains were low at the larvae stage, the third-generation selection led to 21.76% increase in the shell length for juvenile and 18.22% increase for adults. This level of improvement is encouraging and consistent with previous expectations for many bivalve species, whose gains were predicted to be between 10% and 20% per generation (Newkirk, 1980). For example, Nell et al. (1999) reported an average 18% increase in the body weight of the oyster Saccostrea commercialis after two generations of mass selection. In the bay scallop A. irradians irradians Lamarck (1819), the genetic gain in the shell length was 17.56% after twogeneration selection for fast growth (Zheng et al., 2006). In the pearl oyster Pinctada fucata, He et al. (2008) reported that the genetic gain in the shell height was 16.03% ± 4.79% for the second generation. Wang et al. (2011) reported 13.27% genetic gain in the adult shell length over the control after three successive generations of selection for larger shell length in the pearl oyster Pinctada martensii. The results of the third-generation selection for faster growth of H. discus hannai showed a 21% increase in the daily growth compared with the control (Newkirk et al., 1983).
Inbreeding depression can be observed in progeny stocks produced by a small number of breeders when artificial selection programs were carried out. In selection experiments, the inbreeding depression of shellfish has been reported due to the low effective breeding numbers. For example, no positive response was found in one selection experiment of the hard clam M. mercenaria because of the reduced effective population size (Hadley et al., 1991). Zheng et al. (2012) reported that the degree of depression was predicted that a 10% increase in the inbreeding coefficients (F) might result in a 10.48% decrease in hatching, an 11.11% decrease in survival, a 3.38% decrease in larval size on day 10, a 5.85% decrease in spat size at day 50, and a 9.50% decrease in adult size on day 160 for the bay scallop. Interestingly, our results show that the inbreeding depressions of the SS and SC lines were not observed at larvae, juvenile, and adult stages. Gaffney et al. (1992) considered that a large number of parents might be necessary for selective breeding to conserve high heterozygosity and genetic diversity in the brood stocks. Wang et al. (2011) suggested that the inbreeding depression could be avoided effectively when the quantities of the stock were between 50 and 100 individuals per generation in the artificial selection experiments. In our experiment, the effective number of parents was 60 for the SS and SC lines, so the inbreeding depression was not observed during the whole stages.
5 ConclusionsOur study on the additive components of genetic variance for understating the inbreeding depression in the orange line of R. philippinarum shows that the shell length increased about 10% on a large scale. Furthermore, maintaining the selection pressure on the shell length in successive generation could avoid the inbreeding depression and could be an effective approach for genetic improvement of the growth of bivalve mollusks.
AcknowledgementsThis project was supported by the National Key R & D Program of China (No. 2018YFD0901404), Tianjin Agricultural Science and Technology Achievements Transformation and Promotion Project (No. 201903010), Tianjin Modern Agro-Industry Technology Research SystemAquaculture-Shellfish Breeding Positions (No. ITTFRS 2017013), Tianjin Major Project of Seed Science and Technology (No. 17ZXZYNC00020), and Earmarked Fund for Modern Agro-Industry Technology Research System (No. CARS-49).
Falconer, D. S., and Mackay, T. F. C., 1996. Introduction to Quantitative Genetics. 4th edition. Longman, Essex, England, 480pp.
(  0) 0) |
Gaffney, P. M., Davis, C. V. and Hawes, R. O., 1992. Assessment of drift and selection in hatchery populations of oysters (Crassostrea virginica). Aquaculture, 105: 1-20. DOI:10.1016/0044-8486(92)90157-G (  0) 0) |
Hadley, N. H., Dillon, Jr., R. T. and Manzi, J. J., 1991. Realized heritability of growth rate in the hard clam Mercenaria mercenaria. Aquaculture, 93: 109-119. DOI:10.1016/0044-8486(91)90210-X (  0) 0) |
He, M. X., Guan, Y. Y., Yuan, T. and Zhang, H., 2008. Realized heritability and response to selection for shell height in the pearl oyster pinctada fucata (Gould). Aquaculture Research, 39: 801-805. DOI:10.1111/j.1365-2109.2008.01889.x (  0) 0) |
Heffernan, P. B., Walker, R. L. and Crenshaw, Jr., J. W., 1991. Negative larval response to selection for increased growth rate in the northern quahog, Mercenaria mercenaria (Linnaeus, 1758). Journal of Shellfish Research, 1: 199-202. (  0) 0) |
Heffernan, P. B., Walker, R. L. and Crenshaw, Jr., J. W., 1992. Embryonic and larval responses to selection for increased rate of growth in adult bay scallop, Argopecten irradians concentricus (Say, 1822). Journal of Shellfish Research, 11: 21-25. (  0) 0) |
Huo, Z. M., Li, N., Zhang, X. K., Li, Y., Yan, X. W. and Yang, F., 2017. Inbreeding depression on growth and survival of Full-Sib family of Manila clam (Ruditapes philippinarum). Journal of Ocean University of China, 16: 145-150. DOI:10.1007/s11802-017-3081-6 (  0) 0) |
Huo, Z. M., Yan, X. W. and Zhao, L. Q., 2014. Larval and juvenile growth performance of Manila clam hybrids of two full-sib families. Journal of Ocean University of China, 14: 564-568. (  0) 0) |
Ibarra, A. M., Ramirez, J. L., Ruiz, C. A., Cruz, P. and Avila, S., 1999. Realized heritabilities and genetic correlation after dual selection for total weight and shell width in Catarina scallop (Argopecten circularis). Aquaculture, 175: 227-241. DOI:10.1016/S0044-8486(99)00100-3 (  0) 0) |
Langdon, C., Evans, F., Jacobson, D. and Blouin, M., 2003. Yields of cultured Pacific oysters Crassostrea gigas Thunberg improved after one generation of selection. Aquaculture, 220: 227-244. DOI:10.1016/S0044-8486(02)00621-X (  0) 0) |
Li, Q., Wang, Q. Z., Liu, S. K. and Kong, L. F., 2011. Selection response and realized heritability for growth in three stocks the Pacific oyster Crassostrea gigas. Aquaculture, 77: 643-648. (  0) 0) |
Nell, J. A. and Perkins, B., 2005. Evaluation of progeny of fourth generation Sydney rock oyster Saccostrea glomerata (Gould, 1850) breeding lines. Aquaculture Research, 36: 753-757. (  0) 0) |
Nell, J. A. and Perkins, B., 2006. Evaluation of the progeny of third-generation Sydney rock oyster Saccostrea glomerata (Gould, 1850) breeding lines for resistance to QX disease Marteilia sydneyi and winter mortality Bonamia roughleyi. Aquaculture Research, 37: 693-700. DOI:10.1111/j.1365-2109.2006.01482.x (  0) 0) |
Nell, J. A., Smith, I. R. and Sheridan, A. K., 1999. Third generation evaluation of Sydney rock oyster Saccostrea commercialis (Iredale and Roughley) breeding lines. Aquaculture, 170: 195-203. DOI:10.1016/S0044-8486(98)00408-6 (  0) 0) |
Newkirk, G. F., 1980. Review of the genetics and the potential for selective breeding commercially important bivalves. Aquaculture, 19: 209-228. DOI:10.1016/0044-8486(80)90045-9 (  0) 0) |
Newkirk, G. F. and Haley, L. E., 1982. Progress in selection for growth rate in the European oyster Ostrea edulis. Marine Ecology Progress Series, 10: 177-184. (  0) 0) |
Newkirk, G. F. and Haley, L. E., 1983. Selection for growth rate in the European oyster Ostrea edulis: Response of second generation groups. Aquaculture, 33: 149-155. DOI:10.1016/0044-8486(83)90396-4 (  0) 0) |
Nguyen, T. T. T., Hayes, B. J. and Ingram, B. A., 2014. Genetic parameters and response to selection in blue mussel (Mytilus galloprovincialis) using a SNP-based pedigree. Aquaculture, 420: 295-301. (  0) 0) |
Nie, H. T., Liu, L. H., Ding, J. F. and Yan, X. W., 2015a. Characterization of fourteen single nucleotide polymorphism markers in the Manila clam (Ruditapes philippinarum). Conservation Genetics Resources, 7: 1-4. DOI:10.1007/s12686-014-0292-7 (  0) 0) |
Nie, H. T., Niu, H. B., Yan, X. W., Zhao, L. Q. and Yang, F., 2015b. Genetic diversity and structure of Manila clam (Ruditapes philippinarum) populations from Liaodong peninsula revealed by SSR markers. Biochemical Systematics and Ecology, 59: 116-125. DOI:10.1016/j.bse.2014.12.029 (  0) 0) |
Sheridan, A. K., 1997. Genetic improvement of oyster production-A critique. Aquaculture, 153: 165-179. DOI:10.1016/S0044-8486(97)00024-0 (  0) 0) |
Stiles, S., Choromanski, J. and Cooper, C., 1998. Selection studies on growth and survival of bay scallop (Argopecten irradians) from Long Island Sound. Journal of Shellfish Research, 17: 363. (  0) 0) |
Stiles, S., Choromanski, J. and Schweitzer, D., 1997. Early responses to selection for growth in the bay scallop, Argopecten irradians, from Long Island Sound. Journal of Shellfish Research, 16: 295. (  0) 0) |
Wada, K. T., 1986. Genetic selection for shell traits in the Japanese pearl oyster, Pinctada fucata martensii. Aquaculture, 57: 171-176. DOI:10.1016/0044-8486(86)90194-8 (  0) 0) |
Wang, H., Liu, J., Li, Y. H., Zhu, X. W. and Liu, Z. G., 2014. Responses to two-way selection on growth in mass-spawned F-1 progeny of Argopecten Irradians Concentricus (say). Chinese Journal of Oceanology and Limnology, 32(2): 349-357. DOI:10.1007/s00343-014-3153-z (  0) 0) |
Wang, Q. H., Deng, Y. W., Du, X. D., Fu, S. and Lu, Y. Z., 2011. Realized heritability and genetic gains of three generation for superior growth in the pearl oyster Pinctada martensii. Acta Ecologica Sinica, 31: 108-111. DOI:10.1016/j.chnaes.2010.12.001 (  0) 0) |
Yan, X. W., Zhang, Y. H., Huo, Z. M., Sun, H. Q., Pan, F. L., Yang, F. and Zhang, G. F., 2010. Responses to selection and realized heritability in three geographical populations of Manila clam (Ruditapes philippinarum). Journal of Fisheries of China, 34: 704-710 (in Chinese with English abstract). DOI:10.3724/SP.J.1231.2010.06415 (  0) 0) |
Zhang, G. F. and Yan, X. W., 2006. Development of a new threephases culture method for Manila clam Ruditapes philippinarum, farming in northern China. Aquaculture, 258: 452-461. (  0) 0) |
Zhao, L. Q., Yan, X. W., Huo, Z. M., Yang, F. and Zhang, G. F., 2012. Divergent selection for shell length in the Manila clam Ruditapes philippinarum. Journal of the World Aquaculture Society, 43: 878-884. DOI:10.1111/j.1749-7345.2012.00612.x (  0) 0) |
Zheng, H. P., Li, L. and Zhang, G. F., 2012. Inbreeding depression for fitness-related traits and purging the genetic load in the hermaphroditic bay scallop Argopecten irradians irradians (Mollusca: Bivalvia). Aquaculture, 366: 27-33. (  0) 0) |
Zheng, H. P., Zhang, G. F., Liu, X. and Guo, X. M., 2006. Sustained response to selection in an introduced population of the hermaphroditic bay scallop Argopecten irradians irradians Lamarck (1819). Aquaculture, 255: 579-585. DOI:10.1016/j.aquaculture.2005.11.037 (  0) 0) |
Zheng, H. P., Zhang, G. F., Liu, X., Zhang, F. S. and Guo, X. M., 2004. Different responses to selection in two stocks of the bay scallop, Argopecten irradians irradians Lamarck (1819). Journal of Experimental Marine Biology and Ecology, 313: 213-223. (  0) 0) |
Zhu, D. P., Nie, H. T., Qin, Y. J., Li, J., Liu, L. H. and Yan, X. W., 2015. Development and characterization of 38 microsatellite makers for Manila clam (Ruditapes philippinarum). Conservation Genetics Resources, 7: 517-520. DOI:10.1007/s12686-014-0410-6 (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


