2) Powerchina Northwest Engineering Corporation Limited, Xi'an 710065, China
Hypotrichia Stein, 1859 is one of the largest groups in Ciliophora and has garnered great interest from protozoologists in recent times (Borror, 1972; Berger, 2006; Song and Shao, 2017; Wang et al., 2021; Fu et al., 2022; Song et al., 2022). Nevertheless, the hypotrichs are among the most complex groups of ciliates in terms of their systematic characteristics, mainly due to a lack of morphological, morphogenetic, and molecular data for many taxa (Dong et al., 2022; Luo et al., 2022; Omar et al., 2022; Zhang et al., 2022; Bharti and Kumar, 2023).
The urostylids are composed of over 200 species and 60 genera. Studies from the past years revealed that this group is even more diverse than previously supposed (Eigner and Foissner, 1992; Berger, 2006; Foissner, 2016, 2021). The genus Apokeronopsis was erected by Shao et al. (2007), with A. crassa (Claparède and Lachmann, 1858) Shao et al., 2007 as type species. Ever since, five Apokeronopsis species have been studied (Petz, 1995; Li et al., 2008; Long et al., 2008; Shao et al., 2008; Liu et al., 2009; Jung et al., 2011). Apokeronopsis wrighti Long et al., 2008, as originnally reported in a population from Hong Kong, China, was characterized solely on morphology and SSU rRNA sequence (Long et al., 2008). Morphogenetic information, LSU rDNA and ITS1-5.8S-ITS2 genes in A. wrighti have not been reported yet.
In the survey of ciliate biodiversity in Southeast China, a population of Apokeronopsis wrighti was isolated, giving us the opportunity to investigate its ontogenetic processes in detail during binary fission and providing more perspectives to realize its phylogenetic position and relationship among Apokeronopsis species in the meantime.
2 Materials and Methods 2.1 Sampling and Morphological MethodsApokeronopsis wrighti was isolated on 15 November 2022 from Rose Coast (22˚36΄55.2΄΄N, 114˚23΄6.4΄΄E), Shenzhen, China, when the water temperature was 26℃ and the salinity was 30. The cultures were maintained in Petri dishes at room temperature using habitat water. Although clonal cultures were not established, no similar morphotypes were present in the protargol preparations. Hence, it is highly probable that the present morphological, morphogenetic and molecular studies deal with the same species. Cells were observed in vivo using bright field and dif-ferential interference contrast microscopy (Olympus DP74) at (100 – 1000) × magnification. The infraciliature and nuclear apparatus were revealed by protargol staining (Wilbert, 1975). The protargol (Sigma-Aldrich, product number 448 K2787347) silver staining method according to Wilbert (1975) was used to reveal the infraciliature and nuclear apparatus. Six protargol slides containing voucher specimens (registration numbers: LCY2022111504-1, LCY2022111504-2, LCY2022111504-3, LCY2022111504-4, LCY2022111504-5, LCY2022111504-6) were deposited in the Laboratory of Protozoology, Shaanxi Normal University. Counts and measurements were conducted at a magnifications of 1000 ×. Drawings of stained specimens were made with the help of a drawing attachment and photomicrographs. To illustrate the changes during morphogenesis, parental cirri are depicted by contour whereas new ones are shaded black. Taxonomy and systematics are according to Berger (2006) and Shao et al. (2007).
2.2 DNA Extraction and Gene SequencingFirstly, to eliminate any contamination of other organisms, the target species were washed with sterile water. Then, the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) was utilized to obtain whole genomic DNA of Apokeronopsis wrighti. After that, three rDNA genes (SSU rDNA, LSU rDNA, and ITS1-5.8S-ITS2) were amplified with primers 18SF (5'-AAC CTG GTT GAT CCT GCC AGT-3'), 18SR (5'-TGA TCC TTC TGC AGG TTC ACC TAC-3'), F3 (5'-ACCCGCTGAACTTAAGCAT-3'), R2 (5'-AACCTTGG AGACCTGAT-3'), ITS-F (5'-GTAGGTGAACCTGCGG AAGGATCATTA-3'), and ITS-R (5'-TACTGATATGCT TAAGTTCAGCGG-3'), respectively (Medlin et al., 1988; Jerome et al., 1996; Moreira et al., 2007; Gao et al., 2012). Finally, the PCR product was sequenced by Tsingke Biological Technology Company (Beijing, China) and assembled by SeqMan (DNA Star).
2.3 Phylogenetic AnalysesHere, the rDNA sequences of our new isolate and relative taxa (accession numbers shown in Fig.5) are used for phylogenetic study. Among them, four euplotids are chosen as outgroup taxa. Three rDNA databases were aligned and edited using the MAFFT version 7 server (https://mafft.cbrc.jp/alignment/server/), obtaining alignments of 1813 (SSU rDNA) and 4557 (concatenated three-gene data) sites, respectively. Subsequently, the Maximum likelihood (ML) and Bayesian inference (BI) analyses were separately performed using RAxML-HPC2 and MrBayes 3.2.7 at the CIPRES Science Gateway (Ronquist et al., 2012; Stamatakis, 2014). The model of ML was GTRGAMMA and that of BI was GTR + I + G (Nylander, 2004; Miller et al., 2012). Furthermore, 1000 bootstrap replicates were used for ML analysis, while 10000000 generations with sampling every 100 generations and a burn-in of 25000 trees were carried out for BI analysis.
|
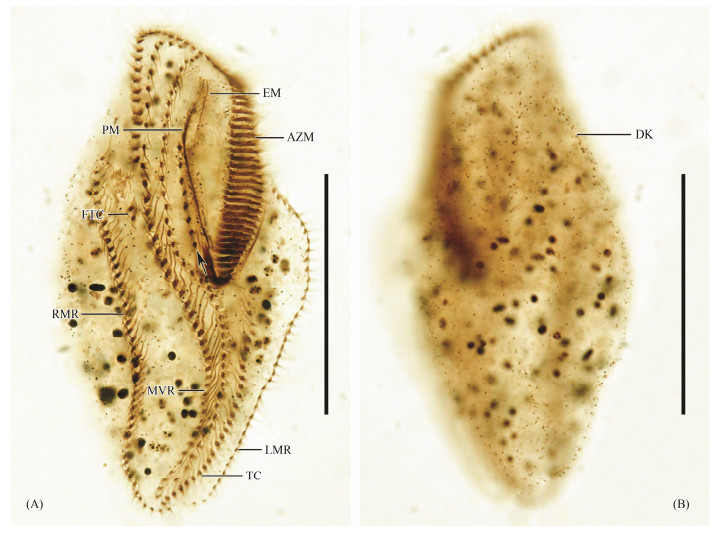
Fig. 1 Photomicrographs of Apokeronopsis wrighti Long et al., 2008 after protargol impregnation. Ventral (A) and dorsal (B) views of the same specimen, showing the ciliary pattern of a non-divider, arrow indicate cirri row arranged along the paroral and endoral. Scale bar = 50 μm. |
|
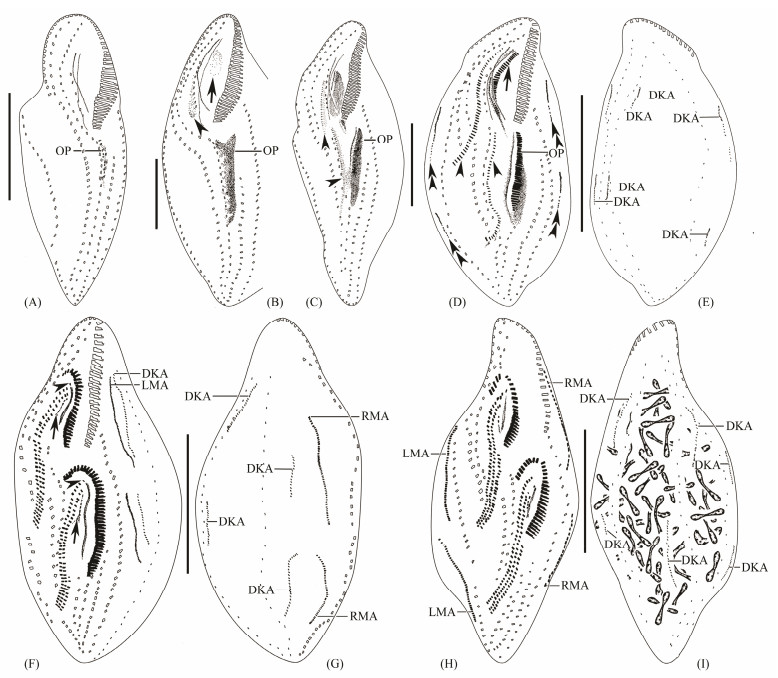
Fig. 2 Early to middle-late morphogenetic stages of Apokeronopsis wrighti Long et al., 2008. (A), ventral view of an early divider, showing the appearance of the oral primordium. (B), ventral view of a slightly later divider, showing the appearance of the FVT-anlagen (arrowhead) and the primordium (arrow) for the proter. (C), ventral view of an early divider, showing the FVT-anlagen in the proter and the opisthe (arrowheads). Ventral (D) and dorsal (E) views of the same divider, showing the development of the FVT-anlagen (arrowheads), newly formed adoral membranelles for the proter (arrow), left and right marginal anlagen (double-arrowheads) and the dorsal kinety anlagen. Ventral (F) and dorsal (G) views of the same divider, arrows indicate the pseudorow separated from several anterior FVT-anlagen, arrowheads mark the leftmost frontal cirrus which is cut off from the undulating membranes anlage. Ventral (H) and dorsal (I) views of a slightly later divider, showing the development and migration of the newly formed cirri. DKA, dorsal kineties anlagen; LMA, left marginal anlagen; OP, oral primordia; RMA, right marginal anlagen. Scale bar = 50 μm. |

|
Fig. 3 Photomicrographs of Apokeronopsis wrighti Long et al., 2008 during morphogenetic process (after protargol stain-ing). (A), ventral view of a very early divider, showing the oral primordium in the opisthe. (B) and (C), ventral views of an early divider, showing the proliferation of basal bodies in oral primordia of the opisthe (B) and FVT-anlagen formed in the proter (C, arrowhead). (D), ventral view of an early opisthe showing the new FVT-anlagen (arrowhead) and the newly formed adoral membranelles (arrow). (E) and (F), ventral views of the same divider, showing the marginal anlage and the dorsal kinety anlage (E, arrowheads) and formation of the FVT-anlagen (F). (G) and (H), ventral views of an early divider, showing the streaks of FVT-anlagen (G) and right marginal anlage (H, arrowhead). (I), ventral view, showing that the pseudorow is separated from several anterior FVT-anlagen (arrowheads). (J), ventral view of a divider, demonstrating that the leftmost frontal cirrus is separated from the undulating membranes anlage (arrowhead). (K), ventral view of a late divider, arrowhead shows the leftmost frontal cirrus. L, partial fusion of the macronuclear nodules. (M) – (P), ventral and dorsal views of a very late divider, showing the migration of the newly formed cirri, arrowheads indicate the frontoterminal cirri (M) and right marginal anlage (P). Scale bars = 20 μm in (A) – (N), (P); scale bar = 50 μm in (O). |
|
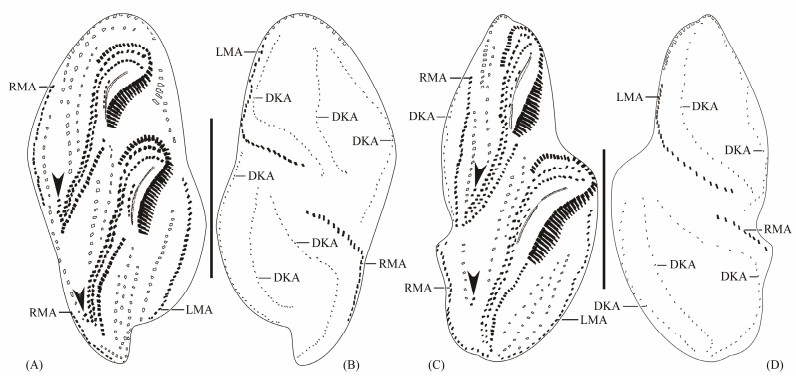
Fig. 4 Late morphogenetic stages of Apokeronopsis wrighti Long et al., 2008. (A) and (B), ventral and dorsal views of the same divider, respectively, and arrowheads demonstrate that the frontoterminal cirri is about to migrate. (C) and (D), ventral and dorsal views of a very late divider, and arrowheads demonstrate the migration of the frontoterminal cirri. DKA, dorsal kineties anlagen; LMA, left marginal anlagen; RMA, right marginal anlagen. Scale bars = 50 μm. |
|
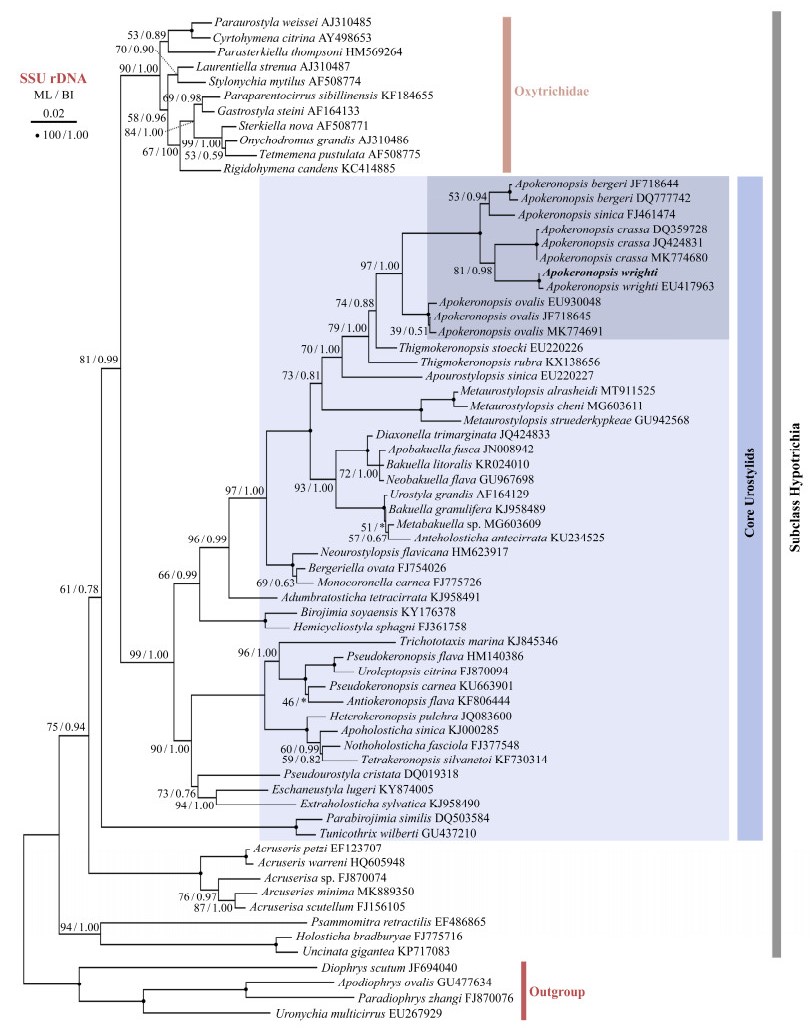
Fig. 5 The maximum likelihood (ML) tree inferred from SSU rDNA sequences. The support values of ML and Bayesian inference (BI) are showed near nodes. Complete support values (100 ML/1.00 BI) are drawn with solid circles. The inconsistent topologies between ML and BI are indicated by asterisks. Scale bar corresponds to 2 substitutions per 100 bases. All branches are drawn to scale. |
Apokeronopsis wrighti – Long et al., 2008, J. Eukaryot. Microbiol., 55: 321 – 322 (original description).
3.1.1 Morphological descriptionCell in vivo (75 – 105) × (15 – 30) μm (n = 5) and (85 – 163) ×(25 – 85) μm after protargol staining (cell size increased due to the dyeing process). Body shape generally elongate-elliptical with anterior end broadly rounded and posterior end slightly tapering; body slightly contractile and flexible. Two kinds of cortical granules: red and blood cell-shaped, 2 μm in diameter, dark-reddish, arranged roughly in three or four rows on ventral side and two rows on dorsal side; rounded in shape, colorless, ca. 0.5 μm across and arranged in short rows. Contractile vacuole positioned at or slightly anterior of equatorial region. More than 100 macronuclear nodules (Table 1).
|
|
Table 1 Morphometric data of Shenzhen population of Apokeronopsis wrighti after protargol staining |
As shown in Fig.1A, adoral zone occupying approximately 40% of body length, consisting of 45 – 59 membranelles. Paroral and endoral intersected at the middle to posterior one fifth in space. The bicorona comprises about 25 frontal cirri. Constantly two frontoterminal cirri. A cirral row arranged along the paroral and endoral, composed of 4 – 10 cirri. Midventral complex terminates caudally. 16 – 29 transverse cirri arranged in a row extending anteriorly near the mid-body. Right marginal row comprising 26 – 43 cirri, left row composed of 23 – 40 cirri (Fig.1A). Invariably three dorsal kineties, almost bipolar, composed of 26 – 41, 22 – 38, 22 – 38 dikinetids, respectively (Fig.1B).
3.1.2 Morphogenesis of Apokeronopsis wrighti 3.1.2.1 Stomatogenesis and development of the frontoventral-transverse cirral anlagenStomatogenesis starts with the formation of small groups of basal bodies close to several intact left midventral cirri (Figs.2A, 3A). These groups subsequently become larger with the proliferation of basal bodies, merger and form a wedge-shaped field which is the oral primordium for the opisthe (Figs.2B, 3B). In the proter, the oral primordium develops on the dorsal wall of the buccal cavity and a set of frontoventral-transverse cirral anlagen (FVT-anlagen) appears to the right of the old cirri row arranged along the paroral and endoral (Figs.2B, 3C).
In the next stage, the undulating membranes anlage (UMA) and FVT-anlagen for the opisthe develop to the right of the oral primordium on the surface of the cell (Figs.2C, 3D). The oral primordium for the proter enlarges and the UMA develops to the right (Fig. 2C). The oral primordium begins to differentiate into membranelles anteriad in each filial product (Figs.2C, 3D).
In the intermediate stage, the FVT-anlagen and the UMA continue to differentiate (Figs.2D, 3F, 3G). New adoral membranelles appear in the right anterior of oral primordia in both daughter cells (Figs.2D, 3F). During this period, the cirral row arranged along the paroral and endoral and the midventral are still maintain integrity, indicating they are not involved in the construction of the new anlagen (Fig.2D).
In the next period, the differentiation of membranelles is almost complete, the new oral structure of proter and opisthe are already formed, the anterior end of the new adoral zone of membranelles bends to the right (Fig.2F). Meanwhile, each undulating membranes anlage cuts off a single frontal cirrus and frontoventral-transverse streaks Ⅱ to Ⅴ-Ⅺ (deduced from morphometric data) each segregates a pair of midventral cirri and a segment on the left (Figs. 2F, 3I, 3J, 3K). The left segments migrate towards the newly formed undulating membranes anlage and become the pseudorow feigning a buccal row (Figs.2F, 3I). Each of the remaining streaks divided into two cirri (midventral pairs) except for the streaks in the posterior half that form three cirri. The left cirri form the transverse cirri (Figs.2F, 3N).
Subsequently, the undulating membranes anlage splits longitudinally to form the paroral and endoral parts (Fig. 2H).
The segregation of cirri of FVT-anlagen is almost complete in the meantime and the pseudorow migrates posteriad alongside the new undulating membranes, while the last FVT-anlage forms two frontoterminal cirri on the right (Fig.4A).
The two frontoterminal cirri gradually move to the anteriad position (Fig.4C). The leftmost cirrus in each of the posterior several FVT-anlagen becomes a transverse cirrus (Fig.4C).
3.1.2.2 Development of marginal rows and dorsal anlagenThe marginal rows anlagen originate de novo on each side of the cell (Figs.2D, 3E). The anlagen develop with further proliferation of basal bodies and gradually lengthen, and finally generate new marginal rows. The parental marginal rows are not involved in the construction of the cirral anlagen and are eventually resorbed (Figs.2F, 2H, 3H, 3P, 4A, 4C).
Three streaks of basal bodies appear de novo in each filial product on the dorsal side (Figs.2E, 3E), then proliferates, and stretch continuously in both directions across almost the whole dorsal surface (Figs.2G, 2I, 3P, 4B, 4D).
3.1.2.3 Division of nuclear apparatusThe replication bands of macronuclear nodule appears in early stage. More than 50 macronuclear segments fuse into many masses prior to division (Figs.2I, 3L).
3.2 Phylogenetic Analyses Based on Single Gene and Concatenated Three-Gene DatasetsThe partial SSU rDNA, ITS1-5.8S-ITS2, and LSU rDNA sequences of Apokeronopsis wrighti are deposited in GenBank with length, G + C content and accession number as follows: SSU rDNA (1531 bp, 45.92%, and OR726041), ITS1-5.8S-ITS2 (465 bp, 45.59%, and OR726042), LSU rDNA (1686 bp, 50.36%, and OR726040).
The topologies of the ML and BI trees constructed ac-cording to SSU rDNA gene were almost identical except for some minor differences, therefore only the ML tree is presented here, including bootstraps and posterior probabilities from both algorithms (Fig.5). Five Apokeronopsis species form a monophyletic clade with a high support (97% ML/1.00 BI). Two populations of A. wrighti group together with each other with a full support (100% ML/1.00 BI), and the sequence similarity of SSU rDNA between two populations is 99.7%. Within the genus of Apokeronopsis, A. wrighti is sister to A. crassa with a high support (81% ML/0.98 BI) and then groups with the clade comprising A. sinica and A. bergeri. Three populations of A. ovalis fall outside of the other species. In our SSU rDNA tree, the genus Apokeronopsis clusters with genus Thigmokeronopsis, thereby forming a sister clade of A. sinica. Three species from Metaurostylopsis group outside of all these taxa.
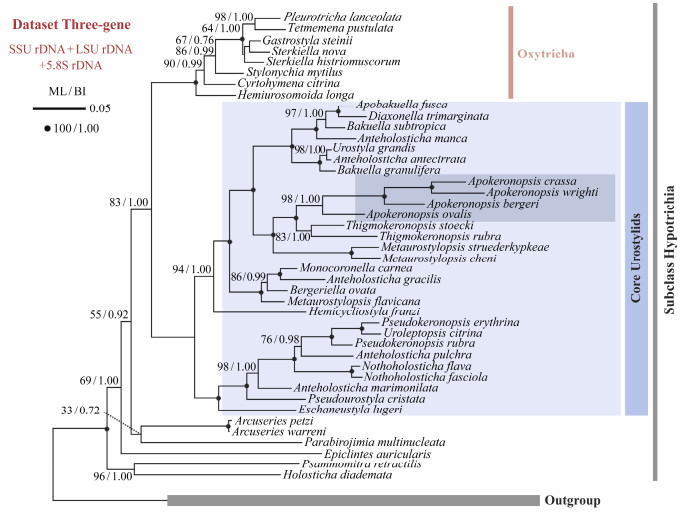
Maximum likelihood (ML) and Bayesian inference (BI) analyses based on a combination of SSU rDNA, LSU rDNA, and ITS1-5.8S-ITS2 sequence data were also performed in this study (Fig.6). The concatenated and SSU rDNA tree exhibit similar topologies: 1) genus Apokeronopsis is monophyletic clade with a high support (98% ML/1.00 BI), 2) Apokeronopsis wrighti groups with A. crassa with a full support (100% ML/1.00 BI) and then group with A. bergeri, with A. ovalis falling outside of this group, and 3) Thigmokeronopsis and Apokeronopsis cluster together, forming a sister group of Metaurostylopsis.
|
Fig. 6 Maximum likelihood (ML) phylogenetic tree inferred from concatenated rDNA data (SSU rDNA, ITS1-5.8S-ITS2, and LSU rDNA). The support values of ML and Bayesian inference (BI) are showed near the nodes. Complete support values (100 ML/1.00 BI) are drawn with solid circles. The inconsistent topologies between ML and BI are indicated by asterisks. Scale bar corresponds to 5 substitutions per 100 bases. All branches are drawn to scale. |
Apokeronopsis wrighti Long et al., 2008, originally de-scribed by Long et al. (2008), was found in coastal marine water in Hong Kong, China. We identified our population as A. wrighti because it shares all following features with the type population: body size and shape, pattern of cortical granules, infraciliature and morphometrics and habitat. Furthermore, the Shenzhen population conforms to the Hong Kong population (EU417963) with a 99.7% similarity in SSU rDNA sequences. Hence, the identification is in no doubt.
4.2 Ontogenetic ComparisonWith latest available data, four Apokeronopsis species, i.e., A. antarctica, A. bergeri, A. crassa, and A. ovalis have been investigated morphogenetically (Petz, 1995; Shao et al., 2007, 2008; Li et al., 2008). They share similar morphogenetic characteristics, namely 1) complete replacement of the old oral apparatus by the newly built structure; 2) the oral primordium for the proter develop on the wall of the buccal cavity; 3) the FVT-anlagen formed de novo; 4) de novo development of marginal and dorsal kineties anlagen; and 5) macronuclear nodules partially fuse during the whole process.
Compared to the congeners, A. wrighti shows similarity to A. bergeri in the formation of the pseudorow arranged along the paroral and endoral, i.e., several anterior FVT-anlagen each contribute a cirrus on the left side, while in A. ovalis and A. crassa (and A. antarctica?) the buccal cirral row is generated from the FVT-anlage Ⅱ.
4.3 Phylogenetic PerspectivesAs shown in the present phylogenetic trees, Apokeronopsis is monophyletic. This is consistent with previous studies (e.g., Chen et al., 2010; Yi and Song, 2011; Huang et al., 2014), in which only SSU rDNA was provided.
Among Apokeronopsis species, A. wrighti is most close-ly related with A. crassa in SSU rDNA and concatenated trees (Figs.5, 6). This corresponds well with the similar morphological characteristics and infraciliature pattern between A. wright and A. crassa: 1) elongated shaped with narrowed posterior end, 2) transverse cirri extend to more than 50% of body length, and 3) the midventral complex terminates at posterior end of cell. However, A. wrighti can be distinguished from A. crassa by the origin of the cirral row arranged along the paroral and endoral during ontogeny (several anterior streaks from the FVT-anlagen which contribute their last segments to form a pseudorow vs. FVT-anlage Ⅱ develops several cirri to form a true buccal row). It appears that the differences in the morphogenetic process should not carry significant weight in phylogenetic analyses.
Additionally, consistent with previous studies (Yi et al., 2008; Chen et al., 2011; Lyu et al., 2018; Ma et al., 2021), the close relationship among the genera Apokeronopsis, Thigmokeronopsis, and Metaurostylopsis is also supported in present phylogenetic analyses.
AcknowledgementsThis work was supported by the Natural Science Foun-dation of Shaanxi Province (Nos. 2023-JC-QN-0214, 2023-JC-QN-0185), the Postdoctoral Science Foundation of Shaanxi Province (No. 2023BSHEDZZ199), and the Fundamental Research Funds for the Central Universities (No. GK202207019).
Berger, H., 2006. Monograph of the Urostyloidae (Ciliophora, Hypotricha). Monographiae Biologicae, 85: 1-1304. DOI:10.1007/1-4020-5273-1_1 (  0) 0) |
Bharti, D., and Kumar, S., 2023. Description of a new oxytri-chid ciliate, Oxytricha buxai n. sp. and redescription of O. quadricirrata Blatterer and Foissner, 1988 based on morphology and 18S rDNA analyses. European Journal of Protistology, 88: 125959. DOI:10.1016/j.ejop.2023.125959 (  0) 0) |
Borror, A. C., 1972. Revision of the order Hypotrichida (Ciliophora, Protozoa). The Journal of Protozoology, 19: 1-23. DOI:10.1111/j.1550-7408.1972.tb03407.x (  0) 0) |
Chen, X., Clamp, J. C., and Song, W., 2011. Phylogeny and sys-tematic revision of the family Pseudokeronopsidae (Protista, Ciliophora, Hypotricha), with description of a new estuarine species of Pseudokeronopsis. Zoologica Scripta, 40: 659-671. DOI:10.1111/j.1463-6409.2011.00492.x (  0) 0) |
Chen, X., Huang, J., and Song, W., 2010. Ontogeny and phylo-geny of Metaurostylopsis cheni sp. n. (Protozoa, Ciliophora), with estimating the systematic position of Metaurostylopsis. Zoologica Scripta, 40: 99-111. DOI:10.1111/j.1463-6409 (  0) 0) |
Dong, J., Liu, Y., Ma, J., Ma, H., Stoeck, T., and Fan, X., 2022. Ultrastructure of Diophrys appendiculata and new systematic consideration of the euplotid family Uronychiidae (Protista, Ciliophora). Marine Life Science & Technology, 4: 551-568. DOI:10.1007/s42995-022-00153-y (  0) 0) |
Eigner, P., and Foissner, W., 1992. Divisional morphogenesis in Bakuella pampinaria nov. spec, and reevaluation of the classification of the urostylids (Ciliophora, Hypotrichida). European Journal of Protistology, 28: 460-470. DOI:10.1016/S0932-4739(11)80011-8 (  0) 0) |
Foissner, W., 2016. Terrestrial and semiterrestrial ciliates (Proto-zoa, Ciliophora) from Venezuela and Galápagos. Denisia, 35: 1-912. (  0) 0) |
Foissner, W., 2021. Taxonomy of soil ciliates (Ciliophora) from Australia and some other parts of the world. Series Monographiae Ciliophorae, 5: 55-345. (  0) 0) |
Fu, J., Chi, Y., Lu, X., Gao, F., Al-Farraj, S. A., Petroni, G., et al., 2022. Doublets of the mono-cellular organism Euplotes vannus (Protozoa, Ciliophora, Euplotida): The morphogenetic pattern of the ciliature and nuclear apparatus during asexual division. Marine Life Science & Technology, 4: 527-535. DOI:10.1007/s42995-022-00150-1 (  0) 0) |
Gao, F., Katz, L. A., and Song, W., 2012. Insights into the phy-logenetic and taxonomy of philasterid ciliates (Protozoa, Ciliophora, Scuticociliatia) based on analyses of multiple molecular markers. Molecular Phylogenetics and Evolution, 64: 308-317. DOI:10.1016/j.ympev.2012.04.008 (  0) 0) |
Huang, J., Chen, Z., Song, W., and Berger, H., 2014. Three-gene based phylogeny of the Urostyloidea (Protista, Ciliophora, Hypotricha), with notes on classification of some core taxa. Molecular Phylogenetics and Evolution, 70: 337-347. DOI:10.1016/j.ympev.2013.10.005 (  0) 0) |
Jerome, C. A., Lynn, D. H., and Simon, E. M., 1996. Descrip-tion of Tetrahymena empidokyrea n. sp., a new species in the Tetrahymena pyriformis sibling species complex (Ciliophora, oligohymenophorea), and an assessment of its phylogenetic position using small-subunit rRNA sequences. Canadian Journal of Zoology, 74: 1898-1906. DOI:10.1139/z96-214 (  0) 0) |
Jung, J. H., Baek, Y. S., and Min, G. S., 2011. New record of two Apokeronopsis species (Ciliophora: Urostylida: Pseudokeronopsidae) from Korea. Animal Systematics, Evolution and Diversity, 27: 115-122. DOI:10.5635/KJSZ.2011.27.2.115 (  0) 0) |
Li, L., Song, W., Warren, A., Al-Rasheid, K. A. S., Roberts, D., Yi, Z., et al., 2008. Morphology and morphogenesis of a new marine ciliate, Apokeronopsis bergeri nov. spec. (Ciliophora, Hypotrichida), from the Yellow Sea, China. European Journal of Protistology, 44: 208-219. DOI:10.1016/j.ejop.2008.01.001 (  0) 0) |
Liu, W., Li, J., Shan, G., Chen, S., and Song, W., 2009. Morpho-logical studies and molecular data on a new marine ciliate, Apokeronopsis sinica n. sp. (Ciliophora: Urostylida), from the South China Sea. Zootaxa, 2005: 57-66. DOI:10.11646/zootaxa.2005.1.5 (  0) 0) |
Long, H., Liu, H., Liu, W., Miao, M., Hu, X., Lin, X., et al., 2008. Two new ciliates from Hong Kong coastal water: Orthodonella sinica n. sp. and Apokeronopsis wrighti n. sp. (Protozoa: Ciliophora). Journal of Eukaryotic Microbiology, 55: 321-330. DOI:10.1111/j.1550-7408.2008.00334.x (  0) 0) |
Luo, X., Huang, J., Ma, H., Liu, Y., Lu, X., and Bourland, W. A., 2022. Hypotrichidium tisiae (Gelei, 1929) Gelei, 1954: A unique hypotrichid ciliate having a highly specialized developmental pattern during binary division. Marine Life Science & Technology, 4: 536-550. DOI:10.1007/s42995-022-00148-9 (  0) 0) |
Lyu, Z., Wang, J., Huang, J., Warren, A., and Shao, C., 2018. Multigene-based phylogeny of Urostylida (Ciliophora, Hypotrichia), with establishment of a novel family. Zoologica Scripta, 47: 243-254. DOI:10.1111/zsc.12267 (  0) 0) |
Ma, J., Zhang, T., Song, W., and Shao, C., 2021. New contributions to the diversity of hypotrichous ciliates: Description of a new genus and two new species (Protozoa, Ciliophora). Frontiers in Microbiology, 12: 712269. DOI:10.3389/fmicb.2021.712269 (  0) 0) |
Medlin, L., Elwood, H. J., Stickel, S., and Sogin, M. L., 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA coding regions. Gene, 71: 491-499. DOI:10.1016/0378-1119(88)90066-2 (  0) 0) |
Miller, M. A., Pfeiffer, W., and Schwartz, T., 2012. The CIPRES science gateway: Enabling high-impact science for phylogenetics researchers with limited resources. In: Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond. Chicago, 1-8, DOI: 10.1145/2335755.2335836.
(  0) 0) |
Moreira, D., von der Heyden, S., Bass, D., López-García, P., Chao, E., and Cavalier-Smith, T., 2007. Global eukaryote phylogeny: Combined small- and large-subunit ribosomal DNA trees support monophyly of Rhizaria, Retaria and Excavata. Molecular Phylogenetics and Evolution, 44: 255-266. DOI:10.1016/j.ympev.2006.11.001 (  0) 0) |
Nylander J. A. A.. 2004. MrModeltest v2. Evolutionary Biology Centre, Uppsala University. DOI:10.1093/sysbio/sys029
(  0) 0) |
2022. A new 'flagship' ci-liate, Pseudostylonychia obliquocaudata n. gen., n. sp. (Ciliophora, Hypotricha), from South Korea. European Journal of Protistology, 84: 125893. DOI:10.1016/j.ejop.2022.125893 (  0) 0) |
1995. Morphology and morphogenesis of Thigmokeronopsis antarctica nov. spec. and T. crystallis nov. spec. (Ciliophora, Hypotrichida) from Antarctic sea ice. European Journal of Protistology, 31: 137-147. DOI:10.1016/S0932-4739(11)80437-2 (  0) 0) |
2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539-542. DOI:10.1093/sysbio/sys029 (  0) 0) |
2007. Morphogenesis in the marine spirotrichous ciliate Apokeronopsis crassa (Claparède & Lachmann, 1858) n. comb. (Ciliophora: Stichotrichia), with the establishment of a new genus, Apokeronopsis n. g. and redefinition of the genus Thigmokeronopsis. Journal of Eukaryotic Microbiology, 54: 392-401. DOI:10.1111/j.1550-7408.2007.00278.x (  0) 0) |
2008. Morphogenesis and morphological redescription of a poorly known ciliate Apokeronopsis ovalis (Kahl, 1932) nov. comb. (Ciliophora: Urostylida). Acta Protozoologica, 47: 363-376. (  0) 0) |
Song W. B., Shao C.. 2017. Ontogenetic Patterns of Hypo-trich Ciliates. Science Press, Beijing, 498pp (in Chinese).
(  0) 0) |
2022. Ontogenesis and systematic position of a new hypotrichous ciliate, Chaetospira sinica sp. nov., with an improved diagnosis of the poorly defined family Chaetospiridae Jankowski, 1985 (Protozoa, Ciliophora, Hypotrichia). Marine Life Science & Technology, 4: 513-526. DOI:10.1007/s42995-022-00146-x (  0) 0) |
2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 (  0) 0) |
2021. A new hypotrich ciliate, Oxytricha xianica sp. nov., with notes on the morphology and phylogeny of a Chinese population of Oxytricha auripunctata Blatterer & Foissner, 1988 (Ciliophora, Oxytrichidae). Marine Life Science & Technology, 3: 303-312. DOI:10.1007/s42995-020-00089-1 (  0) 0) |
1975. Eine verbesserte technik der protargolimprag-nation für ciliaten. Mikrokosmos, 64: 171-179. (  0) 0) |
2011. Evolution of the order Urostylida (Protozoa, Ciliophora): New hypotheses based on multi-gene information and identification of localized incongruence. PLoS One, 6: e17471. DOI:10.1371/journal.pone.0017471 (  0) 0) |
2008. Phylogeny of some systematically uncertain urostyloids – Apokeronopsis, Metaurostylopsis, Thigmokeronopsis (Ciliophora, Stichotrichia) estimated with small subunit rRNA gene sequence information: Discrepancies and agreements with morphological data. European Journal of Protistology, 44: 254-262. DOI:10.1016/j.ejop.2007.12.002 (  0) 0) |
2022. Insights into the phylogeny of the family Deviatidae (Protozoa, Ciliophora, Hypotrichia) based on multi-gene, morphological and ontogenetic information, with the establishment of a new species Deviata multilineae n. sp. Molecular Phylogenetics and Evolution, 177: 107623. DOI:10.1016/j.ympev.2022.107623 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


