2) Laboratory for Marine Fisheries Science and Food Production Processes, Laoshan Laboratory, Qingdao 266237, China
Crossbreeding is a classical and effective breeding method widely utilized for enhancing economic traits and developing varieties. In aquaculture, it is usually used to exploit heterosis (hybrid vigor) in hybrid offspring and combine desirable characteristics found in one species with those of another (de Verdal et al., 2014; Wang et al., 2017; Chaivichoo et al., 2020). Hybrid varieties account for a large proportion of the production of certain farmed species in some countries, including hybrid clarid catfish in Thailand, hybrid striped bass in the United States, hybrid tilapia in Israel, hybrid characids in Venezuela and hybrid scallop in China (Bartley et al., 2001; Wang et al., 2017). Intraspecific hybridization has been extensively used to obtain varieties with superior yield-related traits in oysters (Hedgecock et al., 1995; Hedgecock and Davis, 2007). However, few successes have been reported in interspecific hybrid (Kong et al., 2017). It might be due to outbreeding depression or hybrid breakdown (Stelkens and Seehausen, 2009). There are several reports of superiority of interspecific F1 hybrids in genus Crassostrea, such as C. hongkongensis × C. gigas (Zhang et al., 2016), Crassostrea hongkongensis × C. sikamea (Zhang et al., 2017), and C. gigas × C. nippona (Xu et al., 2019). The success of crossbreeding program relies on knowledge of the genetic structure underlying the phenotypic variation (Bosworth and Waldbieser, 2014; Ariyanto et al., 2022). Because of the lack of such information, the strategies of most hybridization programs for oysters have not been determined.
The genetic components of hybrids primarily include additive genetic variance, non-additive genetic variation and other minor components (Falconer and Mackay, 1996). To estimate these genetic effects, Sprague and Tatum (1942) introduced the concepts of general combining ability (GCA) and specific combining ability (SCA) for diallel crosses, where GCA is a measure of the additive genetic action and SCA is represented as a deviation from additivity. When hybrid performance is mainly affected by GCA, improvement can be performed through selective breeding, and in cases where SCA is dominant, heterosis can be exploited through hybridization between purebred populations (Bosworth and Waldbieser, 2014; Chaivichoo et al., 2020). The combining ability analysis has been extensively used to guide crossbreeding program in aquaculture, such as red swamp crawfish (Bosworth et al., 1994), common carp (Ariyanto et al., 2022), rainbow trout (Wang et al., 2014), Pacific oyster (Hedgecock and Davis, 2007), hybrid catfish Clarias macrocephalus × C. gariepinus (Chaivichoo et al., 2020), and hybrid tilapia among Piaractus mesopotamicus, P. brachypomus and Colossoma macropomum (Costa et al., 2019). The levels of heterosis and non-additive genetic variation vary depending on the environment in which the hybrids are cultured (Thoa et al., 2016; Li et al., 2018). Consequently, the estimates of combining ability are more reliable when they are drawn over diverse environments.
Differences in environmental conditions can lead to genotype × environment (G × E) interactions in which the relative behaviors of different genotypes vary in response to the environment (Gan et al., 2023). G × E interactions appear to be more common in cultured shellfish such as Pacific oyster (Langdon et al., 2003; Dégremont et al., 2005; Evans and Langdon, 2006), Eastern oyster (Newkirk, 1978; Mallet and Haley, 1983; Dégremont et al., 2012; Proestou et al., 2016), hard clam (Rawson and Hilbish, 1991), pearl oyster (Kvingedal et al., 2008, 2010), red abalone (Farías et al., 2017), and crossbred abalone strains (Gan et al., 2023). G × E interactions may require breeders to establish separate breeding programs for each unique breeding environment if improved strains do behave differently depending on the sites (Evans and Langdon, 2006). The progress of genetic breeding programs is also impacted by heritability and genetic correlation between traits (Falconer and Mackay, 1996). The estimates of heritability and genetic correlation contribute to shape breeding strategies and accelerate genetic progress, especially when significant G × E interactions exist (Proestou et al., 2016).
The Pacific oyster C. gigas, native to East Asia, is one of the most important commercial mollusks in the world nowadays. In China, it is cultivated mainly in the north of the Yangtze River, with a total output of approximately 1.93 million tons (BOF, 2023). However, aquaculture of this species has been hindered by Pacific Oyster Mortality Syndrome (POMS) (Mao et al., 2005; Lian et al., 2010; Yang et al., 2021), forcing oyster farmers to use new improved strains. Recent studies indicate that outbreaks of POMS in the field of China are associated with heat stress and Vibrio, rather than viral infections (Bai et al., 2015; Li et al., 2023; Zhang et al., 2023). The Fujian oyster C. angulata, which is mainly cultivated in Fujian province in southern China, has thermal tolerance but slow-growing traits (Ghaffari et al., 2019). Early comparative studies have consistently demonstrated that C. gigas exhibits higher production yield, faster growth rates, increased clearance, elevated oxygen consumption rates, and heightened feeding time activity compared to C. angulata (His, 1972; Goulletquer et al., 1999; Soletchnik et al., 2002; Haure et al., 2003; Batista et al., 2007).
A crossbreeding program for the hybrids between C. gigas and C. angulata was established in 2019 to address declining C. gigas harvests caused by POMS (Jiang et al., 2021a, 2021b, 2023). Due to the absence of genetic parameters, however, the focus and method of the breeding program are still uncertain. The main objectives of the present study were: 1) to evaluate the combining ability for shell height, summer survival, and thermal tolerance of the oyster hybrid population; 2) to determine heritability and relationship among these traits; and 3) to estimate genotype × environment interaction in hybrid oysters. This information can provide a theoretical guidance for shaping optimal breeding and planting strategies.
2 Materials and Methods 2.1 Broodstock CollectionIn April 2020, the C. gigas broodstock was collected from a strain selected for growth over thirteen generations in Rongcheng, Shandong Province, China (37˚11΄N, 122˚35΄E) (Zhang et al., 2018). This strain was selected as broodstock because it is one of the main commercial strains farmed in northern China. The C. angulata broodstock was obtained from a commercial farm population in Zhangzhou, Fujian Province, China (24˚28΄N, 118˚16΄E). The broodstocks were taken into a hatchery in Laizhou, Shandong Province, China (37˚38΄N, 119˚41΄E) and conditioned in a concrete pond with filtered seawater (temperature: 22℃; salinity: 29) for 30 days before spawning. Oysters were fed with fresh Phaeodactylum tricornutum Bohlin at a concentration of approximately 50000 to 80000 cells mL−1. The pond was supplied with forced air via submerged airstones.
2.2 Mating DesignIn May 2020, an artificial crossing program was undertaken to produce purebred (GG – C. gigas ♀× C. gigas ♂ and AA – C. angulata ♀ × C. angulata ♂) and reciprocal hybrid crosses (GA – C. gigas ♀× C. angulata ♂ and AG – C. angulata ♀× C. gigas ♂). For each cross, a complete factorial mating design where three males were mated with five females (every male mated to every female and every female mated to every male) was used to generate 15 fullsib families, including three paternal half-sibs and five maternal half-sibs. Due to the need to evaluate heterosis, two factorial matings were also used to obtain purebred crosses. Therefore, the experiment consisted of four independent complete factorial matings, resulting in a total of 60 full-sib families.
2.3 Nursery RearingLarval rearing was carried out according to the routine culture procedure described by Li et al. (2011). The newly hatched D-larvae of each family were incubated in 70-L polyethylene buckets supplied with diffuse aeration at a density of 2 – 3 individuals per mL. During the larval rearing, the temperature and salinity were kept at 21 – 23℃ and 30 – 31, respectively. From the 1st day after hatching, the larvae were fed with Isochrysis galbana at a concentration of approximately 30000 cells mL−1. After the 7th day, the larvae were fed with a mixture of I. galbana and Platymonas helgolandica at concentrations ranging from 30000 to 80000 cells mL−1, depending on age. When eye-spots became apparent, scallop shells were put into the backets for eyed larvae to set on. Successfully metamorphosed spat were transferred to a land nursery pond for a temporary rearing until they were deployed in the field.
2.4 Field EvaluationA field trial was established on two main commercial farming sites (Rongcheng: 37˚11΄N, 122˚35΄E; Rushan: 36˚45΄N, 121˚42΄E) in Shandong Province from July 2020 to June 2021 for the evaluation of survival and growth. Details of salinity and temperature of the sites were obtained from the National Marine Data Center (http://mds.nmdis.org.cn/). Families were deployed in three different lantern nets (8-layer) suspended from floating buoys at a density of 20 individuals/layer at each site.
The number of live oysters per replicate was counted at the end of the summer months (October 2020). The summer survival rate (SS) was calculated as the ratio of the number of live oysters to the total number of oysters initially deployed. Twenty oysters were randomly selected from each family at harvest, and individual shell height (SH) were directly measured using an electronic vernier caliper (0.01 mm).
2.5 Evaluation of Thermal ToleranceA total of 100 individuals per family were randomly sourced from Rongcheng and Rushan, respectively, in June 2021. The thermal tolerance (TT) of adults from purebred and reciprocal crosses was evaluated separately from the two sites. Sixty oysters per family were randomly placed in triplicate plastic baskets (20 oysters/basket) secured to the bottom of three different polyethylene tanks (ca. 500 L), and each tank contained 12 families. Oysters were acclimated to the experimental conditions for 24 h before heat treatment, at which time, no dead individual was observed. Thereafter, the temperatures of each tank were adjusted from 20 to 28℃ at a rate of 1℃ h−1 using a 1000-watt electric heater with a temperature control device. This temperature represented the highest natural seawater temperatures in coastal areas of Shandong Province (Fig.1a). Mortality was monitored at 6-h interval. An oyster was considered dead when the shell was opened and the oyster did not react to gentle touching with an anatomic needle. Dead oysters were removed, counted and recorded. The experiment was proceeded with daily mortality recording until less than 1% mortality was observed for all crosses. Ten oysters of each family were selected as controls (20℃) and no oyster died during the trial.
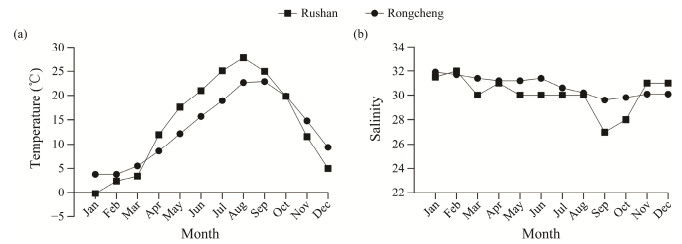
|
Fig. 1 Average temperature (a) and salinity (b) in the two breeding sites in Rongcheng and Rushan in Shandong Province, China. |
The mid-parent heterosis (MPH) and best-parent heterosis (BPH) were calculated using the following formulas (Hallauer et al., 2010):
| $ MPH = \frac{{{X_{{\text{F1}}}} - ({X_{{\text{P1}}}} + {X_{{\text{P2}}}})/2}}{{({X_{{\text{P1}}}} + {X_{{\text{P2}}}})/2}} \times 100\%, $ | (1) |
| $ BPH = \frac{{{X_{{\text{F1}}}} - {X_{{\text{BP}}}}}}{{{X_{{\text{BP}}}}}} \times 100\%, $ | (2) |
where XF1 is the mean SS, SH, and TT of one hybrid F1; XP1 and XP2 are the mean SS, SH, and TT of the parental species; XBP is the mean SS, SH, and TT of the better parent.
The hybrid potence (hp) was calculated using the formula modified from that used in Hedgecock et al. (2007):
| $ {h_{\text{p}}} = \frac{{2[{X_{{\text{F1}}}} - ({X_{{\text{P1}}}} + {X_{{\text{P2}}}})/2]}}{{\left| {{X_{{\text{P1}}}} - {X_{{\text{P2}}}}} \right|}} \times 100\%, $ | (3) |
where all parameters are described above. If hp > 1.0, the hybrid exhibits hybrid vigor or heterosis; if hp < −1.0, the hybrid exhibits hybrid depression; if −1.0 < hp < 1.0, the hybrid does not exhibit obvious hybrid vigor or hybrid depression.
Differences among the four crosses in the mean SS, SH, and TT were analyzed using the one-way analysis of variance (ANOVA) by the SPSS 26.0 software. Tukey test with α = 0.05 was used to evaluate the significance between the different crosses. The general combining ability (GCA) and special combining ability (SCA) for SS, CS, and TT were estimated in ASReml-R3.0 software (Butler et al., 2009) using the following model:
| $ {Y_{hijk}} = \mu + {S_i} + {D_j} + S{D_{ij}} + {e_{hijk}}, $ | (4) |
where Yhijk is the trait value of the kth hybrid progeny, μ is the overall mean, Si is the random effect of the ith sire, Dj is the random effect of the jth dam, SDij is the random effect of the interaction between the ith sire × jth dam, ehijk is the random residual effect.
The following formulas were used to estimate dominant ratio (d2) and heritability (h2) for SS, SH, and TT, written as:
| $ {d^2} = \frac{{4 \times \sigma _{{\text{s}} \times {\text{d}}}^2}}{{\sigma _{\text{s}}^2 + \sigma _{\text{d}}^2 + \sigma _{{\text{s}} \times {\text{d}}}^2 + \sigma _{\text{e}}^2}}, $ | (5) |
| $ {h^2} = \frac{{2 \times (\sigma _{\text{s}}^2 + \sigma _{\text{d}}^2)}}{{\sigma _{\text{s}}^2 + \sigma _{\text{d}}^2 + \sigma _{{\text{s}} \times {\text{d}}}^2 + \sigma _{\text{e}}^2}}, $ | (6) |
where
Pairwise genetic correlations and phenotypic correlations among traits within site were estimated based on bivariate animal model and calculated as follows:
| $ {r_{G/P}} = \frac{{{\text{Co}}{{\text{v}}_{G/P}}(X, Y)}}{{\sqrt {\sigma _{G/P}^2X\sigma _{G/P}^2Y} }}, $ | (7) |
where
Genotype-by-environment (G × E) interactions were estimated through genetic correlations between the trait in Rongcheng and the same trait in Rushan, considered as two different traits in the bivariate analysis (Lynch and Walsh, 1998). Since the G × E interactions were assessed by the differences between 1 and the genetic correlations, the higher the genetic correlations, the smaller the G × E interactions.
3 Results 3.1 Temperature and SalinityBoth sites showed clear and similar seasonal patterns throughout the year (Fig.1). The sea water along Rushan had higher average temperatures and lower average salinities compared to that along Rongcheng over the growing seasons (from April to October). Seasonal fluctuations in temperature and salinity of sea water were more drastic in Rushan.
3.2 Crosses PerformancesThe hybrid crosses outperformed purebred crosses in shell heights (SH), summer survival (SS), and thermal tolerance (TT) in different sites (Fig.2). Better performance was recorded for all crosses in Rushan than in Rongcheng. GA had the highest SH at harvest, with values of 86.65 mm and 82.59 mm in Rushan and Rongcheng, respectively, followed by AG, which was larger than GG (P > 0.05). AA was inferior, with the lowest SH among all crosses (P < 0.05). Variations among crosses for SS and TT showed similar patterns as SH, with the exception that AA was superior to GG.
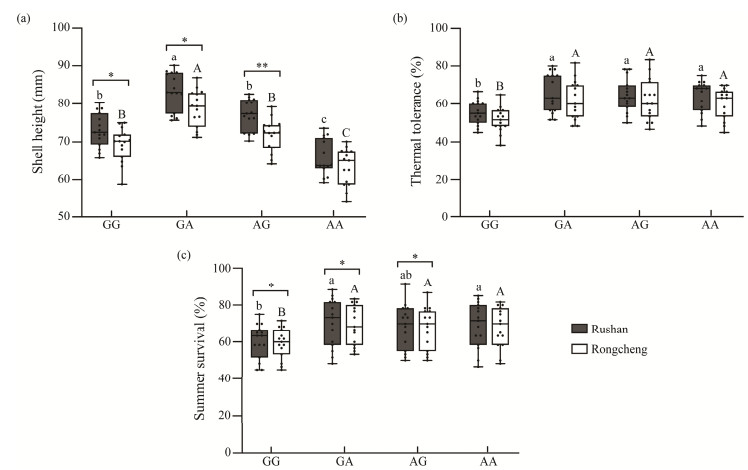
|
Fig. 2 Shell height (a), summer survival (b) and thermal tolerance (c) of GA and AG reared in Rongcheng and Rushan. Different lowercase superscripts indicate significant differences between crosses in Rushan, while different uppercase superscripts indicate significant differences between crosses in Rongcheng (P < 0.05). The difference between different sites in the same cross was indicated by asterisk (* P < 0.05; ** P < 0.01). |
Heterosis of the reciprocal hybrids was evident for SH, SS, and TT (Table 1). Both GA and AG showed favorable MPH for these traits in Rongcheng and Rushan. Compared with AG, however, GA showed higher heterosis for SH (MPH: 19.01% – 20.34%; BPH: 13.89% – 14.32%), SS (MPH: 9.62% – 10.04%; BPH: 2.70% – 2.88%) and TT (MPH: 8.14% – 8.37%; BPH: 1.78% – 1.96%) regardless of the sites. Hybrid potence (hp) values of the two reciprocal hybrids were much greater than 1.0 for SH (GA: 4.22 – 4.50; 1.87 – 2.26) and SS (GA: 2.15 – 2.69; AG: 1.15 – 1.49). The magnitudes of MPH, BPH, and hp for growth traits were slightly higher than those for survival traits in reciprocal hybrids.
|
|
Table 1 Mid-parent heterosis (MPH), best-parent heterosis (BPH) and hybrid potence (hp) for shell height (SH), summer survival rate (SS) and thermal tolerance (TT) of GA and AG reared in Rongcheng (RC) and Rushan (RS) |
The estimates of GCA and SCA for SH, SS and TT were close to zero but positive, except for sire C. gigas for these traits in Rushan which were negative (Table 2). The male C. gigas had better GCA for SH in Rongcheng whereas for SS and TT, male C. angulata was good general combiner in Rongcheng and Rushan. Specific combining ability was much higher than GCA for SH and lower than GCA for TT. Consistently the values of dominant ratio (d2) were larger for SH in GA (0.46 – 0.74) and AG (0.33 – 0.40), but smaller for SS and TT (Table 3). Additionally, the estimates of SCA for the cross GA were higher than that of AG for all traits. The results showed that the top-performing cross, GA, combined positive and higher GCA and SCA than AG for all traits, which was in line with the results of heterosis.
|
|
Table 2 General combining ability (GCA) and specific combining ability (SCA) for shell height, summer survival rate and thermal tolerance of GA and AG reared in Rongcheng and Rushan |
|
|
Table 3 Heritabilities (h2) and dominant ratios (d2) for shell height, summer survival rate and thermal tolerance of GA and AG reared in Rongcheng and Rushan |
The estimated heritabilities were unexpectedly low for SH in the hybrid GA (0.12 – 0.19) and AG (0.10 – 0.12), which was approximately three-fold lower than dominant ratio (Table 3). The smallest heritability values were noted in SS (GA: 0.06 – 0.09; AG: 0.06 – 0.12), with all the other traits ranging upwards from there. The estimates of heritability for TT were low to moderate, with values of 0.13 – 0.20 and 0.13 – 0.16 for GA and AG, respectively.
The estimates of genetic (GA: 0.70 – 0.81; AG: 0.78 – 0.82) and phenotypic correlation (GA: 0.80 – 0.89; AG: 0.78 – 0.90) between SS and TT were positive and high at two sites (Table 4). The SH was moderately correlated with TT at genetic level (GA: 0.43 – 0.63; AG: 0.32 – 0.54). However, the genetic correlations between SH and SS were not stable, with a minimum of −0.09 in GA and a maximum of 0.56 in AG.
|
|
Table 4 Genetic (below diagonal) and phenotypic (above diagonal) correlations among the traits for shell height, summer survival rate and thermal tolerance of GA and AG reared in Rushan and Rongcheng |
The relative performance of the reciprocal crosses was site dependent (Table 5). Genetic correlations of AG for SH (rG = 0.24) and GA for SS (rG = 0.55) between Rongcheng and Rushan were especially low, which suggested high G × E interactions. They are relatively low (rG < 0.8) in GA for SH and in AG for SS between the two sites, revealing the moderate G × E interactions. In contrast, high correlations were observed for TT (rG > 0.98) between two sites, which was indicative of the absence of G × E interaction.
|
|
Table 5 Genetic correlations between the groups in Rongcheng and Rushan for shell height, summer survival rate and thermal tolerance of GA and AG |
Interspecific hybridization between genera and/or species is a traditional breeding approach that improves traits by exploiting genomic differences between species. The development of successful oyster crossbreeding strategies requires genetic insights into economic traits related to growth, survival, yield, stress tolerance and disease resistance. Despite its economic importance, the present study is the first to evaluate genetic components of the C. gigas × C. angulata hybrid. The results furnish crucial insights for developing crossbreeding strategies to concurrently enhance the growth rate and summer survival of hybrids derived from C. gigas and C. angulata.
4.1 Heterosis in Reciprocal Hybrids Between C. gigas and C. angulataThere are many reports of no heterosis in the F1 C. gigas × C. angulata (Menzel, 1974; Huvet et al., 2002; Soletchnik et al., 2002; Batista et al., 2007). However, this does not appear to be the case for the reciprocal hybrids. Consistent with several researches conducted in China (Zheng et al., 2012; Tan et al., 2020; Jiang et al., 2021a, 2021b), this study confirmed that the reciprocal hybrids showed positive heterosis in terms of growth, summer survival and thermal tolerance. Two main factors that are generally thought to be responsible for heterosis are partial to complete dominance within loci and differences in allele frequencies between parental populations (Falconer and Mackay, 1996). In Europe, oyster aquaculture was initially based on C. angulata, which is believed to have been introduced from Taiwan of China in the late 16th century, as indicated by genetic analyses (Boudry et al., 1998; O'Foighil et al., 1998). In the 1970s, C. gigas from Japan were introduced into France after C. angulata became extinct caused by an iridovirus (Grizel and Héral, 1991). However, the C. gigas and C. angulata used in this study originated from Shandong Province and Fujian Province, respectively. Adaptation to different local conditions and differential gene flow lead to differences in gene frequencies between geographical populations of C. gigas and C. angulata, potentially explaining the two conflicting results of heterosis.
This case showed that the GA cross had larger heterosis than the AG cross in both testing environments. Such result might be due to the maternal effects, sex-linked genes, cytoplasmic inheritance, or paternal effects (Bosworth et al., 1994; Bentsen et al., 1998; Kong et al., 2017). Similar results between a pair of reciprocal crosses were also observed in other farmed shellfish (Cruz and Ibarra, 1997; Hedgecock and Davis, 2007; Deng et al., 2010; Han et al., 2020). Considering both the performance of growth and survival, it can be concluded that the hybrid GA can serve as a potential donor in breeding programs.
4.2 Developing Breeding Strategies Based on Combining AbilitiesKnowledge of combining abilities enables breeders to exploit additive effects to match their actual production situations and further increase the productivity by non-additive genetic effects (Ariyanto et al., 2022). In line with the results of heterosis, the cross GA possessed larger SCA values for all tested traits than the AG. This result revealed the importance of high dominance values for heterosis. On the other hand, it indicated that higher heterotic effects can be obtained when GA is selected as the superior combiner. In terms of SH, the larger SCA relative to GCA implied the importance of non-additive gene action was important for growth traits. This phenomenon may occur because one parent performed relatively better than the other parent in this trait. However, the low GCA values for SH in C. gigas probably reflected its population history. The C. gigas broodstock used herein has gone through thirteen generations of mass selection for SH. Inbreeding is prone to accumulate in mass-selected populations, resulting in a decrease in additive genetic variation (Falconer and Mackay, 1996). On the contrary, our results showed that C. angulata was the species with the larger GCA values for SS and TT, and also the species that contributed more to these traits. Selective breeding to enhance the survival of parental strains is available to improve these traits because they are mainly controlled by additive genes (Bosworth and Waldbieser, 2014; Amissah et al., 2019; Chaivichoo et al., 2020). It should be noted that due to the specific genetic backgrounds of the two parental stocks, the C. gigas and C. angulata used in this study may have limited representativeness of the species.
One key issue is which method is the most suitable for developing hybrid varieties combining fast growth and high survival characteristics. The SCA values and dominant ratios indicated that heterosis breeding may be a good option for improving SH. In heterosis breeding, the F1 progenies are the production units, which implies that dominance effects could be utilized in breeding programs (Hallerman, 1994). Theoretically, heterosis can be quickly accumulated in the hybrids because of the accumulation of a number of different non-additive genetic variations in the populations, when the parental stocks are individually selected for several generations (Sheridan, 1997). Moreover, the sale of an F1 enables breeders to preserve their improved pure-species germplasm through F2-breakdown (Bosworth and Waldbieser, 2014; Joshi et al., 2018). In practice, however, the main drawbacks of this method are the high cost and long time-consuming due to the need to maintain and breed different pure parental populations or complete set lines (de Verdal et al., 2014).
Given the common influence of additive and non-additive components on SS and TT, improving the survival of F1 hybrids through traditional crossbreeding or combination breeding seems to be the most likely approach. The essence of these methods is hybridization followed by selection, which can harness both additive and non-additive effects (Kumari et al., 2015). Moreover, these methods also decrease cost and time to a breeding program, accelerating the achievement of genetic gains. In the present case, the reciprocal hybrids between C. gigas and C. angulata showed positive heterosis in SH, SS, and TT, implying excellent species complementarity. Thus, combination breeding would be expected to develop hybrids between the two oyster species and then implement directed selection to simultaneously increase growth and survival. In aquaculture, many hybridizations were often used in combination with selection to obtain superior varieties combining desirable characteristics derived from two parent species (de Verdal et al., 2014; Wang et al., 2017).
4.3 Impact of Genetic Parameters on Breeding StrategiesIn breeding strategies based on hybridization before selection, it is indispensable for the evaluation of genetic parameters. In this study, the estimates of heritability for TT in hybrid oysters were 0.13 – 0.20, and were within the range (0.08 – 0.47) of estimates in other aquatic animals (Perry et al., 2005; Zhang et al., 2014; Camara et al., 2017; Han et al., 2022). In this sense, sustained directional selection could be beneficial to improve the TT of hybrid oysters. Furthermore, most studies have demonstrated low-to-moderate heritability for growth traits of oysters (Evans and Langdon, 2006; Li et al., 2011; Zhang et al., 2018; Han et al., 2022). The low heritability estimates of SH in this study may be due to the presence of dominant or nonadditive genetic effects. However, our estimates were based on 15 families for each cross only and thus must be taken with caution as they may reflect a relatively large dominant genetic variance in this particular population. There is doubt about whether the inheritances such as heritability can be properly interpreted due to potential genetic disequilibrium in interspecific hybrids (Gordon, 1999). Volker et al. (2008) pointed out that additive genetic components in pure and hybrid crosses are theoretically incomparable.
Correlation analysis revealed that the estimation of SS was correlated in positive direction with TT. In the context of lower heritability of SS, selection for TT may be used as an indirect means to passively improve SS. Hershberger et al. (1984) noted that selection for tolerance to elevated temperatures had significantly increased resistance to summer mortality as a correlated response in C. gigas. The goal of our breeding program is to increase both survival rate and growth rate. The genetic correlation between SH and SS ranged from −0.09 to 0.56, and the genetic correlation between SH and TT ranged from 0.32 to 0.63; hence both traits can be improved simultaneously. Similarly, a low but positive genetic correlation between growth and summer survival was also found in C. gigas (Dégremont et al., 2007).
Two sites that were considered to be representative environments for C. gigas along the coast of Shandong Province were selected to ascertain the magnitude of G × E interaction. Studies have indicated that if the genetic correlation between sites is below 0.8, the G × E interactions are assumed to exist; however, if the value is higher, it can be ignored (Robertson, 1959; Farías et al., 2017). In this study, genetic correlations among sites were low between the two groups in Rongcheng and Rushan (SH: 0.24 – 0.70; SS: 0.55 – 0.78), suggesting high G × E interaction effects. The significant G × E interactions across sites demonstrated that adopting separate breeding methods, rather than a single breeding method, would be more effective in improving yield-related traits of hybrid oysters farmed in different sites. In oysters, G × E interaction on growth and survival traits has been observed in several species (Newkirk, 1978; Mallet and Haley, 1983; Dégremont et al., 2012; Proestou et al., 2016; Jiang et al., 2022). G × E interaction is probably attributed to the heterogeneity of genetic components and heritability across environments and the magnitude of correlations between traits in different locations (Lynch and Walsh, 1998). In general, the probability of undergoing a G × E interacttion for a trait is expected to increase as the heterogeneity of environmental conditions increases (Evans and Langdon, 2006). It is plausible that the higher temperature and lower salinity from April to October in Rushan leads to different rankings of the hybrid families.
4.4 Considerations for Commercializing Hybrid StrainsA concern for the commercialization of hybrid varieties is the potential genetic contamination. The production of sterile triploids is an effective approach to reduce impacts on local stocks in the events of hybrid escapes (Garcia-Abiado et al., 2002; Jiang et al., 2022). Interestingly, the production of sterile triploid hybrids could be advantageous to improve growth rate by avoiding channeling energy into reproduction (Bartley et al., 2001) and make full use of non-additive variation by incorporating SCA into the tetraploid lines (Hedgecock and Davis, 2007). In fish, hybridization has been widely used with polyploidization to produce offspring with sterility and improved yield characteristics (Bartley et al., 2001). Therefore, breeding hybrid triploid oysters is also an attractive option in future commercial hybridization programs.
5 ConclusionsThe present study proves the importance of assessing the fundamental genetic parameters underlying the performance in crossbreeding programs. The hybrid GA showed positive heterosis in SH, SS and TT, making it a potential donor parent. Because non-additive and additive gene actions play an important role in these traits, combination breeding may be used to harness both additive and nonadditive genetic effects, as well as the complementarity of species. The existence of correlations between SH and TT suggests that both of these traits can be improved simultaneously. The significant G × E interactions demonstrate the need to undertake site-specific breeding programs in dissimilar environments. After a few generations of improvement, however, it is necessary to evaluate the selection responses and monitor genetic diversity.
AcknowledgementsThis research was founded by the National Key R & D Program of China (No. 2022YFD2400305), the Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province (Nos. 2022LZGCQY010, 2021LZGC027 and 2021ZLGX03), and the China Agriculture Research System Project (No. CARS-49).
Author Contributions
Gaowei Jiang: investigation, conceptualization, formal analysis, and writing-original draft. Qi Li: supervision, conceptualization, resources, writing-review & editing, and funding acquisition. Chengxun Xu: supervision and resources.
Data Availability
The data and references presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate
All methods used in this study were conducted according to the guiding principles of the Chinese Legislation on the Use and Care of Laboratory Animals. The Academic Council approved the animal protocol of the Ocean University of China.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests. Qi Li is one of the Editorial Board Members, but he was not involved in the journal's review of, or decision related to, this manuscript.
Amissah, S., Osekre, E. A., Nyadanu, D., Akromah, R., Afun, J. V. K., Adu Amoah, R., et al., 2019. Inheritance and combining ability studies on grain yield and resistance to maize weevil (Sitophilus zeamais, Motschulsky) among extra early quality protein maize inbred lines. Ecological Genetics and Genomics, 12: 100043. DOI:10.1016/j.egg.2019.100043 (  0) 0) |
Ariyanto, D., Carman, O., Sulistyowati, D. T., Zairin Jr., M., Syukur, M., Suharyanto, et al., 2022. Analysis of combining ability and heterotic estimation on a complete diallel cross involving five strains of common carp (Cyprinus carpio) from different geographical regions in West Java, Indonesia. Aquaculture Research, 53: 6900-6909. DOI:10.1111/are.16155 (  0) 0) |
Bai, C., Wang, C., Xia, J., Sun, H., Zhang, S., and Huang, J., 2015. Emerging and endemic types of Ostreid herpesvirus 1 were detected in bivalves in China. Journal of Invertebrate Pathology, 124: 98-106. DOI:10.1016/j.jip.2014.11.007 (  0) 0) |
Bartley, D. M., Rana, K., and Immink, A. J., 2001. The use of inter-specific hybrids aquaculture and fisheries. Review in Fish Biology and Fisheries, 10: 325-337. (  0) 0) |
Batista, F. M., Leitão, A., Fonseca, V. G., Ben-Hamadou, R., Ruano, F., Henriques, M. A., et al., 2007. Individual relationship between aneuploidy of gill cells and growth rate in the cupped oysters Crassostrea angulata, C. gigas and their reciprocal hybrids. Journal of Experimental Marine Biology and Ecology, 352: 226-233. DOI:10.1016/j.jembe.2007.07.009 (  0) 0) |
Bentsen, H. B., Eknath, A. E., Palada-de Vera, M. S., Danting, J. C., Bolivar, H. L., Reyes, R. A., et al., 1998. Genetic improvement of farmed tilapias: Growth performances in a complete diallel cross experiment with eight strains of Oreochromis niloticus. Aquaculture, 160(1-2): 145-173. DOI:10.1016/S0044-8486(97)00230-5 (  0) 0) |
BOF (Bureau of Fisheries), 2023. China Fisheries Statistic Yearbook 2023. Agriculture Press, Beijing, 159pp (in Chinese).
(  0) 0) |
Bosworth, B. G., Wolters, W. R., and Saxton, A. M., 1994. Analysis of a diallel cross to estimate effects of crossing on performance of red swamp crawfish, Procambarus clarkii. Aquaculture, 121(4): 301-312. DOI:10.1016/0044-8486(94)90266-6 (  0) 0) |
Bosworth, B., and Waldbieser, G., 2014. General and specific combining ability of male blue catfish (Ictalurus furcatus) and female channel catfish (Ictalurus punctatus) for growth and carcass yield of their F1 hybrid progeny. Aquaculture, 420-421: 147-153.
(  0) 0) |
Boudry, P., Heurtebise, S., Collet, B., Cornette, F., and Gérard, A., 1998. Differentiation between populations of the Portuguese oyster, Crassostrea angulata (Lamark) and the Pacific oyster. Crassostrea gigas (Thunberg), revealed by mtDNA RFLP analysis. Journal of Experimental Marine Biology and Ecology, 226: 279-291.
(  0) 0) |
Butler, D. G., Cullis, B. R., Gilmour, A. R., and Gogel, B. J., 2009. ASReml-R Reference Manual. Queensland Department of Primary Industries and Fisheries, NSW Department of Primary Industries, Brisbane.
(  0) 0) |
Camara, M. D., Yen, S., Kaspar, H. F., Kesarcodi-Watson, A., King, N., Jeffs, A. G., et al., 2017. Assessment of heat shock and laboratory virus challenges to selectively breed for Ostreid herpesvirus 1 (OsHV-1) resistance in the Pacific oyster, Crassostrea gigas. Aquaculture, 469: 50-58. DOI:10.1016/j.aquaculture.2016.11.031 (  0) 0) |
Chaivichoo, P., Koonawootrittriron, S., Chatchaiphan, S., Srimai, W., and Na-Nakorn, U., 2020. Genetic components of growth traits of the hybrid between ♂ North African catfish (Clarias gariepinus Burchell, 1822) and ♀ bighead catfish (C. Macrocephalus Günther, 1864). Aquaculture, 521: 735082.
(  0) 0) |
Costa, A. C., Botelho, H. A., Gomes, R. C. D., Campos, S. A. D., Neto, R. V. R., Balestre, M., et al., 2019. General and specific combining ability in Serrasalmidae. Aquaculture Research, 50: 717-724. (  0) 0) |
Cruz, P., and Ibarra, A. M., 1997. Larval growth and survival of two catarina scallop (Argopecten circularis, Sowerby, 1985) populations and their reciprocal crosses. Journal of Experimental Marine Biology and Ecology, 212: 95-110. DOI:10.1016/S0022-0981(96)02742-6 (  0) 0) |
de Verdal, H., Rosario, W., Vandeputte, M., Muyalde, N., Morissens, P., Baroiller, J. F., et al., 2014. Response to selection for growth in an interspecific hybrid between Oreochromis mossambicus and O. niloticus in two distinct environments. Aquaculture, 430: 159-165. DOI:10.1016/j.aquaculture.2014.03.051 (  0) 0) |
Dégremont, L., Bedier, E., Soletchnik, P., Ropert, M., Huvet, A., Moal, J., et al., 2005. Relative importance of family, site, and field placement timing on survival, growth, and yield of hatchery-produced Pacific oyster spat (Crassostrea gigas). Aquaculture, 249(1-4): 213-229. DOI:10.1016/j.aquaculture.2005.03.046 (  0) 0) |
Dégremont, L., Ernaude, B., Bédier, E., and Boudry, P., 2007. Summer mortality of hatchery-reared Pacific oyster (Crassostrea gigas). I. Estimation of genetic parameters for survival and growth. Aquaculture, 262: 41-53. DOI:10.1016/j.aquaculture.2006.10.025 (  0) 0) |
Dégremont, L., Garcia, C., Frank-Lawale, A., and Allen, S. K., 2012. Triploid oysters in the Chesapeake Bay: Comparison of diploid and triploid Crassostrea virginica. Journal of Shellfish Research, 31: 21-31. DOI:10.2983/035.031.0103 (  0) 0) |
Deng, Y., Liu, X., Zhang, G., and Wu, F., 2010. Heterosis and combining ability: A diallel cross of three geographically isolated populations of Pacific abalone Haliotis discus hannai Ino. Chinese Journal of Oceanology and Limnology, 28: 1195-1199. DOI:10.1007/s00343-010-9903-7 (  0) 0) |
Evans, S., and Langdon, C., 2006. Effects of genotype × environment interactions on the selection of broadly adapted Pacific oysters (Crassostrea gigas). Aquaculture, 261(2): 522-534. DOI:10.1016/j.aquaculture.2006.07.022 (  0) 0) |
Falconer, D. S., and Mackay, T. F. C., 1996. Introduction to Quantitative Genetics. 4th edition. Pearson Education Ltd., Essex, 464pp.
(  0) 0) |
Farías, W. J., Winkler, F. M., and Brokordt, K. B., 2017. Genotype by environment interactions, heritabilities and genetic correlations for productive traits of Haliotis rufescens. Aquaculture, 473: 407-416. DOI:10.1016/j.aquaculture.2017.02.030 (  0) 0) |
Gan, Y., Wang, Y., Yu, F., Xiao, Q., Luo, X., Han, Z., et al., 2023. Genotype by environment interactions for productive traits of purebred and crossbred abalone strains under different rearing modes. Aquaculture, 563: 738966. DOI:10.1016/j.aquaculture.2022.738966 (  0) 0) |
Garcia-Abiado, M. A. R., Lynch, W. E., Dabrowski, K., Czesny, S., and Rinchard, J., 2002. Juvenile growth and survival of heat-shocked triploid hybrid saugeyes, Stizostedion vitreum × S. canadense. Fisheries Management and Ecology, 9: 105-110. DOI:10.1046/j.1365-2400.2002.00291.x (  0) 0) |
Ghaffari, H., Wang, W., Li, A., Zhang, G. F., and Li, L., 2019. Thermotolerance divergence revealed by the physiological and molecular responses in two oyster subspecies of Crassostrea gigas in China. Frontiers in Physiology, 10: 1137. DOI:10.3389/fphys.2019.01137 (  0) 0) |
Gordon, J., 1999. Quantitative genetics of intraspecies hybrids. Heredity, 83: 757-764. DOI:10.1046/j.1365-2540.1999.00634.x (  0) 0) |
Goulletquer, P., Wolowicz, M., Latala, A., Geairon, P., Huvet, A., and Boudry, P., 1999. Comparative analysis of oxygen consumption rates between cupped oyster spat of Crassostrea gigas of French, Japanese, Spanish and Taiwanese origins. Aquatic Living Resource, 12: 271-277. DOI:10.1016/S0990-7440(00)86638-3 (  0) 0) |
Grizel, H., and Héral, M., 1991. Introduction into France of the Japanese oyster (Crassostrea gigas). Journal du Conseil/Conseil International pour l'Exploration de la Mer, 47: 399-403. DOI:10.1093/icesjms/47.3.399 (  0) 0) |
Hallauer, A. R., Carena, M. J., and Filho, J. B. M., 2010. Heterosis. In: Quantitative Genetics in Maize Breeding. Handbook of Plant Breeding. Vol 6. Springer, New York, 477-529.
(  0) 0) |
Hallerman, E. M., 1994. Toward coordination and funding of longterm genetic improvement programs for striped bass and hybrid bass Morone sp.. Journal World Aquaculture Society, 25: 360-365. DOI:10.1111/j.1749-7345.1994.tb00219.x (  0) 0) |
Han, Z., Guo, X., Lu, Z., Song, Y., Chen, R., Han, X., et al., 2022. Heritability estimates for gonadal development traits and their genetic correlations with growth and heat tolerance traits in the Fujian oyster Crassostrea angulata. Frontier in Marine Science, 9: 986441. DOI:10.3389/fmars.2022.986441 (  0) 0) |
Han, Z., Li, Q., Liu, S., and Kong, L., 2020. Crossbreeding of three different shell color lines in the Pacific oyster reveals high heterosis for survival but low heterosis for growth. Aquaculture, 529: 735621. DOI:10.1016/j.aquaculture.2020.735621 (  0) 0) |
Haure, J., Huvet, A., Palvadeau, H., Nourry, M., Penisson, C., Martin, J. L. Y., et al., 2003. Feeding and respiratory time activities in the cupped oysters Crassostrea gigas, Crassostrea angulata and their hybrids. Aquaculture, 218: 539-551. DOI:10.1016/S0044-8486(02)00493-3 (  0) 0) |
Hedgecock, D., and Davis, J. P., 2007. Heterosis for yield and crossbreeding of the Pacific oyster Crassostrea gigas. Aquaculture, 272: S17-S29. DOI:10.1016/j.aquaculture.2007.07.226 (  0) 0) |
Hedgecock, D., McGoldrick, D. J., and Bayne, B. L., 1995. Hybrid vigor in Pacific oysters: An experimental approach using crosses among inbred lines. Aquaculture, 137: 285-298. DOI:10.1016/0044-8486(95)01105-6 (  0) 0) |
Hershberger, W. K., Perdue, J. A., and Beattie, J. H., 1984. Genetic selection and systematic breeding in Pacific oyster culture. Aquaculture, 39: 237-245. DOI:10.1016/0044-8486(84)90269-2 (  0) 0) |
His, E., 1972. Premiers éléments de comparaison entre l'huître portugaise et l'huître japonaise. Bulletin de l'Institut des Pêches Maritimes, 219: 1-9. (  0) 0) |
Huvet, A., Gerard, A., Ledu, C., Phelipot, P., Heurtebise, S., and Boudry, P., 2002. Is fertility of hybrids enough to conclude that the two oysters Crassostrea gigas and C. angulata are the same species?. Aquatic Living Resource, 15: 45-52. DOI:10.1016/S0990-7440(01)01148-2 (  0) 0) |
Jiang, G., Li, Q., and Xu, C., 2022. Growth, survival and gonad development of two new types of reciprocal triploid hybrids between Crassostrea gigas and C. angulata. Aquaculture, 559: 738451. DOI:10.1016/j.aquaculture.2022.738451 (  0) 0) |
Jiang, G., Li, Q., and Xu, C., 2023. Genetic parameters and response to selection for thermal tolerance, summer survival and growth in hybrid oyster (Crassostrea gigas ♀ × C. angulata ♂). Aquatic Living Resource, 36: 30. DOI:10.1051/alr/2023026 (  0) 0) |
Jiang, G., Li, Q., Xu, C., and Liu, S., 2021a. Effects of temperature on the growth and survival of reciprocal hybrids of two oyster species, Crassostrea gigas and Crassostrea angulata. Journal of Fishery Sciences of China, 28: 29-36 (in Chinese with English abstract). (  0) 0) |
Jiang, G., Li, Q., Xu, C., Liu, S., Kong, L., and Yu, H., 2021b. Reciprocal hybrids derived from Crassostrea gigas and C. angulata exhibit high heterosis in growth, survival and thermotolerance in northern China. Aquaculture, 545: 737173.
(  0) 0) |
Joshi, R., Woolliams, J. A., Meuwissen, T. H. E., and Gjøen, H. M., 2018. Maternal, dominance and additive genetic effects in Nile tilapia; influence on growth, fillet yield and body size traits. Heredity, 120: 452-462. DOI:10.1038/s41437-017-0046-x (  0) 0) |
Kong, L., Song, S., and Li, Q., 2017. The effect of interstrain hybridization on the production performance in the Pacific oyster Crassostrea gigas. Aquaculture, 472: 44-49. DOI:10.1016/j.aquaculture.2016.07.018 (  0) 0) |
Kumari, J., Dikshit, H. K., Singh, B., and Singh, D., 2015. Combining ability and character association of agronomic and biochemical traits in pea (Pisum sativum L.). Scientific Horticulture, 181: 26-33. DOI:10.1016/j.scienta.2014.10.051 (  0) 0) |
Kvingedal, R., Evans, B. S., Lind, C. E., Taylor, J. J. U., Dupont-Nivet, M., and Jerry, D. R., 2010. Population and family growth response to different rearing location, heritability estimates and genotype × environment interaction in the silver-lip pearl oyster (Pinctada maxima). Aquaculture, 304(1-4): 1-6. DOI:10.1016/j.aquaculture.2010.02.035 (  0) 0) |
Kvingedal, R., Evans, B. S., Taylor, J., Knauer, J., and Jerry, D. R., 2008. Family by environment interactions in shell size of 43-day old silver-lip pearl oyster (Pinctada maxima), five families reared under different nursery conditions. Aquaculture, 279: 23-28. DOI:10.1016/j.aquaculture.2008.04.022 (  0) 0) |
Langdon, C., Evans, F., Jacobson, D., and Blouin, M., 2003. Yields of cultured Pacific oysters Crassostrea gigas Thunberg improved after one generation of selection. Aquaculture, 220(1-4): 227-244. DOI:10.1016/S0044-8486(02)00621-X (  0) 0) |
Li, Q., Wang, Q., Liu, S., and Kong, L., 2011. Selection response and realized heritability for growth in three stocks of the Pacific oyster Crassostrea gigas. Fisheries Science, 77: 643-648. DOI:10.1007/s12562-011-0369-0 (  0) 0) |
Li, X., Shi, C., Yang, B., Li, Q., and Liu, S., 2023. High temperature aggravates mortalities of the Pacific oyster (Crassostrea gigas) infected with Vibrio: A perspective from homeostasis of digestive microbiota and immune response. Aquaculture, 568: 739309. DOI:10.1016/j.aquaculture.2023.739309 (  0) 0) |
Li, Z., Coffey, L., Garfin, J., Miller, N. D., White, M. R., Spalding, E. P., et al., 2018. Genotype-by-environment interactions affecting heterosis in maize. PLoS One, 14(8): e0219528. (  0) 0) |
Lian, W., Weng, H. S., Mao, Y. Z., and Fang, J. G., 2010. Study on the relationship between Pacific oyster Crassostrea gigas summer mortality with culture environment and organism condition. Progress in Fishery Science, 31: 92-100 (in Chinese with English abstract). (  0) 0) |
Lynch, M., and Walsh, B., 1998. Genetics and Analysis of Quantitative Traits. Sinauer Associates, Inc., Sundeland, MA, 980pp.
(  0) 0) |
Mallet, L., and Haley, L. E., 1983. Growth rate and survival in pure population matings and crosses of the oyster Crassostrea virginica. Canadian Journal of Fisheries and Aquatic Sciences, 40: 948-954. DOI:10.1139/f83-121 (  0) 0) |
Mao, Y. Z., Zhou, Y., Yang, H. S., Yuan, X. T., Wen, H. X., and Wang, R. C., 2005. Seasonal variation in metabolic rate of Pacific oyster, Crassostrea gigas and its implication to summer mortality. Oceanology and Limnology Sinica, 36: 445-451 (in Chinese with English abstract). (  0) 0) |
Menzel, R. W., 1974. Portuguese and Japanese oysters are the same species. Journal of the Fisheries Research Board of Canada, 31: 453-456. DOI:10.1139/f74-074 (  0) 0) |
Newkirk, G., 1978. Interaction of genotype and salinity in larvae of the oyster Crassostrea virginica. Marine Biology, 48: 227-234. DOI:10.1007/BF00397149 (  0) 0) |
O'Foighil, D., Gaffney, P. M., Wilbur, A. E., and Hilbish, T. J., 1998. Mitochondrial cytochrome oxidase I gene sequences support an Asian origin for the Portuguese oyster Crassostrea angulata. Marine Biology, 131: 497-503. DOI:10.1007/s002270050341 (  0) 0) |
Perry, G. M. L., Martyniuk, C. M., Ferguson, M. M., and Danzmann, R. G., 2005. Genetic parameters for upper thermal tolerance and growth-related traits in rainbow trout (Oncorhynchus mykiss). Aquaculture, 250: 120-128. DOI:10.1016/j.aquaculture.2005.04.042 (  0) 0) |
Proestou, D. A., Vinyard, B. T., Corbett, R. J., Piesz, J., Allen, S. K., Small, J. M., et al., 2016. Performance of selectively-bred lines of eastern oyster, Crassostrea virginica, across eastern US estuaries. Aquaculture, 464: 17-27. DOI:10.1016/j.aquaculture.2016.06.012 (  0) 0) |
Rawson, P. D., and Hilbish, T. J., 1991. Genotype × environment interaction for juvenile growth in the hard clam Mercenaria mercenaria (L.). Evolution, 45: 1924-1935. (  0) 0) |
Robertson, A., 1959. The sampling variance of the genetic correlation coefficient. Biometrics, 15: 469-485. DOI:10.2307/2527750 (  0) 0) |
Sheridan, A. K., 1997. Genetic improvement of oyster production – A critique. Aquaculture, 153: 165-179. DOI:10.1016/S0044-8486(97)00024-0 (  0) 0) |
Soletchnik, P., Huvet, A., Moine, O. L., Razet, D., Geairon, P., Faury, N., et al., 2002. A comparative field study of growth, survival and reproduction of Crassostrea gigas, C. angulata and their hybrids. Aquatic Living Resource, 15: 243-250. DOI:10.1016/S0990-7440(02)01175-0 (  0) 0) |
Sprague, G. F., and Tatum, L. A., 1942. General vs. specific combining ability in single crosses of corn. Journal of the American Society of Agronomy, 34: 923-932. DOI:10.2134/agronj1942.00021962003400100008x (  0) 0) |
Stelkens, R., and Seehausen, O., 2009. Genetic distance between species predicts novel trait expression in their hybrids. Evolution, 64: 884-897. (  0) 0) |
Tan, K., Liu, H., Ye, T., Ma, H., and Li, S., 2020. Growth, survival and lipid composition of Crassostrea gigas, C. angulata and their reciprocal hybrids cultured in southern China. Aquaculture, 516: 734524. DOI:10.1016/j.aquaculture.2019.734524 (  0) 0) |
Thoa, N. P., Ninh, N. H., Hoa, N. T., Knibb, W., Diep, N. H., and Nguyen, N. H., 2016. Additive genetic and heterotic effects in a 4 × 4 complete diallel cross-population of Nile tilapia (Oreochromis niloticus, Linnaeus, 1758) reared in different water temperature environments in northern Vietnam. Aquaculture Research, 47: 708-720. DOI:10.1111/are.12530 (  0) 0) |
Volker, P. W., Potts, B. M., and Borralho, N. M. G., 2008. Genetic parameters of intra- and inter-specific hybrids of Eucalyptus globulus and E. nitens. Tree Genetics & Genomes, 4: 445-460. (  0) 0) |
Wang, B., Gu, W., Gao, H., Hu, G., and Yang, R., 2014. Longitudinal genetic analysis for growth traits in the complete diallel cross of rainbow trout (Oncorhynchus mykiss). Aquaculture, 430: 173-178. DOI:10.1016/j.aquaculture.2014.04.004 (  0) 0) |
Wang, C., Liu, B., Liu, X., Ma, B., Zhao, Y., Zhao, X., et al., 2017. Selection of a new scallop strain, the Bohai Red, from the hybrid between the bay scallop and the Peruvian scallop. Aquaculture, 479: 250-255. DOI:10.1016/j.aquaculture.2017.05.045 (  0) 0) |
Xu, H. Q., Li, Q., Han, Z. Q., Liu, S. K., Yu, H., and Kong, L. F., 2019. Fertilization, survival and growth of reciprocal crosses between two oysters, Crassostrea gigas and Crassostrea nippona. Aquaculture, 507: 91-96. DOI:10.1016/j.aquaculture.2019.04.012 (  0) 0) |
Yang, B., Zhai, S. Y., Li, X., Tian, J., Li, Q., Shan, H., et al., 2021. Identification of Vibrio alginolyticus as a causative pathogen associated with mass summer mortality of the Pacific oyster (Crassostrea gigas) in China. Aquaculture, 535: 736363. DOI:10.1016/j.aquaculture.2021.736363 (  0) 0) |
Zhang, J., Li, Q., and Xu, C., 2018. Estimates of genetic parameters of growth-related traits in Crassostrea gigas 'Haida No. 1'. Journal of Fishery Sciences of China, 25: 998-1003 (in Chinese with English abstract).
(  0) 0) |
Zhang, T., Kong, J., Liu, B., Wang, Q., Cao, B., Luan, S., et al., 2014. Genetic parameter estimation for juvenile growth and upper thermal tolerance in turbot (Scophthalmus maximus Linnaeus). Acta Oceanologica Sinica, 33(8): 106-110. DOI:10.1007/s13131-014-0460-3 (  0) 0) |
Zhang, X., Huang, B. W., Zheng, Y. D., Xin, L. S., Chen, W. B., Yu, T., et al., 2023. Identification and characterization of infectious pathogens associated with mass mortalities of Pacific oyster (Crassostrea gigas) cultured in northern China. Biology, 12: 759.
(  0) 0) |
Zhang, Y. H., Li, J., Zhang, Y., Ma, H. T., Xiao, S., Xiang, Z. M., et al., 2017. Performance evaluation of reciprocal hybrids derived from the two brackish oysters, Crassostrea hongkongensis and Crassostrea sikamea in southern China. Aquaculture, 473: 310-316. DOI:10.1016/j.aquaculture.2017.02.031 (  0) 0) |
Zhang, Y. H., Zhang, Y., Li, J., Xiao, S., Xiang, Z. M., Wang, Z. P., et al., 2016. Artificial interspecific backcrosses between the hybrid of female Crassostrea hongkongensis × male C. gigas and the two parental species. Aquaculture, 450: 95-101. DOI:10.1016/j.aquaculture.2015.07.013 (  0) 0) |
Zheng, H., Wang, D., Lin, Q., Sun, Z., Zhang, T., and Chen, X., 2012. Hybridization between the two close related species Crassostrea gigas and C. angulata and heterosis for growth and survival at early stage of life history. Journal of Fisheries of China, 36(2): 210-215 (in Chinese with English abstract). (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


