2) Guangdong Provincial Key Laboratory of Aquatic Product Processing and Safety, Guangdong Ocean University, Zhanjiang 524000, China;
3) Collaborative Innovation Center of Provincial and Ministerial Co-Construction for Marine Food Deep Processing, Dalian Polytechnic University, Dalian 116034, China
Lipases, which refer to as triacylglycerol acyl hydrolases (E.C. 3.1.1.3), catalyze reversible triacylglycerol hydrolysis to diacylglycerol, monoacylglycerol, fatty acid (FA), and glycerol (Casas-Godoy et al., 2012). Lipases have numerous uses in a variety of industrial fields, such as food, pharmaceuticals, biofuels, and biotechnology. Recent years have witnessed a great interest in modifying lipids for the production of triacylglycerols enriched with n-3 polyunsaturated fatty acids (n-3 PUFAs). n-3 PUFAs have a variety of biological activities (Li et al., 2019; Gu et al., 2020; Gao et al., 2021; Zhu et al., 2022), and long-chain PUFAs are highly sensitive to oxidation. Enzymatic catalytic reactions can prevent lipid oxidation and trans isomer production due to their selectivity and mild reaction conditions. Various studies were conducted using microbial lipases for the production and concentration of n-3 PUFAs due to their regiospecificity and stereospecificity (Rubio-Rodríguez et al., 2010; Kralovec et al., 2012). Considering the unusual diets and habitats of fish, their lipases may exhibit unique regioselectivity or FA selectivity compared with lipases from mammals and microbes. Thus far, lipases have been purified and characterized from grey mullet (Smichi et al., 2013), sardine (Smichi et al., 2010), sea bream (Smichi et al., 2017), cod (Gjellesvik et al., 1992), Chinook salmon, and New Zealand hoki (Kurtovic et al., 2010). However, the isolation and application of lipases from golden pompano (Trachinotus ovatus) have not been reported.
Golden pompano is widely cultured in the South China Sea (Wang et al., 2013) and has become a good candidate species in the aquaculture industry due to its rapid growth, high flesh quality, and easiness of farming (Ma et al., 2016). Golden pompano processing generates large quantities of wastes that are usually discarded or reduced to fish meal.
Fish viscera can be recycled into valuable biomolecules, such as enzymes (Kurtovic et al., 2009; Zhao et al., 2011) and n-3 PUFAs, or used in biodiesel production. They can also be used as the growth medium of microorganisms. Many high-level lipases come from microorganisms in viscera, such as Staphylococcus xylosus and S. epidermidis (Ben Rebah and Miled, 2013). The efficient extraction of enzymes from golden pompano viscera has the potential to generate economic and environmental benefits to the fishery processing and enzyme industries. Despite the availability of large amounts of visceral waste, the isolation and usage of the enzyme from golden pompano have not been reported. Thus, fundamental studies on golden pompano enzymes are warranted.
This study was conducted to isolate and purify the lipase from golden pompano viscera (GPL), characterize its properties, and prepare n-3 PUFA concentrates through the lipase-assisted hydrolysis of golden pompano viscera oil-derived triglycerides.
2 Materials and Methods 2.1 MaterialsViscera from golden pompano were obtained from the gutting line at Hainan Xiangtai Fishery Co., Ltd. (Hainan, China). They were frozen immediately and transported to the laboratory. Chemicals and reagents of analytical grade were purchased from Guangzhou Chemical Co. (Guangzhou, China).
2.2 Extraction of Crude Enzyme and Purification of LipaseViscera samples were cut into small pieces and powdered in liquid nitrogen with a Waring blender, followed by lyophilization and degreasing using the method of Iijima et al. (1998). A homogenate was acquired from the prepared 10-time volume of extraction buffer (25 mmol L−1 phosphate buffer, 150 mmol L−1 NaCl, 2 mmol L−1 benzamidine, pH 7.0). The extract was stirred for 45 min and centrifuged in 10000 g at 4℃ for 30 min. The supernatant was then filtered through a Buchner funnel and collected for the measurement of lipase activity and protein concentration.
Protein precipitates were acquired using ammonium sulfate at ratios of 0 – 30%, 30% – 60%, and 60% – 90% saturation. A comparison was conducted for the protein content, specific enzyme activity, purification factor, and protein molecular weight of the precipitates with different saturation levels. The result showed that most of the target proteins can be precipitated at 60% – 90% saturation. Therefore, the fraction of 60% – 90% saturation was gathered through centrifugation (10000 g, 30 min, 4℃). The sediment was resuspended in 25 mmol L−1 phosphate buffer (pH 7.0), dialyzed, and further wiped off through centrifugation. The above operations were carried out at 4℃.
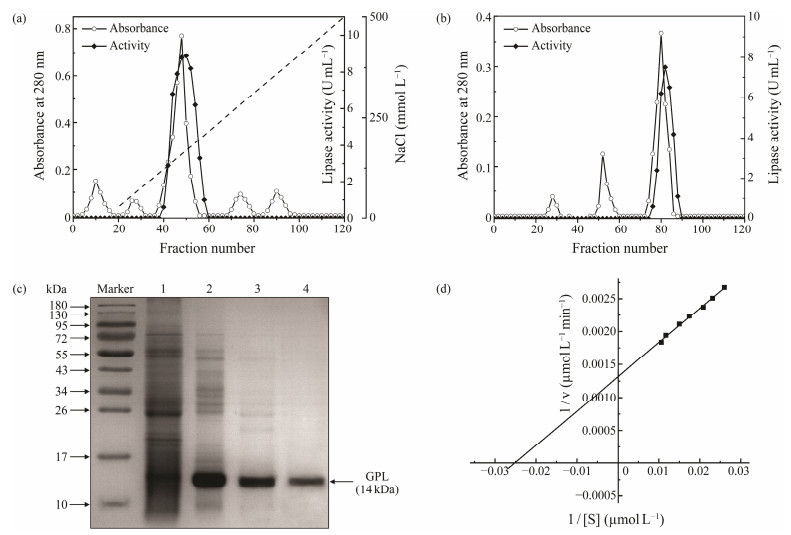
The dialyzed supernatant containing the lipase was loaded into a DEAE-Sepharose Fast Flow column (1.6 cm × 30 cm, GE Healthcare) equilibrated with buffer A (25 mmol L−1 Tris-HCl pH 8.0, 25 mmol L−1 NaCl, 2 mmol L−1 benzamidine) at 300 mL h−1 (Xia et al., 2015; Hei et al., 2019). Nonabsorbed proteins were removed with one bed volume of buffer A. The adsorbed substance was then eluted with a linear gradient of NaCl (25 – 500 mmol L−1), and 3 mL of fractions were gathered each time. Lipase was collected between 110 and 185 mmol L−1 NaCl (Fig.1a).
|
Fig. 1 (a) DEAE-Sepharose Fast Flow column purification profile of the crude enzyme extract precipitated using 60% – 90% ammonium sulfate. (b) Gel Filtration purification profile of fractions with lipase activity issued from the DEAE-Sepharose Fast Flow column. (c) SDS-PAGE analysis of GPL purification steps. Lane 1, crude extract; Lane 2, fraction collected by (NH4)2SO4 precipitation; Lane 3, peak fraction following DEAE-Sepharose Fast Flow; Lane 4, peak fraction following Sephadex G-75. (d) Kinetics of hydrolysis of p-NPP by GPL. The concentrations of p-NPP range from 0 to 100 μmol L−1. Km and Vmax values are from Lineweaver-Burk plot and expressed as the means of three independent experiments. |
Pooled fractions with lipase activity obtained from the DEAE-Sepharose Fast Flow column were desalted, concentrated, and loaded on a Sephadex G-75 column (1.6 cm × 100 cm, GE Healthcare) prebalanced with buffer A. Elution was carried out with buffer A at a rate of 35 mL h−1, and the elution components with lipase were pooled and lyophilized (Fig.1b).
2.3 Lipase Activity DeterminationGolden pompano lipase (GPL) activity was measured according to the hydrolysis of p-nitrophenyl palmitate (p-NPP) (Winkler and Stuckmann, 1979). The substrate buffer solution was composed of 0.1 mmol L−1 p-NPP in 25 mmol L−1 Tris-HCl buffer (pH 8.0, containing 4 mmol L−1 sodium deoxycholate and 0.01% gum Arabic). In brief, 20 µL of the GPL was mixed with the substrate buffer solution to a total volume of 3 mL and allowed to react at 40℃ for 15 min. The heat-inactivated sample was employed to build blank activity, and absorbance was determined at 410 nm. One unit of activity was defined as 1 µmol L−1 p-nitrophenol (p-NP) freed in 1 min under the test conditions.
2.4 SDS-PAGE Analysis of LipaseProtein analysis was performed following the classical method of Laemmli (1970) using 15% acrylamide gel stained with Coomassie blue. The protein content was determined as described by Bradford (1976).
2.5 Kinetic ParametersWith the p-NPP substrate concentration range set to 0 – 100 µmol L−1 using the standard lipase assay, the kinetic parameters (Km and Vmax values) of GPL were computed from the Lineweaver-Burk plot.
2.6 Effects of pH and Temperature on GPL Activity and StabilityThe substrate solution was obtained from different buffers with pH values from 7.0 to 11.0 to measure GPL activity at different pH levels. GPL was first incubated at pH 3.0 – 12.0 for 30 min at room temperature, and the residual activity was measured to assess the effect of pH on GPL stability.
GPL activity was measured under different temperatures (20℃ to 60℃). GPL was incubated at varying temperatures (0℃ to 80℃) for 30 min and then rapidly cooled to 0℃ prior to the assay to assess its thermal stability.
2.7 Effects of Salt, Metal Ions, Bile Salt, Organic Solvents, and Orlistat on GPL ActivityGPL was incubated with NaCl ranging from 0 to 1 mol L−1, sodium deoxycholate (NaDC) ranging from 0 to 10 mmol L−1, or other tested substances ranging from 1 to 5 mmol L−1 at 25℃ for 30 min followed by activity measurements. The sample incubated without a chemical served as the control.
GPL stability in organic solvents was investigated according to the method of Aryee et al. (2007). GPL was incubated with various organic solvents (30% in Tris-HCl buffer) at 25℃ for 30 min. The residual activity was then measured after the organic solvents were removed through centrifugation (12000 g, 5 min, room temperature).
Orlistat was used as a specific inhibitor to assess whether GPL is a serine lipase. GPL was incubated with 1, 2, 5, 10, or 20 μg mL−1 of orlistat for 1 h at room temperature, and the residual lipase activity was then determined.
2.8 Preparation of n-3 PUFA Concentrates from Golden Pompano Viscera Oil by Lipase-Catalyzed HydrolysisOil extraction from golden pompano viscera was carried out using the method of Senphan and Benjakul (2015). A homogenate was prepared with minced viscera and an equal volume of distilled water containing 0.01% butylated hydroxytoluene as the antioxidant (1:1 w/v). Hydrolysis was carried out by adding trypsin at 50℃ and pH 9.0 for 120 min with constant shaking. Oil extract was collected through centrifugation at 5000 g for 10 min at 4℃ and stored in nitrogen.
After triglyceride hydrolysis was carried out with GPL, the alteration of FA composition at various time intervals (4 – 16 h) was analyzed. The reaction solution comprised 20 g of viscera oil, 60 mL of phosphate buffer (0.1 mol L−1, pH 8.0; 0.03% w/v gum Arabic), and lipase (800 U g−1 viscera oil) and was flushed with nitrogen. The reaction was conducted at 35℃ with constant shaking. Methanolic KOH solution (0.5 mol L−1) was used to neutralize the free FAs released during hydrolysis. Acylglycerols were separated from the solution by n-hexane after the pH was controlled to 1.0 by HCl (Chakraborty et al., 2010; Akanbi and Barrow, 2017).
FA methyl esters (FAMEs) were obtained using the AOCS (2007) method Ce-1b 89 and measured by GC-MS (Agilent 7890B) with an HP-5MS capillary column of 30 m × 0.25 mm × 0.25 µm film thickness). In brief, 1 µL of the sample was injected with a split ratio of 70:1 using helium as the carrier gas. The instrument details were as follows: injector temperature of 250℃, detector temperature of 280℃, initial oven temperature of 60℃ held for 2 min, programmed temperature increased by 6℃ min−1, and a final temperature of 300℃ held for 10 min. FAMEs were identified by comparing the retention time and the mass spectra with the Mast Hunter NIST11 MS library. The results were relatively quantified and shown as a percentage of total FAs.
2.9 Statistical AnalysisResults were shown as means ± standard deviation (SD) of three replicates. Data were compared by one-way ANOVA and Tukey's test using SPSS Statistics 17.0 (Mei et al., 2021; Meng et al., 2021). The difference was considered significant when P < 0.05.
3 Results and Discussion 3.1 GPL PurificationHere, a lipase was successfully purified from the crude
enzyme extracts of golden pompano viscera following the purification procedure detailed above. As summarized in Table 1, a final purification factor of 27.50 and an activity recovery rate of 18.31% were achieved in the purification. The fractions showing GPL activity after each purification step were analyzed with SDS-PAGE (Fig.1c). The results showed that GPL was homogeneously pure and possessed an apparent molecular weight (Mw) of 14 kDa. Previous studies reported that the Mw values of lipases isolated from other fishes are 35 – 79.6 kDa (Gjellesvik et al., 1992; Kurtovic et al., 2010; Smichi et al., 2013). GPL has lower Mw than most reported lipases and can be identified as a new type of lipase.
|
|
Table 1 Flow sheet of lipase purification |
The Km and Vmax values of GPL were calculated at 40.16 μmol L−1 and 769.23 μmol L−1 min−1 for the p-NPP substrate (Fig.1d). The Km value was relatively low, which indicated a high affinity to the p-NPP substrate compared with several lipases from other fishes (Gjellesvik et al., 1992; Aryee et al., 2007; Kurtovic et al., 2010; Görgün and Akpınar, 2012).
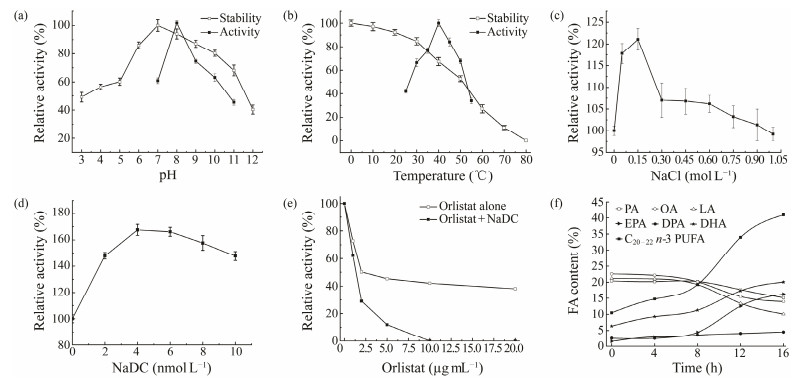
3.3 Effects of pH and Temperature on GPL Activity and StabilityGPL activity was maximized at pH 8.0 (Fig.2a), and was reduced when the enzyme reaction was performed at a pH level higher or lower than 8.0. Lipases from other fishes also show optimal activity at neutral to moderately alkaline pH, such as the digestive lipase from grey mullet at pH 8.0 (Smichi et al., 2013), the digestive lipase from Chinook salmon at pH 8.0, and New Zealand hoki at pH 8.5 (Kurtovic et al., 2010). GPL maintained higher than 80% of its activity between pH 6.0 and 10.0 and was most stable at pH 7.0 when incubated for 30 min at room temperature. The pH range stability of GPL was similar to that of lipases from other fish species such as grey mullet (Smichi et al., 2013) and Chinook salmon (Kurtovic et al., 2010).
|
Fig. 2 Effects of pH (a), temperature (b), NaCl (c), bile salt (d), and orlistat (e) on GPL activity. (f) Changes in the FA profile of golden pompano oil throughout the GPL-catalyzed hydrolysis. Reaction conditions: 20 g of oil, 60 mL of phosphate buffer, 16000 U of lipase, 35℃, 150 oscillations per minute. PA, C16:0; OA, C18:1 n-9; LA, C18:2 n-6; EPA, 20:5 n-3; DHA, 22:6 n-3; DPA, 22:5 n-3; C20 – 22 n-3 PUFA, EPA + DPA + DHA. |
GPL showed optimum activity against p-NPP at 40℃ (Fig. 2b). The optimum temperature of fish digestive enzymes depends greatly on fish species (warm or cold water species) and is typically close to 35℃ to 40℃ (Noriega-Rodríguez et al., 2009; Kurtovic et al., 2010; Smichi et al., 2010). Fish species from warm water habitats have higher optimum temperatures than those from cold water habitats (Kurtovic et al., 2009). GPL showed excellent stability between 0℃ and 30℃, which may be related to the optimum growth temperature of golden pompano at 26℃ to 29℃ (Yang, 2016). GPL maintained more than 60% of its activity in the temperature range of 0℃ to 40℃, and its stability decreased remarkably when the temperature was increased above 50℃ after a 30 min incubation period. The dramatic decrease in thermal stability is attributed to the denaturation of lipase and the destruction of its structure. A comparison of the optimum pH and temperature for the 15 fish lipases as reported showed that, similar to most fish lipases, GPL maintained its high activity in alkaline environments and at 35℃ to 40℃.
3.4 Effects of Salt, Metal Ions, Bile Salt, Organic Solvents, and Orlistat on GPL ActivityThe effect of high salinity on GPL activity was investigated (Fig.2c). GPL showed a good salinity tolerance of up to 1 mol L−1. The activity was peaked when incubated with 150 mmol L−1 NaCl. The activation of lipase by NaCl might be related to the habits of golden pompano, a euryhaline fish whose suitable growth condition is within the salinity range of 10 to 34 (Ma et al., 2016). Only a few studies have been conducted on the effect of salt concentration on lipase activity in fish. Some reports showed that fish lipases are highly sensitive to high salt concentrations. Sardine digestive lipase (Noriega-Rodríguez et al., 2009) loses 50% of its activity in the presence of 1 mol L−1 NaCl, and annular seabream (Smichi et al., 2017) loses 45%. In the present work, GPL was found to be highly salt-tolerant and thus may have potential application value in catalyzing some lipolysis reactions at high salt concentrations.
Bile salts are amphiphilic molecules, which can increase the contact area between lipase and substrate (Kortner et al., 2013). Thus, bile salts are conducive to substrate hydrolysis. In addition, bile salts may induce the conformational changes of a lipase that expose its active site and thus increase affinity to the substrate (Moore et al., 2001). In this experiment, the bile salt NaDC showed an activating effect on GPL activity within the measured concentration range (Fig.2d). GPL exhibited maximum activity in 4 mmol L−1 NaDC, which differed from that of pancreatic lipases that are greatly inhibited by bile salts (Smichi et al., 2013, 2015). Similar results have been reported for lipases from seabream (Nolasco et al., 2011), grey mullet (Aryee et al., 2007), and cod (Gjellesvik et al., 1992). Orlistat is a selective inhibitor of gastrointestinal lipases and interacts with nucleophilic serine residues (Tiss et al., 2010). Here, the effect of orlistat on GPL activity was investigated to determine whether GPL is a serine enzyme (Fig.2e). GPL lost half of its lipase activity in the presence of 2 µg mL−1 orlistat and maintained 38% of its activity in the presence of 20 µg mL−1 orlistat. The combination of orlistat and NaDC showed a more potent inhibitory effect than orlistat alone. The inhibition of GPL activity was significantly accelerated by the addition of 4 mmol L−1 NaDC. The inhibitory effect of orlistat on GPL activity indicated that the serine residue is required for its catalytic activity. Therefore, GPL is a serine hydrolase, similar to all known lipases isolated from different origins.
Metal ions are important in the catalytic function of enzymes; they may bind with the specific sites of enzymes and exhibit a structural role. As presented in Table 2, GPL was inhibited by Cu2+ and Zn2+, enhanced by Mn2+, and was not affected by Mg2+ to a great extent. Ca2+ at 1 mmol L−1 caused a slight 5% increase in GPL activity, and Ca2+ at 5 mmol L−1 had no appreciable effect on GPL activity. Thus, GPL catalysis does not require Ca2+, similar to any other lipases (Nolasco et al., 2011; Smichi et al., 2015). This property is different with the lipases isolated from Chinook salmon and New Zealand hoki (Kurtovic et al., 2010), whose activities depend on Ca2+.
|
|
Table 2 Effect of selected chemicals on GPL stability |
GPL activity was decreased 11% by 1 mmol L−1 EDTA. The increased EDTA concentration of up to 5 mmol L−1 showed a similar inhibition effect on GPL activity, implying that GPL is not a metalloenzyme. As a serine protease inhibitor, PMSF inhibited GPL activity, and this result was in agreement with the lipases obtained from grey mullet (Aryee et al., 2007), Chinook salmon, and New Zealand hoki (Kurtovic et al., 2010). This phenomenon might be due to the involvement of a typical catalytic triad in the function of serine proteases and lipases. Surfactants can lower the surface tension when applied in water at limited concentrations, thus influencing enzyme catalysis (Aryee et al., 2007). The anionic surfactant of SDS and the nonionic surfactants, including Triton X-100 and Tween-80, reduced the GPL activity.
Whether a lipase can be utilized in the catalysis of synthetic reactions depends on its stability in organic solvents. GPL was stable in the chosen organic solvents, except in acetone, isopropanol, and butanol (Table 3). Acetone forms strong hydrogen bonds, which may induce the loss of enzymatic activity (Pogorevc et al., 2002). GPL maintained complete activity in chloroform and n-hexane. The enzyme might have been in an open conformation in the presence of water-immiscible organic solvents. Similar behavior was found for the lipases from grey mullet (Aryee et al., 2007), Chinook salmon, and New Zealand hoki (Kurtovic et al., 2010). The apparent stability of GPL in lipophilic solvents implies its possible use in reactions that require low water content, for example, synthesis reactions and lipid modifications.
|
|
Table 3 GPL stability in the presence of various organic solvents |
The FA composition of golden pompano viscera oil is given in Table 4. The dominant FA in the substrate was oleic acid (C18:1), accounting for 22.6% of total FAs, followed by linoleic acid (C18:2 n-6, 21.17%) and palmitic acid (C16:0, 20.43%). The total n-3 PUFA content was 11.57%.
|
|
Table 4 FA profiles of crude and lipase hydrolysates of golden pompano viscera oil at four different time durations (4, 8, 12, and 16 h) using GPL |
The changes in the FA profile of golden pompano viscera oil throughout the lipase-catalyzed hydrolysis are illustrated in Table 4. ∑SFA and ∑MUFA decreased significantly during the hydrolysis by GPL mainly due to the removal of SFA and MUFA such as C16:0 and C18:1. By contrast, ∑PUFA showed an increase from 34.99% to 56.99% with the time up to 16 h. The reduction in SFA and MUFA after GPL hydrolysis may be due to the high selectivity of GPL for SFA and MUFA, therefore releasing these FAs while recovering PUFAs in the glyceride fraction. In addition, GPL hydrolysis released abundant linoleic acid, leading to a decrease in the acylglycerol fraction of this FA. Lipases normally show specificity in hydrolyzing FAs with a particular acyl chain length (Chakraborty et al., 2010). GPL seems to be specific in hydrolyzing C18 acyl chain. Meanwhile, the total C20 – 22 n-3 PUFA content was enriched to 40.9%, almost quadrupling its initial level. The hydrolysis of sardine oil by Bacillus circulans lipase led to a twofold rise in EPA concentration to 37.7% but was ineffective in extracting DHA and DPA (Chakraborty et al., 2010). The concentration results of Candida cylinderacea lipase on marine microalgae also showed that the concentration effect was mainly aimed at EPA, and the effects on DHA and DPA were not evident (Jacob and Mathew, 2017). In the present study, GPL catalyzed the hydrolysis of golden pompano oil and enriched the EPA. EPA content was increased from 1.57% to 16.3% and was concentrated to 10.4-fold of its original level after 16 h of hydrolysis. Meanwhile, DHA and DPA contents were enriched to 3.2 and 1.8 folds, respectively. On the basis of these results, GPL is less reactive toward FAs higher than C20 n-3. Therefore, n-3 PUFAs with acyl chain lengths longer than C20, including EPA, DHA, and DPA, can be enriched in the acylglycerol portion. The initial level of n-3 PUFA concentration is relatively low when byproducts are utilized. In this study, GPL-catalyzed hydrolysis effectively enriched the n-3 PUFAs in the oil obtained from golden pompano processing waste. These results suggested the potential use of GPL in the oleochemical industry.
4 ConclusionsIn this study, a lipase from golden pompano viscera was successfully purified using three consecutive purification steps to achieve apparent homogeneity. GPL is a monomeric enzyme with an estimated Mw of 14 kDa and exhibits interesting properties, especially its remarkable stability against organic solvents (methanol, ethanol, chloroform, and hexane) and high salt concentrations. Bile salt NaDC acts as an activator of GPL. GPL can significantly enrich EPA, DHA, and DPA in golden pompano oil through hydrolysis, suggesting its potential as an enzyme for the preparation of n-3 PUFA concentrates of acyl chain length longer than C20. These results indicate the high potential of GPL for oleochemical industrial applications.
AcknowledgementsThis work was supported by the National Key R & D Programs of China (No. 2018YFD0901103), the Program of the Hainan Association for Science and Technology Plans to Youth R & D Innovation (No. QCXM20 2003), and the Hainan Provincial Natural Science Foundation of China (No. 2019RC093).
Akanbi T. O., Barrow C. J.. 2017. Candida antarctica lipase A effectively concentrates DHA from fish and thraustochytrid oils. Food Chemistry, 229: 509-516. DOI:10.1016/j.foodchem.2017.02.099 (  0) 0) |
Aryee A. N. A., Simpson B. K., Villalonga R.. 2007. Lipase fraction from the viscera of grey mullet (Mugil cephalus). Enzyme and Microbial Technology, 40: 394-402. DOI:10.1016/j.enzmictec.2006.07.009 (  0) 0) |
Ben Rebah F., Miled N.. 2013. Fish processing wastes for microbial enzyme production: A review. 3 Biotech, 3: 255-265. DOI:10.1007/s13205-012-0099-8 (  0) 0) |
Bradford M. M.. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254. DOI:10.1016/0003-2697(76)90527-3 (  0) 0) |
Casas-Godoy L., Duquesne S., Bordes F., Sandoval G., Marty A.. 2012. Lipases: An overview. Methods in Molecular Biology, 861: 3-30. DOI:10.1007/978-1-61779-600-5_1 (  0) 0) |
Chakraborty K., Vijayagopal P., Chakraborty R. D., Vijayan K. K.. 2010. Preparation of eicosapentaenoic acid concentrates from sardine oil by Bacillus circulans lipase. Food Chemistry, 120: 433-442. DOI:10.1016/j.foodchem.2009.10.032 (  0) 0) |
Gao X., Yi X., Liu Z., Dong X., Xia G., Zhang X., et al. 2021. Comparative study on curcumin loaded in golden pompano (Trachinotus blochii) head phospholipid and soybean lecithin liposomes: Preparation, characteristics and anti-inflammatory properties. Molecules, 26: 2328. DOI:10.3390/molecules26082328 (  0) 0) |
Gjellesvik D. R., Lombardo D., Walther B. T.. 1992. Pancreatic bile salt dependent lipase from cod (Gadus morhua): Purification and properties. Biochimica et Biophysica Acta (BBA) – Lipids and Lipid Metabolism, 1124: 123-134. DOI:10.1016/0005-2760(92)90088-D (  0) 0) |
Görgün S., Akpınar M. A.. 2012. Purification and characterization of lipase from the liver of carp, Cyprinus carpio L. (1758), living in Lake Tödürge (Sivas, Türkiye). Turkish Journal of Fisheries and Aquatic Sciences, 12: 207-215. DOI:10.4194/1303-2712-v12_2_03 (  0) 0) |
Gu Z., Zhu Y., Jiang S., Xia G., Li C., Zhang X., et al. 2020. Tilapia head glycolipids reduce inflammation by regulating the gut microbiota in dextran sulphate sodium-induced colitis mice. Food & Function, 11: 3245-3255. DOI:10.1039/d0fo00116c (  0) 0) |
Hei Z., Zhao M., Tian Y., Chang H., Shen X., Xia G., et al. 2019. Isolation and characterization of a novel sialoglycopeptide promoting osteogenesis from gadus morhua eggs. Molecules, 25: 156. DOI:10.3390/molecules25010156 (  0) 0) |
Jacob J. P., Mathew S.. 2017. Effect of lipases from Candida cylinderacea on enrichment of PUFA in marine microalgae: Lipases on microalgae. Journal of Food Processing and Preservation, 41: e12928. DOI:10.1111/jfpp.12928 (  0) 0) |
Kortner T. M., Gu J., Krogdahl Å., Bakke A. M.. 2013. Transcriptional regulation of cholesterol and bile acid metabolism after dietary soyabean meal treatment in Atlantic salmon (Salmo salar L.). British Journal of Nutrition, 109: 593-604. DOI:10.1017/S0007114512002024 (  0) 0) |
Kralovec J. A., Zhang S., Zhang W., Barrow C. J.. 2012. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chemistry, 131: 639-644. DOI:10.1016/j.foodchem.2011.08.085 (  0) 0) |
Kurtovic I., Marshall S. N., Zhao X., Simpson B. K.. 2009. Lipases from mammals and fishes. Reviews in Fisheries Science, 17: 18-40. DOI:10.1080/10641260802031322 (  0) 0) |
Kurtovic I., Marshall S. N., Zhao X., Simpson B. K.. 2010. Purification and properties of digestive lipases from Chinook salmon (Oncorhynchus tshawytscha) and New Zealand hoki (Macruronus novaezelandiae). Fish Physiology and Biochemistry, 36: 1041-1060. DOI:10.1007/s10695-010-9382-y (  0) 0) |
Li Y., Li P., Xia G., Li C., Shen X.. 2019. Analysis and identification of Golden Pompano (Trachinotus blochii) head phospholipid molecular species by liquid chromatography-mass spectrometry. Journal of Oleo Science, 68: 1187-1197. DOI:10.5650/jos.ess19189 (  0) 0) |
Ma Z., Guo H., Zheng P., Wang L., Jiang S., Zhang D., et al. 2016. Effect of salinity on the rearing performance of juvenile golden pompano Trachinotus ovatus (Linnaeus 1758). Aquaculture Research, 47: 1761-1769. DOI:10.1111/are.12633 (  0) 0) |
Mei F., Meng K., Gu Z., Yun Y., Zhang W., Zhang C., et al. 2021. Arecanut (Areca catechu L.) seed polyphenol-ameliorated osteoporosis by altering gut microbiome via LYZ and the immune system in estrogen-feficient rats. Journal of Agricultural and Food Chemistry, 69: 246-258. DOI:10.1021/acs.jafc.0c06671 (  0) 0) |
Meng K., Mei F., Zhu L., Xiang Q., Quan Z., Pan F., et al. 2021. Arecanut (Areca catechu L.) seed polyphenol improves osteoporosis via gut-serotonin mediated Wnt/β-catenin pathway in ovariectomized rats. Journal of Functional Foods, 84: 104598. DOI:10.1016/j.jff.2021.104598 (  0) 0) |
Moore S. A., Kingston R. L., Loomes K. M., Hernell O., Bläckberg L., Baker H. M., et al. 2001. The structure of truncated recombinant human bile salt-stimulated lipase reveals bile saltindependent conformational flexibility at the active-site loop and provides insights into heparin binding. Journal of Molecular Biology, 312: 511-523. DOI:10.1006/jmbi.2001.4979 (  0) 0) |
Nolasco H., Moyano-López F., Vega-Villasante F.. 2011. Partial characterization of pyloric-duodenal lipase of gilthead seabream (Sparus aurata). Fish Physiology and Biochemistry, 37: 43-52. DOI:10.1007/s10695-010-9414-7 (  0) 0) |
Noriega-Rodríguez J. A., Gámez-Meza N., Alanis-Villa A., Medina-Juárez L. A., Tejeda-Mansir A., Angulo-Guerrero O., et al. 2009. Extraction and fractionation of lipolytic enzyme from viscera of Monterey sardine (Sardinops sagax caerulea). International Journal of Food Science and Technology, 44: 1223-1228. DOI:10.1111/j.1365-2621.2009.01951.x (  0) 0) |
Pogorevc M., Stecher H., Faber K.. 2002. A caveat for the use of log P values for the assessment of the biocompatibility of organic solvents. Biotechnology Letters, 24: 857-860. DOI:10.1023/A:1015598523282 (  0) 0) |
Rubio-Rodríguez N., Beltrán S., Jaime I., de Diego S. M., Sanz M. T., Carballido J. R.. 2010. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innovative Food Science & Emerging Technologies, 11: 1-12. DOI:10.1016/j.ifset.2009.10.006 (  0) 0) |
Senphan T., Benjakul S.. 2015. Impact of enzymatic method using crude protease from Pacific white shrimp hepatopancreas on the extraction efficiency and compositions of lipids. Food Chemistry, 166: 498-506. DOI:10.1016/j.foodchem.2014.06.054 (  0) 0) |
Smichi N., Fendri A., Chaâbouni R., Rebah F. B., Gargouri Y., Miled N.. 2010. Purification and biochemical characterization of an acid-stable lipase from the pyloric caeca of sardine (Sardinella aurita). Applied Biochemistry and Biotechnology, 162: 1483-1496. DOI:10.1007/s12010-010-8920-5 (  0) 0) |
Smichi N., Fendri A., Gargouri Y., Miled N.. 2015. A high salt-tolerant thermoactive esterase from golden grey mullet: Purification, characterization and kinetic properties: Characterization of a grey mullet thermoactive esterase. Journal of Food Biochemistry, 39: 289-299. DOI:10.1111/jfbc.12129 (  0) 0) |
Smichi N., Gargouri Y., Miled N., Fendri A.. 2013. A grey mullet enzyme displaying both lipase and phospholipase activities: Purification and characterization. International Journal of Biological Macromolecules, 58: 87-94. DOI:10.1016/j.ijbiomac.2013.03.056 (  0) 0) |
Smichi N., Miled N., Gargouri Y., Fendri A.. 2017. A newly thermoactive and detergent-stable lipase from annular sea bream (Diplodus annularis): Biochemical properties. Biotechnology and Applied Biochemistry, 64: 79-86. DOI:10.1002/bab.1445 (  0) 0) |
Tiss A., Lengsfeld H., Verger R.. 2010. A comparative kinetic study on human pancreatic and Thermomyces lanuginosa lipases: Inhibitory effects of tetrahydrolipstatin in the presence of lipid substrates. Journal of Molecular Catalysis B: Enzymatic, 62: 19-26. DOI:10.1016/j.molcatb.2009.08.011 (  0) 0) |
Wang F., Han H., Wang Y., Ma X.. 2013. Growth, feed utilization and body composition of juvenile golden pompano Trachinotus ovatus fed at different dietary protein and lipid levels. Aquaculture Nutrition, 19: 360-367. DOI:10.1111/j.1365-2095.2012.00964.x (  0) 0) |
Winkler U. K., Stuckmann M.. 1979. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. Journal of Bacteriology, 138: 663-670. DOI:10.1128/jb.138.3.663-670.1979 (  0) 0) |
Xia G., Yu Z., Zhao Y., Wang Y., Wang S., He M., et al. 2015. Sialoglycoproteins isolated from the eggs of Carassius auratus prevents osteoporosis by suppressing the activation of osteoclastogenesis related NF-κB and MAPK pathways. Journal of Functional Foods, 17: 491-503. DOI:10.1016/j.jff.2015.05.036 (  0) 0) |
Yang Q., Ma Z., Zheng P., Jiang S., Qin J., Zhang Q.. 2016. Effect of temperature on growth, survival and occurrence of skeletal deformity in the golden pompano Trachinotus ovatus larvae. Indian Journal of Fisheries, 63: 74-82. DOI:10.21077/ijf.2016.63.1.51490-10 (  0) 0) |
Zhao L., Budge S. M., Ghaly A. E., Brooks M. S., Dave D.. 2011. Extraction, purification and characterization of fish pepsin: A critical review. Journal of Food Processing and Technology, 2: 126. DOI:10.4172/2157-7110.1000126 (  0) 0) |
Zhu Y., Liu S., Mei F., Zhao M., Xia G., Shen X.. 2022. Tilapia nilotica head lipids improved bone loss by regulating inflammation and serum metabolism through gut microbiota in ovariectomized rats. Frontiers in Nutrition, 8: 792793. DOI:10.3389/fnut.2021.792793 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


