2) Research Center for Safety, Metrology, and Nuclear Quality Technology, National Research and Innovation Agency Republic of Indonesia (BRIN), Jakarta 10210, Indonesia;
3) Research Center for Fisheries, National Research and Innovation Agency Republic of Indonesia (BRIN), Cibinong, Bogor, Jawa Barat 45264, Indonesia
Tropical coastal ecosystems, which host economically important species such as fish, shellfish, and seaweed, are increasingly threatened by human activities and inadequate enforcement of environmental regulations. Waste-water discharge from domestic, industrial, agricultural, and aquaculture sources contributes significantly to pollution, particularly nutrient runoff, which leads to a process known as eutrophication (Sindern et al., 2016; Areco et al., 2021; Priya et al., 2021). Eutrophication has regions globally, with significant cases observed in Indonesia and other Southeast Asian countries (Damar et al., 2021; Lao et al., 2021; Luo et al., 2022). This process fundamentally alters seawater chemistry, disrupting nutrient balances, increasing turbidity, accelerating acidification, and depleting oxygen, all of which pose serious threats to marine ecosystems (Kessouri et al., 2021). Moreover, these changes ecologically foster phytoplankton and harmful algal blooms (HABs), which negatively affect vital species such as seagrasses, corals, and other coastal organisms (Brown et al., 2019; Jiang et al., 2022). The consequences are severe, endangering marine food security, degrading environmental health, and threatening key economic sectors like tourism and fisheries (Ngatia et al., 2019).
Given these challenges, regular environmental quality monitoring is crucial for supporting the sustainability of coastal ecosystems. However, the initial setup costs can be substantial due to the need for sophisticated instruments and specialized expertise. Monitoring involves regular sampling and analysis of water, sediments, and organisms, often necessitating cutting-edge analytical equipment, which results in significant financial burdens (Jha et al., 2023). Additionally, expert involvement across various fields is necessary. For example, Beiras et al. (2011) noted that five research teams participated in a multiyear study of coastal pollution in the Iberian Peninsula. Recent innovations, such as molecular techniques like metabolomics, have shown promise in detecting metabolic changes in organisms responding to environmental stress (Lettieri et al., 2023). Metabolomics offers a high-throughput screening method, capable of quickly detecting changes in both biotic and abiotic environmental conditions, making it an effective initial stage for investigating coastal pollution.
Research on the application of metabolomics as a tool for studying environmental pollution has been widely conducted using various indicator species, particularly marine bivalves. Bivalves are considered reliable bioindicators because, as filter feeders, they continuously process water and accumulate pollutants from their surroundings, providing a localized reflection of pollution levels (Ward et al., 2019). Metabolomic studies have extensively examined how bivalves respond to stressors such as hypoxia, heavy metals, microplastics, pesticides, and Polycyclic Aromatic Hydrocarbons (PAHs). Those studies have been conducted on various species, including Ruditapes philippinarum, Perna viridis, Mytilus edulis, Dreissena polymorpha, and Anadara granosa (Liu et al., 2010; Niiyama et al., 2012; Juhel et al., 2016; Chan and Wang, 2018; Ward et al., 2019; Hani et al., 2021; Almulhim et al., 2022; López-Pedrouso et al., 2022). However, metabolomic studies on eutrophication-induced stress in coastal environments are still limited. While Gidman et al. (2007) and Campillo et al. (2019) suggested that bivalve metabolic responses could indicate eutrophication, the specific metabolites correlating with nutrient levels have not yet been identified.
Among marine bivalves, Anadara granosa is a potential organism for tropical metabolomic research. This species is widely distributed across the Indo-Pacific region and inhabits muddy sediments in intertidal zones, where it feeds on phytoplankton and organic detritus (Mirzaei et al., 2014; Peng et al., 2015). Ecologically, A. granosa serves as a food source for various predators and plays a crucial role in shaping benthic communities, with its presence serving as an important indicator of coastal ecosystem health (Clune and Harrison, 2009). The biological characteristics of A. granosa is influenced by various environmental factors, including temperature, salinity, and sediment composition (Khalil, 2018). Moreover, elevated nutrient levels in coastal waters promote food availability for marine bivalves (Renitasari et al., 2023). However, eutrophication also poses physiological risks, such as biotoxin accumulation and hypoxia due to harmful algal blooms (Dai et al., 2023). Research on A. granosa related to the environment usually highlighted the capacity for bioaccumulation, making it a useful indicator for assessing environmental health and pollution levels in marine ecosystems (Mirsadeghi et al., 2013). Metabolic changes in A. granosa as a response to these environmental stressors can be detected using metabolomic techniques.
Therefore, this study aims to identify potential metabolites in A. granosa as biomarkers of eutrophic conditions in tropical coastal environments. This species is widely distributed in tropical coastal regions and has significant economic value as a marine resource. The research was conducted along the coast of Cirebon Bay, one of Indonesia's most major areas for marine bivalve production. The growth of key economic sectors in this area, including aquaculture, agriculture, and food processing, may lead to environmental consequences for eutrophication in Cirebon Bay (Suratih et al., 2019; Sanjoto et al., 2021; Mardhatilla et al., 2022). These factors make it an ideal location for metabolomic research.
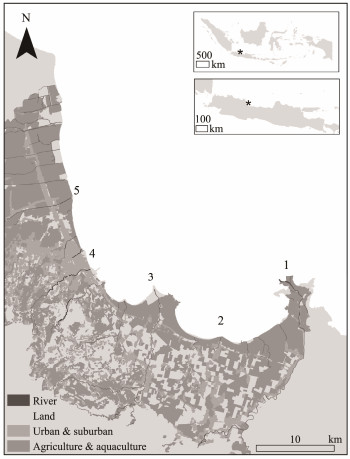
2 Materials and Methods 2.1 Study Stations and Sample CollectionThis study was conducted in Cirebon Bay, located in West Java Province, Indonesia, covering the area from the eastern side to the northern side (Fig.1). Five sampling stations were selected based on preliminary in situ dissolved oxygen analyses to capture varying levels of eutrophication among the observation sites. On the eastern side, Stations 1, 2, and 3 are situated in the Losari, Gebang, and Pengarengan Districts, respectively. Losari and Gebang Districts are characterized by aquaculture along the coast and agriculture in the terrestrial areas. The key distinction is that Losari District is located at the estuary of the major Cisanggarung River, which flows from the upstream region in central West Java. In contrast, Gebang District is a coastal area with smaller rivers that serve as tributaries to the surrounding Cirebon area. Meanwhile, aquaculture and agriculture are also the primary livelihoods of the residents in Pengarengan District, though on a smaller scale due to its proximity to a Coal-Fired Steam Power Plant with a capacity of 1660 MW, which supplies electricity to West Java Province. Meanwhile, on the northern side of Cirebon Bay, Station 4 is located in the waters of Kejawanan District, part of the urban core of Cirebon City, which serves as the capital of Cirebon Regency. The final station, Station 5, is situated in Mundu District at the northernmost point. Mundu District is significant for both aquaculture and agriculture along Cirebon Bay, and Station 5 functions as the estuary for several rivers that flow into the marine waters. The positions of the sampling locations were recorded using a Garmin GPSMAP 64s and then plotted onto a geospatial map using ArcGIS 10.8 software. Five kilograms of wet A. granosa samples were randomly collected from each site, specifically at locations where local fishermen commonly capture this bivalve. All samples were stored in an ice chest at low temperatures for transportation to the laboratory.
|
Fig. 1 Sampling stations at Losari (1), Gebang (2), Pengarengan (3), Kejawanan (4), and Mundu (5) Districts at Cirebon Bay, Indonesia. |
Initially, water samples at the bottom layer of water column (habitat of A. granosa), were collected in triplicate from each location using a Nansen bottle. These samples were then analyzed for water transparency, pH, dissolved oxygen, and salinity using Secchi disk, HACH HQ40d device equipped with durable probes, and Eutech Salt +6 digital salt-meter aboard the sampling boat. Electrode calibration for total pH measurement was performed using tris-buffer in synthetic seawater, following the pH calculation method outlined by Nemzer and Dickson (2005). After the collection of water samples, nutrient levels, specifically phosphate, nitrate, and ammonia, were assessed at a base camp near the study stations. Prior to nutrient analysis, all water samples were filtered through a 0.45 μm filter. The nutrient analysis was conducted using colorimetric techniques with HACH DR-890 instruments.
2.3 Metabolic AnalysisThe preparation of samples for metabolomic analysis followed the methods established by Anissah et al. (2021) in earlier Proton-NMR (Nuclear Magnetic Resonance) metabolomics studies. In the laboratory, the specimens were thoroughly cleaned to remove any attached mud and debris. Soft tissues from 100 individual specimens were collected from each location and subsequently homogenized. The samples were then dried in a vacuum oven at 50℃ for three days. A total of 200 mg of sample from each location (with 9 replications) was extracted using 70% methanol three times, evaporated to dryness, and then redissolved in 0.8 mL of deuterated methanol. To ensure accurate quantification, 0.2 mL of a 6 mg mL−1 solution of 1, 2, 4, 5-Tetrachloro-3-nitrobenzene (in deuterated methanol) was added as an internal standard for the Proton-NMR (Nuclear Magnetic Resonance) metabolomics experiment. The Proton-NMR analysis was performed using a 400 MHz Jeol NMR spectrometer at 2048 scans, with chemical shift identification based on the methodologies of Wu and Wang (2010), Kwon et al. (2012), Cappello et al. (2013) Rochfort et al. (2013), and. The quantification of each compound was calculated based on the integration level of the internal standard, following the method of Shumilina et al. (2015).
2.4 Data ProcessingWater quality characteristics were tabulated to assess the trophic status at each station, following the criteria set up by Kadiri et al. (2021). According to this study, eutrophication in coastal regions is defined by thresholds exceeding 0.21 mg L−1 for total inorganic nitrogen (the sum of nitrate and ammonia) and 0.024 mg L−1 for total phosphate. One-way ANOVA (analysis of variance) with a Duncan post-test was performed to analyze the differences in the concentrations of each variable among the observed sites. Data transformation was applied according to Templeton (2011), and the Kolmogorov-Smirnov test was used to analyze the normal distribution of each variable. Statistical multivariate discriminant analysis and one-way ANOSIM (analysis of similarity) were employed to find metabolites that could discriminate between the metabolic profiles of mussels collected from different sites. In the final stage, canonical correspondence analysis, MANOVA (multivariate analysis of variance) and regression analysis were conducted to find a specific metabolite that might serve as a biomarker for eutrophication conditions. The result from regression analysis was evaluated by determination coefficient (R2), which showed categorized as weak (0.25 – 0.5), moderate (0.51 – 0.74), and substantial (0.75 – 1.0), according to Hair et al. (2011). All statistical multivariate analyses were performed using the Statistical Software Past V3.17 (Hammer et al., 2001). Meanwhile the regression analysis was performed by SPSS version 15.
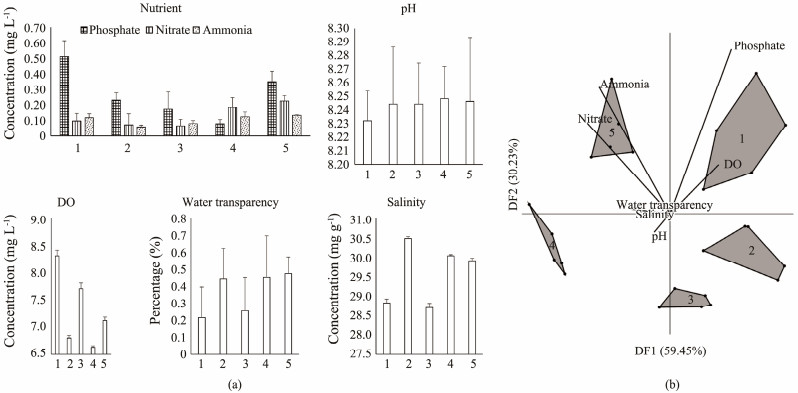
3 Results 3.1 Water Quality of Cirebon BayThe analysis of nutrient ions revealed that phosphorus was the dominant ion at nearly all observation stations, except at Station 4, Kejawanan (Fig.2). Additionally, elevated total nitrogen levels (exceeding 0.2 mg L−1) were seen in the northern stations, particularly at Kejawanan (Station 4) and Mundu (Station 5). Other chemical parameters, except for pH and water transparency, also showed significant variation (P < 0.05). Dissolved oxygen (DO) concentrations were highest at the outermost stations, specifically in Losari (Station 1), Mundu (Station 5), and at the coastal cape station of Pengarengan (Station 3). Conversely, salinity levels were significantly lower at stations located near river mouths, including Losari (Station 1) and Pengarengan (Station 3). According to eutrophication threshold values, Kejawanan (Station 4) and Mundu (Station 5) can be classified as eutrophic waters, with total inorganic nitrogen levels (the sum of nitrate and ammonia) exceeding 0.2 mg L−1, and total phosphate levels surpassing 0.024 mg L−1.
|
Fig. 2 Water quality characteristics (a) and multivariate discriminant analysis of water quality (b) from Losari (1), Gebang (2), Pengarengan (3), Kejawanan (4), and Mundu (5) Districts at Cirebon Bay, Indonesia. |
Multivariate discriminant analysis revealed that dissolved oxygen and nutrients (phosphate, ammonia, and nitrate) were the key factors differentiating seawater characteristics across stations. The first discriminant factor (DF 1) was primarily driven by phosphate and dissolved oxygen, accounting for 54.12% of the variance, while the second discriminant factor, driven by ammonia and nitrate, explained 33.94% of the variance. ANOSIM, with a 95% significance level, found three distinct groups of Anadara granosa habitats based on seawater characteristics. The first group included northern stations Kejawanan (Station 4) and Mundu (Station 5), characterized by higher concentrations of dissolved inorganic nitrogen (nitrate and ammonia). The second group consisted of Losari (Station 1), distinguished by higher levels of dissolved oxygen and total phosphate. The third group comprised Gebang (Station 2) and Pengarengan (Station 3), both of which showed lower nutrient and dissolved oxygen levels compared to the other stations.
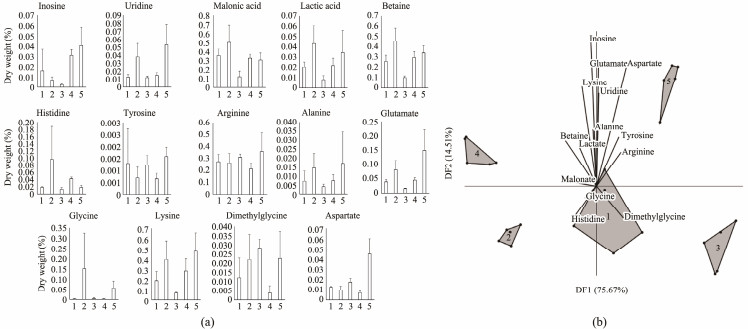
3.2 Metabolic Profile of Anadara granosaQuantitative metabolomic analysis using Proton-NMR detected 13 types of metabolites. These included nine amino acids (histidine, tyrosine, arginine, alanine, glutamate, glycine, lysine, dimethylglycine, and aspartate), two organic acids (malonic acid and lactic acid), one biogenic amine (uridine), one nucleoside (inosine), and betaine (Fig.3). ANOVA revealed that the concentrations of nearly all compounds exhibited significant variation (P < 0.05), except for alanine. The large standard deviations among samples suggested that alanine levels did not significantly differ among A. granosa populations from different stations. Meanwhile, discriminant analysis and ANOSIM indicated two distinct grouping patterns based on the metabolite composition. The first group included stations located in quadrants 1 and 2 of the multivariate discriminant dendrogram, namely Losari (Station 1), Gebang (Station 2), and Pengarengan (Station 3), situated on the eastern side of the observation area in Cirebon Bay. This group was characterized by higher levels of two amino acids, which were histidine and dimethylglycine. The second group consisted of Kejawanan (Station 4) and Mundu (Station 5), located on the northern side of Cirebon Bay. Apart from histidine and dimethylglycine, nearly all other detected metabolites were found at higher concentrations at these two stations.
|
Fig. 3 Metabolites profile of Anadara granosa (a) and multivariate discriminant analysis of A. granosa metabolites (b) from Losari (1), Gebang (2), Pengarengan (3), Kejawanan (4), and Mundu (5) Districts at Cirebon Bay, Indonesia. |
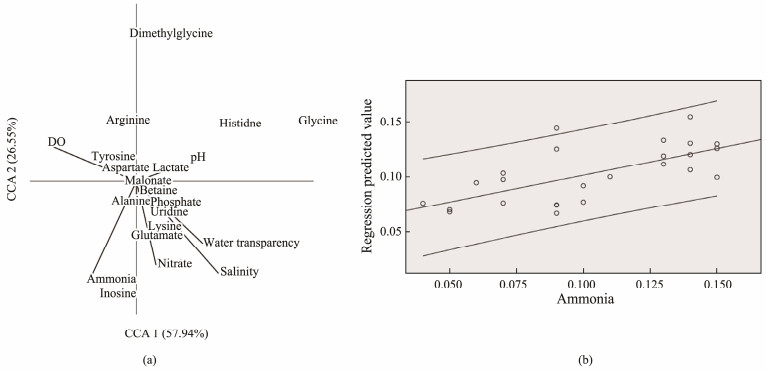
The dendrogram of multivariate canonical correspondence analysis revealed variation in the metabolite's composition of A. granosa in relation to the water quality characteristics (Fig.4). The pattern found several compounds as potential biomarkers for eutrophic conditions characterized by inorganic nitrogen ions (ammonia and nitrate). Inosine was positively correlated with the ammonia and nitrate vector, while histidine and dimethylglycine were negatively correlated. Further regression analysis showed that only inosine and dimethylglycine showed a significant correspondence (P < 0.05) with ammonia concentrations in the water. The coefficient of determination (R2) from the regression model comparing predicted ammonia values with actual concentrations was 0.631, indicating a moderate relationship between environmental ammonia levels with inosine and dimethylglycine concentrations in A. granosa.
|
Fig. 4 Canonical correspondence analysis (a) and regression of ammonia to inosine and dimethylglycine content (b) in Anadara granosa from Cirebon Bay, Indonesia. |
The concentrations of dissolved inorganic phosphate and inorganic nitrogen are key contributors to eutrophication in coastal waters. Coastal ecosystems generally exhibit higher phosphate levels due to the natural release of nutrients from sediments, leading to elevated dissolved phosphate concentrations compared to freshwater systems (Blomqvist et al., 2004). As a result, the dynamics of inorganic nitrogen often play a more dominant role in driving eutrophication and are strongly correlated with increases in phytoplankton biomass (Topcu et al., 2021; He et al., 2023).
The eutrophic conditions in northern coastal waters are likely attributable to runoff from terrestrial activities. Geospatial data (Fig.1) show that northern Cirebon Bay is an urbanized region with extensive aquaculture and agriculture, contrasting with the less-developed eastern coast. Runoff from these human activities likely elevates inorganic nitrogen levels, promoting eutrophication (Schutte et al., 2015; Wang et al., 2020a; Mustafa et al., 2022). Similar findings have also been reported in earlier studies, higher pollution levels on the coast and rivers at northern side were detected, compared to the the eastern side of Cirebon Bay (Zahroh et al., 2019; Hidayah et al., 2021).
Increased nutrient levels have also been linked to higher phytoplankton biomass, which is further reflected in the distinct salinity patterns between the northern and eastern parts of Cirebon Bay. Salinity fluctuates significantly in the eastern region, particularly between Losari, Gebang, and Pengarengan, due to dynamic freshwater inputs from rivers. In contrast, salinity in Kejawanan and Mundu waters was relatively stable despite freshwater inflows. The increase in phytoplankton biomass can lead to dense surface layers that inhibit vertical mixing with deeper waters, allowing salinity concentrations in bottom layer to remain similar with open seawater salinity even near freshwater sources (Šilović et al., 2012; Li et al., 2018). Elevated phytoplankton levels in northern Cirebon Bay have also been associated with recurring episodes of Paralytic Shellfish Poisoning, caused by biotoxins from Pyrodinium bahamense (Nurlina, 2018; Rachman et al., 2019).
These distinct environmental conditions have been shown to affect the metabolite composition of A. granosa. Multivariate discriminant analysis revealed that A. granosa from stations with higher nutrient levels clustered in the upper quadrant of the horizontal axis, consistent with the water quality data. The results showed increased metabolite production in nutrient-rich environments, except for histidine and dimethylglycine. In such environments, the rise in phytoplankton populations, a primary food source for bivalves, stimulates increased physiological activity and metabolite production (Moruf et al., 2020). Meanwhile, histidine and dimethylglycine, found at higher concentrations in A. granosa from low-nutrient areas, play essential roles in stabilizing cellular structures and maintaining osmotic balance, particularly in fluctuating salinity conditions (Liu et al., 2024). The broader salinity range in eastern waters likely subjects A. granosa to osmotic stress, contributing to the higher levels of these metabolites.
Further multivariate and regression analyses identified a correlation between ammonia concentrations and the metabolism of inosine and dimethylglycine. Inosine, a breakdown product of ATP (adenosine triphosphate), the primary energy storage molecule, increased in eutrophic waters where food availability is greater due to phytoplankton blooms. This suggests that A. granosa experiences heightened cellular activity and energy demands in such environments (Wang et al., 2020b; Zhang et al., 2020). Similar findings were reported by Roznere et al. (2014), who found that nucleosides, including inosine, can serve as biomarkers of nutritional status in bivalves. Inosine also has bioactive properties that mitigate oxidative stress caused by hypoxia, a common result of eutrophic conditions (Haider et al., 2020). Thus, the analysis suggests that inosine and dimethylglycine could serve as effective biomarkers for assessing coastal eutrophic conditions. High inosine levels may indicate an abundant food supply, while lower dimethylglycine levels, reflecting low salinity variation in the bottom water layer, may point to the prevalence of eutrophic conditions in the coastal habitats.
5 ConclusionsThe results of this study showed that eutrophication in Cirebon Bay is primarily driven by terrestrial runoff, resulting in increased levels of dissolved inorganic nitrogen and phosphate in northern coastal waters. These nutrient enrichments promote phytoplankton blooms, leading to stratification of water layers and reduced salinity fluctuations. The metabolic response of Anadara granosa to these environmental conditions reflects the organism's adaptation to nutrient availability and osmotic stress. Metabolites such as inosine and dimethylglycine were identified as potential biomarkers of eutrophic conditions, with inosine levels correlating with increased cellular energy demands in nutrient-rich waters, and dimethylglycine contributing to osmotic regulation in low-nutrient environments with greater salinity fluctuations. Overall, the study underscores the importance of monitoring nutrient dynamics and the metabolic responses of bivalves to assess the ecological health of coastal ecosystems affected by eutrophication. Effective management strategies are needed to mitigate nutrient pollution and its impacts on marine biodiversity and food security in coastal regions like Cirebon Bay.
AcknowledgementsWe express our gratitude to the officers of the Fisheries Product Quarantine, Quality Control, and Food Safety in Cirebon District for their help during the field activities.
Author Contributions
Hedi Indra Januar: conceptualization, laboratory analysis, and writing-original draft. Izhamil Hidayah: sampling activities and raw material preparation. Sutomo Sutomo: conceptualization, supervision, writing, and review. Eko Pujiono: data curation, writing, and review. Muhammad Hadi Saputra: data analysis, and review. Nida Humaida: data analysis, writing, and editing. Etik Erna Wati Hadi: data analysis, review, and editing. Hery Kurniawan: data curation, data analysis, and review. Relawan Kuswandi: data curation, writing, and review. Jalma Giring Sukmawait: sample preparation and laboratory preparation. Wahyu Retno Prihatiningish: sampling activities and sample preparation. Sri Iswani: sampling activities and laboratory analysis.
Data Availability
The data and references presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests.
Almulhim, F., Rossbach, S., Emwas, A., Kharbatia, N. M., Jaremko, Ł., Jaremko, M., et al., 2022. Metabolomic study on tridacna maxima giant clams reveals metabolic fingerprint of environmental pollutants. Frontiers in Marine Science, 9: 813404. DOI:10.3389/fmars.2022.813404 (  0) 0) |
Anissah, U., Ariyani, F., Barokah, G., and Januar, H. I., 2021. NMR metabolomics of saurida tumbil fish treated with formaldehyde solution as misconduct food preservation method. Journal of Aquatic Food Product Technology, 30: 263-270. DOI:10.1080/10498850.2021.1880510 (  0) 0) |
Areco, M. M., Salomone, V. N., and dos Santos Afonso, M., 2021. Ulva lactuca: A bioindicator for anthropogenic contamination and its environmental remediation capacity. Marine Environmental Research, 171: 105468. DOI:10.1016/j.marenvres.2021.105468 (  0) 0) |
Beiras, R., Durán, I., Parra, S., Urrutia, M., Besada, V., Bellas, J., et al., 2011. Linking chemical contamination to biological effects in coastal pollution monitoring. Ecotoxicology, 21(1): 9-17. (  0) 0) |
Blomqvist, S., Gunnars, A., and Elmgren, R., 2004. Why the limiting nutrient differs between temperate coastal seas and freshwater lakes: A matter of salt. Limnology and Oceanography, 49(6): 2236-2241. DOI:10.4319/lo.2004.49.6.2236 (  0) 0) |
Brown, A. R., Lilley, M. K. S., Shutler, J. D., Lowe, C. D., Artioli, Y., Torres, R., et al., 2019. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Reviews in Aquaculture, 12(3): 1663-1688. (  0) 0) |
Campillo, J. A., Sevilla, A., González-Fernández, C., Bellas, J., Bernal, C., Cánovas, M., et al., 2019. Metabolomic responses of mussel Mytilus galloprovincialis to fluoranthene exposure under different nutritive conditions. Marine Environmental Research, 144: 194-202. DOI:10.1016/j.marenvres.2019.01.012 (  0) 0) |
Cappello, T., Mauceri, A., Corsaro, C., Maisano, M., Parrino, V., Paro, G. L., et al., 2013. Impact of environmental pollution on caged mussels Mytilus galloprovincialis using NMR-based metabolomics. Marine Pollution Bulletin, 77: 132-139. DOI:10.1016/j.marpolbul.2013.10.019 (  0) 0) |
Chan, C. Y., and Wang, W. X., 2018. Seasonal and spatial variations of biomarker responses of rock oysters in a coastal environment influenced by large estuary input. Environmental Pollution, 242: 1253-1265. DOI:10.1016/j.envpol.2018.08.013 (  0) 0) |
Clune, G., and Harrison, R., 2009. Coastal shell middens of the Abydos coastal plain, Western Australia. Archaeology in Oceania, 44(S1): 70-80. DOI:10.1002/j.1834-4453.2009.tb00069.x (  0) 0) |
Dai, M., Chai, F., Chen, M., Chen, N., Chen, Y., Cheng, D., et al., 2023. Persistent eutrophication and hypoxia in the coastal ocean. Cambridge Prisms: Coastal Futures, 1: e19. DOI:10.1017/cft.2023.7 (  0) 0) |
Damar, A., Ervinia, A., Kurniawan, F., and Rudianto, B. Y., 2021. Eutrophication in a tropical estuary: Is it good or bad? IOP Conference Series: Earth and Environmental Science, 744: 012010.
(  0) 0) |
Gidman, E. A., Jones, M. L. M., Bussell, J. A., Malham, S. K., Reynolds, B., Seed, R., et al., 2007. A methodology for screening haemolymph of intertidal mussels, Mytilus edulis, using FT-IR spectroscopy as a tool for environmental assessment. Metabolomics, 3: 465-473. DOI:10.1007/s11306-007-0060-8 (  0) 0) |
Haider, F., Falfushynska, H. I., Timm, S., and Sokolova, I. M., 2020. Effects of hypoxia and reoxygenation on intermediary metabolite homeostasis of marine bivalves Mytilus edulis and Crassostrea gigas. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 242: 110657. (  0) 0) |
Hair, J. F., Ringle, C. M., and Sarstedt, M., 2011. PLS-SEM: Indeed, a silver bullet. Journal of Marketing Theory and Practice, 19(2): 139-152. DOI:10.2753/MTP1069-6679190202 (  0) 0) |
Hammer, O., Harper, D., and Ryan, P., 2001. PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontologia Electronica, 4: 1-9. (  0) 0) |
Hani, Y. M. I., Prud'Homme, S. M., Nuzillard, J. M., Bonnard, I., Robert, C., Nott, K., et al., 2021. 1H-NMR metabolomics profiling of zebra mussel (Dreissena polymorpha): A field-scale monitoring tool in ecotoxicological studies. Environmental Pollution, 270: 116048. (  0) 0) |
He, G., Lao, Q., Jin, G., Zhu, Q., and Chen, F., 2023. Increasing eutrophication driven by the increase of phosphate discharge in a subtropical bay in the past 30 years. Frontiers in Marine Science, 10: 1184421. (  0) 0) |
Hidayah, I., Januar, H. I., Dwiyitno, D., and Prihantini, N. B., 2021. Seasonal and spatial water pollution status at the river's estuaries in Cirebon region. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology, 16: 75-82. DOI:10.15578/squalen.505 (  0) 0) |
Jha, D. K., Wu, M., Thiruchitrambalam, G., and Marimuthu, P. D., 2023. Coastal and marine environmental quality assessments. Frontiers in Marine Science, 10: 1141278. DOI:10.3389/fmars.2023.1141278 (  0) 0) |
Jiang, Z., Liu, S., Cui, L., He, J., Fang, Y., Premarathne, C., et al., 2022. Sand supplementation favors tropical seagrass Thalassia hemprichii in eutrophic bay: Implications for seagrass restoration and management. BMC Plant Biology, 22(1): 296. DOI:10.1186/s12870-022-03647-0 (  0) 0) |
Juhel, G., Bayen, S., Goh, C. C. M., Lee, W. K., and Kelly, B. C., 2016. Use of a suite of biomarkers to assess the effects of carbamazepine, bisphenol A, atrazine, and their mixtures on green mussels, Perna viridis. Environmental Toxicology and Chemistry, 36(2): 429-441. DOI:10.1002/etc.3556 (  0) 0) |
Kadiri, M., Zhang, H., Angeloudis, A., and Piggott, M. D., 2021. Evaluating the eutrophication risk of an artificial tidal lagoon. Ocean & Coastal Management, 203: 105490. (  0) 0) |
Khalil, M., 2018. The effect of environmental condition on the spawning period of blood cockle Anadara granosa (Bivalvia: Arcidae) in Lhokseumawe, the northern straits of Malacca. Jurnal Agrium, 10(2): 69-76. DOI:10.29103/agrium.v10i2.499 (  0) 0) |
Kessouri, F., McWilliams, J. C., Bianchi, D., Sutula, M., Renault, L., Deutsch, C., et al., 2021. Coastal eutrophication drives acidification, oxygen loss, and ecosystem change in a major oceanic upwelling system. Proceedings of the National Academy of Sciences, 118(21): e2018856118. (  0) 0) |
Kwon, Y. K., Jung, Y. S., Park, J. C., Seo, J., Choi, M. S., and Hwang, G. S., 2012. Characterizing the effect of heavy metal contamination on marine mussels using metabolomics. Marine Pollution Bulletin, 64: 1874-1879. DOI:10.1016/j.marpolbul.2012.06.012 (  0) 0) |
Lao, Q., Liu, G., Shen, Y., Su, Q., and Lei, X., 2021. Biogeochemical processes and eutrophication status of nutrients in the northern Beibu Gulf, South China. Journal of Earth System Science, 130: 1-14. DOI:10.1007/s12040-020-01500-2 (  0) 0) |
Lettieri, G., Marinaro, C., Brogna, C., Montano, L., Lombardi, M., Trotta, A., et al., 2023. A metabolomic analysis to assess the responses of the male gonads of Mytilus galloprovincialis after heavy metal exposure. Metabolites, 13(12): 1168. DOI:10.3390/metabo13121168 (  0) 0) |
Li, Q. P., Zhou, W., Chen, Y., and Wu, Z., 2018. Phytoplankton response to a plume front in the northern South China Sea. Biogeosciences, 15(8): 2551-2563. DOI:10.5194/bg-15-2551-2018 (  0) 0) |
Liu, X., Zhang, L., You, L., Yu, J., Zhao, J., Li, L., et al., 2010. Differential toxicological effects induced by mercury in gills from three pedigrees of Manila clam Ruditapes philippinarum by NMR-based metabolomics. Ecotoxicology, 20(1): 177-186. (  0) 0) |
Liu, Y., Teng, X., Chen, L., Wu, S., Xue, C., and Li, Z., 2024. Changes in flavor-related biomarkers in Pacific oysters (Cras-Sostrea gigas) following microplastic exposure. Foods, 13(5): 765. (  0) 0) |
López-Pedrouso, M., Lorenzo, J. M., Varela, Z., Fernández, J. Á., and Franco, D., 2022. Finding biomarkers in antioxidant molecular mechanisms for ensuring food safety of bivalves threatened by marine pollution. Antioxidants, 11: 369. (  0) 0) |
Luo, J., Sun, Z., Lu, L., Xiong, Z., Cui, L., and Mao, Z., 2022. Rapid expansion of coastal aquaculture ponds in Southeast Asia: Patterns, drivers and impacts. Journal of Environmental Management, 315: 115100. (  0) 0) |
Mardhatilla, F., Atmaja, I. S. W., Hartono, E., Hidayat, F., and Ferdianto, F., 2022. The contribution of the agricultural sector to community welfare in the cirebon district. KnE Life Sciences, 2022: 239-244. (  0) 0) |
Mirsadeghi, S., Zakaria, M., Yap, C., and Gobas, F., 2013. Evaluation of the potential bioaccumulation ability of the blood cockle (Anadara granosa L.) for assessment of environmental matrices of mudflats. Science of the Total Environment, 454-455: 584-597. (  0) 0) |
Mirzaei, M. R., Yasin, Z., and Shau-Hwai, A. T., 2014. Periodicity and shell microgrowth pattern formation in intertidal and subtidal areas using shell cross sections of the blood cockle, Anadara granosa. Egyptian Journal of Aquatic Research, 40(4): 459-468. (  0) 0) |
Moruf, R. O., Okunade, G. F., and Elegbeleye, O. W., 2020. Bivalve mariculture in two-way interaction with phytoplankton: A review of feeding mechanism and nutrient recycling. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Animal Science and Biotechnologies, 77(2): 1-8. (  0) 0) |
Mustafa, A., Paena, M., Athirah, A., Ratnawati, E., Asaf, R., Suwoyo, H. S., et al., 2022. Temporal and spatial analysis of coastal water quality to support application of whiteleg shrimp Litopenaeus vannamei intensive pond technology. Sustainability, 14: 2659. (  0) 0) |
Nemzer, B. V., and Dickson, A. G., 2005. The stability and reproducibility of Tris buffers in synthetic seawater. Marine Chemistry, 96: 237-242. (  0) 0) |
Ngatia, L., Grace III, J. M., Moriasi, D., and Taylor, R., 2019. Nitrogen and phosphorus eutrophication in marine ecosystems. Monitoring of Marine Pollution, 1: 1-17. (  0) 0) |
Niiyama, T., Toyohara, H., and Tanaka, K., 2012. Cellulase activity in blood cockle (Anadara granosa) in the Matang Mangrove Forest Reserve, Malaysia. Japan Agricultural Research Quarterly, 46(4): 355-359. (  0) 0) |
Nurlina, A., 2018. Exceptional paralytic shellfish poisoning on green mussel consumption which contaminated with saxitoxin at Cirebon District, Indonesia di Kabupaten Cirebon, Indonesia. Proceeding of Indonesian National Seminar on Health Research, 1: 134-141. (  0) 0) |
Peng, C., Zhao, X., Han, Y., Shi, W., Liu, S., and Liu, G., 2015. Toxic effects of chronic sub-lethal Cu2+, Pb2+ and Cd2+ on antioxidant enzyme activities in various tissues of the blood cockle, Anadara granosa. Journal of Residuals Science and Technology, 12(3): 125-131. (  0) 0) |
Priya, A. K., Antony, S., Kumar, G. S., Sivamoorthi, S., and Vineesh, S., 2021. Anthropogenic impacts on the contamination in the coastal region: A review. Materials Today: Proceedings, 37: 2236-2238. (  0) 0) |
Rachman, A., Thoha, H., Sianturi, O. R., Bayu, M. D., Fitriya, N., Sidabutar, T., et al., 2019. Distribution of Pyrodinium bahamense cysts in modern sediments of Sukalila water, Cirebon, Indonesia. Philippine Journal of Natural Sciences, 24: 104-115. (  0) 0) |
Renitasari, D. P., Ihwan, I., and Syahrir, M., 2023. Minimizing waste and vannamei shrimp ponds by utilizing blood mussel biofilters (Anadara granosa). Indonesian Journal of Tropical Aquaculture, 7(1): 139-145. (  0) 0) |
Rochfort, S. J., Ezernieks, V., Maher, A. D., Ingram, B. A., and Olsen, L., 2013. Mussel metabolomics – Species discrimination and provenance determination. Food Research International, 54: 1302-1312. (  0) 0) |
Roznere, I., Watters, G. T., Wolfe, B. A., and Daly, M., 2014. Nontargeted metabolomics reveals biochemical pathways altered in response to captivity and food limitation in the freshwater mussel Amblema plicata. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 12: 53-60. (  0) 0) |
Sanjoto, T. B., Sari, H. A., and Hardati, P., 2021. Fishermen adaptation to climate change in Mertasinga Village, Gunungjati Sub-District, Cirebon Regency. International Journal of Sustainable Development and Planning, 16(5): 869-874. (  0) 0) |
Schutte, C. A., Joye, S. B., Wilson, A. M., Evans, T. B., Moore, W. S., and Casciotti, K. L., 2015. Intense nitrogen cycling in permeable intertidal sediment revealed by a nitrous oxide hot spot. Global Biogeochemical Cycles, 29(10): 1584-1598. (  0) 0) |
Shumilina, E., Ciampa, A., Capozzi, F., Rustad, T., and Dikiy, A., 2015. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4℃. Food Chemistry, 184: 12-22. (  0) 0) |
Šilović, T., Balagué, V., Orlić, S., and Pedrós-Alió, C., 2012. Picoplankton seasonal variation and community structure in the northeast Adriatic coastal zone. FEMS Microbiology Ecology, 82(3): 678-691. (  0) 0) |
Sindern, S., Tremöhlen, M., Dsikowitzky, L., Gronen, L., Schwarzbauer, J., Siregar, T. H., et al., 2016. Heavy metals in river and coast sediments of the Jakarta Bay region (Indonesia) – Geogenic versus anthropogenic sources. Marine Pollution Bulletin, 110: 624-633. (  0) 0) |
Sutarih, A., Junaedi, J., and Baehaqi, M. F. A., 2019. Management of hazardous and toxic waste: A legal study of environmental health of fish and shrimp feed industry in PT Suri Tani Pemuka Cirebon West Java Indonesia. Proceedings of the International Symposium on Social Sciences, Education, and Humanities, ISSEH, 2018: 95-99.
(  0) 0) |
Templeton, G., 2011. A two-step approach for transforming continuous variables to normal: Implications and recommendations for IS research. Communications of the Association for Information Systems, 28: 4. (  0) 0) |
Topcu, D., and Brockmann, U., 2021. Consistency of thresholds for eutrophication assessments, examples and recommendations. Environmental Monitoring and Assessment, 193: 1-15. (  0) 0) |
Wang, M., Kroeze, C., Strokal, M., van Vliet, M. T., and Ma, L., 2020a. Global change can make coastal eutrophication control in China more difficult. Earth's Future, 8(4): e2019EF001280. (  0) 0) |
Wang, T., Gnanaprakasam, J. R., Chen, X., Kang, S., Xu, X., Sun, H., et al., 2020b. Inosine is an alternative carbon source for CD8+-T-cell function under glucose restriction. Nature Metabolism, 2(7): 635-647. (  0) 0) |
Ward, J. E., Zhao, S., Holohan, B. A., Mladinich, K., Griffin, T. W., Wozniak, J., et al., 2019. Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): Implications for using bivalves as bioindicators of microplastic pollution. Environmental Science & Technology, 53(15): 8776-8784. (  0) 0) |
Wu, H., and Wang, W. X., 2010. NMR-based metabolomic studies on the toxicological effects of cadmium and copper on green mussels Perna viridis. Aquatic Toxicology, 100: 339-345. (  0) 0) |
Zahroh, A., Riani, E., and Anwar, S., 2019. Analysis of water quality for green mussel cultivation in Cirebon Regency, West Java. Journal of Natural Resources and Environmental Management, 9(1): 86-91. (  0) 0) |
Zhang, X., Lin, S., and Chen, L., 2020. Transcriptomic and physiological responses of skeletonema costatum to ATP utilization. Environmental Microbiology, 22(5): 1861-1869. (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


