2) Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
The Bohai Sea (BS) and Yellow Sea (YS) are typical semi-enclosed marginal shallow seas of China, with complex hydrodynamics and large inputs of land-based materials. The annual organic carbon flux from the Yellow River and other rivers to the YS and BS is (1210 ± 240) × 104 ton (t) yr−1, accounting for 79% of all organic carbon entering these seas (Liu et al., 2015a), while atmospheric deposition contributes < 2% (Qiao et al., 2017). In addition, the Yellow Sea Warm Current (YSWC) transports about 106 t yr−1 of Yangtze River sediments to the North Yellow Sea (NYS) (Gao et al., 1996). The tracing of sedimentary organic matter (SOM) sources improves understanding of the organic matter cycle in aquatic environments (Hedges et al., 1997). SOM is a complex mixture of marine and terrestrial organic compounds, and it is difficult to quantitatively distinguish its sources at the edge of the continental shelf. Normal (n-) alkanes are commonly applied as biomarkers given their widespread occurrence in marine and terrestrial environments, and can be preserved in marine sediments. Compositions and distributions of n-alkanes from different biological sources are generally different. In addition, compared with fatty acids and alkanols, structure of n-alkanes is relatively stable and has strong anti-degradation ability (Mead et al., 2005). Previous studies have shown that n-alkanes may be effective in characterizing sources of SOM in coastal marine systems (Xing et al., 2011; Wang et al., 2013). Long-chain n-alkanes derived from terrestrial higher plants are most abundant in C27, C29, and C31 n-alkanes (Bray and Evans, 1961). The freshwater and marine non-emergent macrophytes and sphagnum mosses are enriched in mid-chain n-alkanes (Pancost et al., 2002; Mead et al., 2005; Mügler et al., 2008; Bush and McInerney, 2013). Short-chain n-alkanes with odd carbon predominance such as C17 n-alkane are generally considered to be derived from aquatic algae and photosynthetic bacteria(Meyers and Ishiwatari, 1993; Silliman and Schelske, 2003; Liu et al., 2012). Additionally, petroleum-derived hydrocarbons also contribute short-chain n-alkanes, with no obvious odd/even predominance (Hostettler et al., 1999). The long-chain (≥C24), n-alkanes have clear odd carbon-number predominance in the NYS, indicating predominant input of terrestrial higher plant material (Lu et al., 2011). Analyses of n-alkanes in sediments of the Yellow River Estuary indicate that SOM originates mainly from terrigenous inputs, while marine microorganisms contribute to short-chain (C12–C22) n-alkanes offshore (Wang et al., 2018). Compositional analysis of n-alkanes in surface sediments of the central South Yellow Sea (SYS) indicates that SOM is derived mainly from terrestrial higher plant input from the modern and old Yellow rivers, with the contribution of herbaceous to woody plants is comparable (Zhang et al., 2014).
Similar chemical n-alkane compositions have been found in different types of organisms, which may confuse their interpretation (Ficken et al., 2000; Mead et al., 2005; Sikes et al., 2009). However, stable carbon isotopic compositions of individual n-alkanes from different sources in marine sediments are generally distinctive and may therefore constrain their sources (Hayes et al., 1990; Mead et al., 2005; Ankit et al., 2017), with n-alkane compositions and stable carbon isotopic characteristics together having been used to identify SOM sources (Eglinton, 1969; Jeng and Huh, 2008; Hu et al., 2013). Previous studies have shown that stable carbon isotopic compositions of long-chain n-alkanes in surface soils of eastern China can be used as an indicator of C3/C4 plant proportions in overlying vegetation (Rao et al., 2008). The analysis of long-chain n-alkanes and their stable carbon isotopic compositions in sediments of Qinghai Lake indicates that δ13C values of C31 n-alkanes are consistent with those of modern land plants around the lake, and can therefore be used as a reliable tracer of C3/C4 compositions of terrestrial vegetation (Liu et al., 2015b). A recent study found that δ13C values of organic matter indicate that terrestrial organic carbon from the Yellow River accumulates mainly at the river mouth and in two muddy areas around it (Sun et al., 2018a).
Until now, little has been known of the spatial distribution of n-alkane stable carbon isotopes in sediments of the BS and NYS. The aim of this study was to elucidate the stable carbon isotopic composition of SOM n-alkanes and the sources of n-alkanes in surface sediments of the BS and NYS.
2 Study Area and Methods 2.1 Study AreaThe BS is a shallow, semi-enclosed epicontinental sea. About 90% of its sediment input is supplied by the surrounding rivers, especially the Yellow River. The YS is a shallow, semi-enclosed, continental margin West Pacific sea, with an area of 400, 000 km2 and an average water depth of 44 m, joining the BS in the north and the East China Sea in the south. The YS is divided into southern and northern parts by the Shandong and Korean peninsulas. The overall topography of the NYS seabed is inclined southward. Water depths in the BS and NYS are generally < 60 m. In the study area, surface currents include coastal currents and the northwestward YSWC (Fig. 1). The YSWC is a branch of the Tsushima Current with warm and saline water. There is no major direct local riverine input to the YS although, over time, fine-grained riverine sediment can be resuspended and transported from the BS to the YS by coastal currents. Analyses of sediment sources indicate that fine-grained sediments within the NYS originate mainly in the modern and old Yellow Rivers (Alexander et al., 1991; Lim et al., 2007). From 1976 to 2005, runoff and sediments from the Yellow River averaged 140.36×108 m3 yr−1 and 3.31×108 t yr−1, respectively (Cui and Li, 2011), with most sedimentary materials being deposited in front edge of the delta and estuary, and finer grained sediment transported to the coastal and shelf areas outside the Yellow River estuary. Muddy sediments along the north coast of Shandong Peninsula are considered to be directly and indirectly from the Yellow River (Yang and Liu, 2007). In addition to riverine input, coastal erosion from the old Yellow River Delta also contributes to sediments (Hu et al., 1998).
|
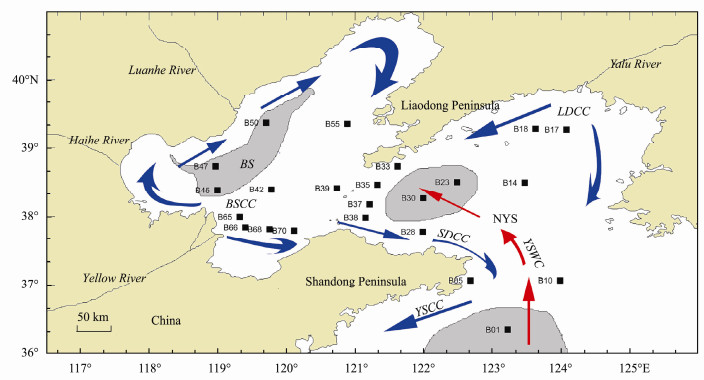
Fig. 1 Sampling sites and surface currents in the BS and NYS. BSCC, Bohai Sea Coastal Current; SDCC, Shandong Coastal Current; YSCC, Yellow Sea Coastal Current; YSWC, Yellow Sea Warm Current; LDCC, Liaodong Peninsula Coastal Current. Blocks represent the sites. Shading indicates the muddy areas. |
Surface sediments (0–3 cm depth) were collected from 23 sites in the BS and NYS using a box corer deployed from R/V Dong Fang Hong 2 during a cruise sponsored by the National Natural Science Foundation of China (NSFC) in June 2011 (Fig. 1). Sediment samples were wrapped in aluminum foil and stored at −20℃ until analysis.
2.3 Analytical Methods 2.3.1 Lipid extraction and purification of n-alkanesFreeze-dried, powdered, and homogenized sediment samples were extracted four times with dichloromethane/ methanol (DCM/MeOH; 3:1, v/v) with ultrasonication (15 min each time), after adding internal standards containing n-C24D50. Extracts of the samples were dried in a N2 stream and hydrolyzed with 6% KOH in MeOH. Nonpolar fractions containing n-alkanes were separated using activated silica gel column chromatography with elution by n-hexane, and dried in a N2 stream.
To accurately measure the δ13C values of individual n-alkanes, n-alkanes need to be further purified. Zeolite molecular sieve is a commonly used and high recovery method for n-alkanes purification. The extracted n-alkanes were transferred to a column with AgNO3-Silica gel and molecular sieve, and then eluted with n-hexane to ensure the components of the inner wall of the AgNO3-Silica gel glass column were completely transferred into the molecular sieve. After elution of about 1.5 mL, the upper AgNO3-Silica gel column was removed and the molecular sieve column was baked in an oven at 40℃ for > 12 h. Subsequently, the zeolite power was transferred to a 10 mL Teflon bottle for digestion with HF to release n-alkanes. A preheating Pasteur column (6 mm, i.d. × 2 cm) filled with Na2SO4 was used to remove residual HF before the n-alkanes were extracted with n-hexane (four times) and then was dried in a gentle N2 stream pending instrumental analyses. The average recovery rate of long-chain (≥ C26), mid-chain (C21–C25), short-chain (C15–C20) n-alkanes in all samples was 63%, 53%, 57%, respectively.
2.3.2 n-alkanes analysisThe n-alkane compositions were determined by an Agilent 6890N gas chromatography, with chromatographic separation on an HP-1 capillary column (50 m × 0.32 mm i.d.× 0.17 μm film thickness, J & W Scientific) using H2 as a carrier gas (1.2 mL min−1). Samples were injected in splitless mode with an injector temperature of 300℃. Oven temperature was programmed from 80℃ to 200℃ at 25℃ min−1, 200℃ to 250℃ at 3℃ min−1, 250℃ to 300℃ at 1.8℃ min−1, 300 to 310℃ at 5℃ min−1, and holding at 310℃ for 5 min. Quantification of compounds was performed by peak area integration in FID GC (Agilent 6890N) relative to the internal standards. The average relative standard deviation in concentrations was < 10%.
The average chain length (ACL; Cranwell et al., 1987), the terrigenous/aquatic ratio (TAR; Bourbonniere and Meyers, 1996), the Pmar-aq (odd mid-chain alkanes/odd mid- and long-chain alkanes; Ficken et al., 2000; Mead et al., 2005) of n-alkanes were calculated as follows:
| ${\rm{ACL}} = \frac{{21 \times {{\rm{C}}_{21}} + 23 \times {{\rm{C}}_{23}} + 25 \times {{\rm{C}}_{25}} + 27 \times {{\rm{C}}_{27}} + 29 \times {{\rm{C}}_{29}} + 31 \times {{\rm{C}}_{31}} + 33 \times {{\rm{C}}_{33}}}}{{{{\rm{C}}_{21}} + {{\rm{C}}_{23}} + {{\rm{C}}_{25}} + {{\rm{C}}_{27}} + {{\rm{C}}_{29}} + {{\rm{C}}_{31}} + {{\rm{C}}_{33}}}}, $ | (1) |
| ${\rm{TAR}} = \frac{{{{\rm{C}}_{{\rm{27}}}} + {{\rm{C}}_{{\rm{29}}}}{\rm{ + }}{{\rm{C}}_{{\rm{31}}}}}}{{{{\rm{C}}_{{\rm{15}}}} + {{\rm{C}}_{{\rm{17}}}}{\rm{ + }}{{\rm{C}}_{{\rm{19}}}}}}, $ | (2) |
| ${{\rm{P}}_{{\rm{mar - aq}}}} = \frac{{{{\rm{C}}_{{\rm{23}}}} + {{\rm{C}}_{{\rm{25}}}}}}{{{{\rm{C}}_{{\rm{25}}}} + {{\rm{C}}_{{\rm{27}}}}{\rm{ + }}{{\rm{C}}_{{\rm{29}}}}{\rm{ + }}{{\rm{C}}_{{\rm{31}}}}}}.$ | (3) |
Gas chromatography isotope ratio mass spectrometry (GC-IRMS; on an HP 6890 GC coupled with a Thermo Delta-V system.) was used to measure stable carbon isotopic compositions of n-alkanes. Chromatographic separation was achieved using a DB-1MS capillary column (60 m× 0.32 mm i.d. × 0.25 μm film thickness, J & W Scientific). The GC oven temperature was programmed from 60℃ to 200℃ at 15℃ min–1, 200℃ to 250℃ at 4℃ min–1, 250℃ to 300℃ at 1.8℃ min–1, 300 to 310℃ at 5℃ min–1, and holding at 310℃ for 5 min. The authentic standard was analyzed under the same conditions after every seven samples. The standard deviation for duplicate analysis of the standard was 0.3‰. Isotopic ratios were expressed as δ13C values (per mil) relative to the Vienna Pee Dee Belemnite (VPDB).
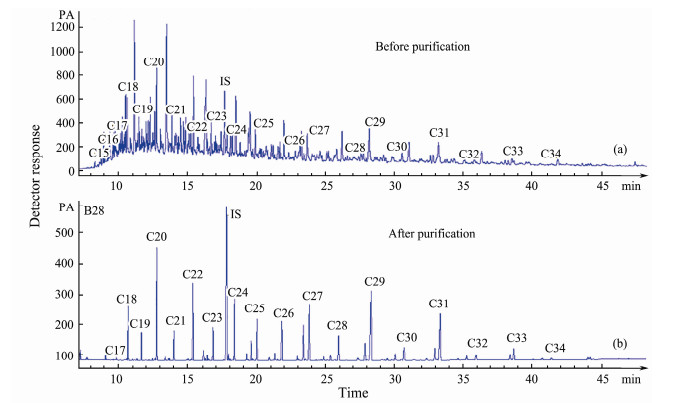
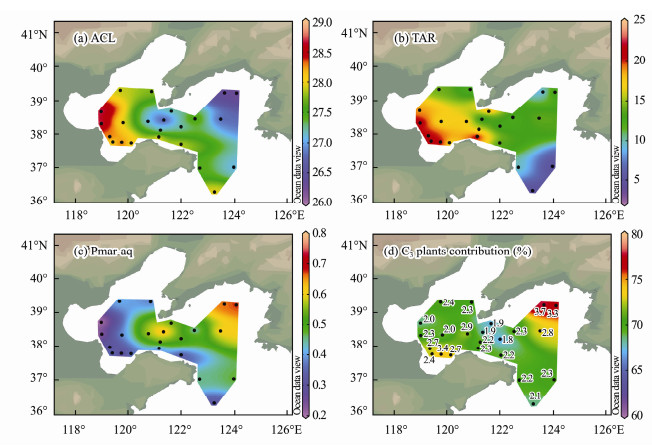
3 Results 3.1 Composition of n-Alkanes and the Hydrocarbon IndicesThe GC-FID chromatograms of n-alkanes showed that n-alkanes were effectively purified after using the molecular sieve (Fig. 2). Total n-alkane contents (∑C15–35) ranged from 456 to 3837 ng g−1 (average = 1897 ng g−1). The contents of long-chain n-alkanes (∑C25–35) ranged from 267 to 2826 ng g−1 (average = 1300 ng g−1). In addition, the average percentage of long-chain, mid-chain, short-chain n-alkanes in samples was 58%, 27%, 14%, respectively. Furthermore, the total n-alkane contents of samples from muddy areas (average = 2666 ng g−1) were significantly higher than those from non-muddy areas (average = 1683 ng g–1). The ACL values varied between 26.1 and 28.9 (Fig. 4a). The values of TAR and Pmar-aq ranged from 3.4 to 25.7, from 0.2 to 0.7 (Figs. 4b, 4c), respectively.
|
Fig. 2 GC-FID chromatograms for n-alkanes of surface sediments (site B28): (a), Before purification; (b), After purification. |
|
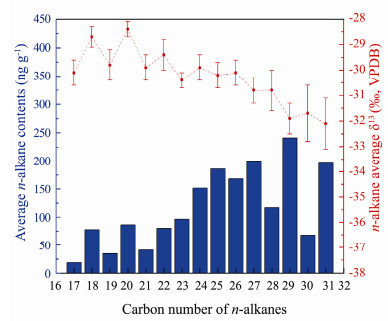
Fig. 3 Compound-specific average δ13C values for the individual n-alkanes (C17-C31) from 23 BS and NYS samples. |
|
Fig. 4 Spatial distribution of n-alkane indices: (a), ACL; (b), TAR; (c), Pmar-aq and (d), C3 plants contribution to n-alkanes and C3/C4 ratio in surface sediments, based on the end-member modeling of compound-specific δ13C values in the study area. |
Compound-specific average δ13C values of n-alkanes in surface sediments were shown in Fig. 3, with average individual values for C17–C31 of −30.1‰ ± 0.5‰, −28.7‰ ± 0.4‰, −29.8‰ ± 0.6‰, −28.4‰ ± 0.3‰, −29.9‰ ± 0.5‰, −29.4‰ ± 0.6‰, −30.4‰ ± 0.3‰, −29.9‰ ± 0.5‰, −30.2‰ ± 0.5‰, −30.1‰ ± 0.5‰, −30.8‰ ± 0.5‰, −30.8‰ ± 0.8‰, −31.9‰ ± 0.6‰, −31.7‰ ± 1.1‰, and −32.1‰ ± 1.0‰, respectively. In both the BS and NYS, δ13C values of mid-chain n-alkanes (C21–C23) varied within a narrow range, while those of short- and long-chain n-alkanes were more variable. Furthermore, for short- and mid-chain n-alkanes, δ13C values of even-carbon-numbered cases were more positive than those of odd-carbon-numbered cases.
4 Discussion 4.1 Long-Chain n-AlkanesThe contents of long-chain n-alkanes were relatively high and exhibited a strong odd carbon predominance in C27, C29, and C31 homologues (Fig. 3), consistent with terrestrial higher plant sources. The ACL describes the average number of carbon atoms in odd carbon n-alkanes in higher plants (Cranwell et al., 1987). The ACL values of BS and NYS surface sediments ranged from 26.1 to 28.9 (average = 27.5). ACL value of about 29 in sediments near the Yellow River estuary suggests an origin of terrestrial higher plants (Fig. 4a). The relative contribution of terrestrial n-alkanes to marine sediments can be assessed using the TAR index. TAR values of BS and NYS surface sediments ranged from 3.4 to 25.7, with an average value of 14.1 (Fig. 4b). This indicates a predominance of terrigenous n-alkanes input (Ankit et al., 2017). Furthermore, compositional analysis of n-alkanes in surface sediments of the BS and NYS also indicates that long-chain n-alkanes are derived mainly from terrestrial higher plant input (Cao, et al., 2018). Hence, Long-chain n-alkanes in the study areas were thus mainly derived from such plants.
The δ13C values of long-chain n-alkanes produced by C3 and C4 plants typically range from −31.0‰ to −39.0‰ and −18.0‰ to −25.0‰, respectively (Collister et al., 1994; Schefuẞ et al., 2003). Modern terrestrial higher plants from eastern China are characterized by n-alkane δ13C values of −21.9‰ to −34.8‰, −25.3‰ to −36.1‰, and −22.9‰ to −36.7‰ for C27, C29, and C31 components (Rao et al., 2008), consistent with our corresponding average δ13C values of −30.8% ± 0.5‰, −31.9% ± 0.6‰, and −32.1% ± 1.0‰ (Fig. 3), respectively, and indicating that long-chain n-alkanes are mainly derived from terrigenous sources. Generally, odd-carbon-numbered long-chain n-alkanes are somewhat 13C-enriched than those of even-carbon-numbered long-chain n-alkanes in terrestrial higher plants (Chikaraishi and Naraoka, 2003). However, our results showed δ13C values of even-carbon-numbered long-chain n-alkanes (C26–30) were more positive than those of odd-carbon-numbered long-chain n-alkanes (C27–31) in the study area (Fig. 3). This implies there may be different sources of even-carbon-numbered long-chain n-alkanes. A previous study reported 14C ages for n-C29+31 alkanes (Δ14C = −288‰ to −612‰) of 2670 to 7550 14C yr, which differ markedly from those of strongly 14C-depleted n-C26+28+30+32 alkanes (Δ14C = −700‰ to −961‰) ages of 9600 to 26050 14C yr for Yellow River suspended particulate matter, implying ancient organic carbon inputs (Tao et al., 2015). This may indicate that even-carbon-numbered long-chain n-alkanes in the BS and NYS are derived from ancient organic carbon.
Weighted mean average δ13C of long-chain n-alkanes from sediment samples were determined to calculate the changes in biomass of C3 and C4 plants in historical periods (Kuang et al., 2013). A binary end-member mixing model was used to estimate the relative contributions of long-chain n-alkanes from C3 and C4 plants (Garcin et al., 2014), with δ13C values of −36.0‰ and −21.0‰ being used as end-members for these plants, respectively (Collister et al., 1994; Zhang et al., 2003). Calculations were performed as follows:
| $ M = ({{\rm{ \mathit{ δ} }}^{13}}{{\rm{C}}_{27}} \times {{\rm{C}}_{27}} + {{\rm{ \mathit{ δ} }}^{13}}{{\rm{C}}_{29}} \times {{\rm{C}}_{29}} + {{\rm{ \mathit{ δ} }}^{13}}{{\rm{C}}_{31}} \times {{\rm{C}}_{31}})/({{\rm{C}}_{27}} + {{\rm{C}}_{29}} + {{\rm{C}}_{31}}) = \left({ - 36.0‰} \right) \times X + \left({ - 21.0‰} \right) \times (100\% - X), $ | (4) |
where M is the weighted mean average δ13C value of long-chain n-alkanes, and X is the C3 contribution (%).
End-member estimations for the BS and NYS indicated that terrestrial C3 plants were dominant n-alkane sources, with relative contributions of 64%–79% (Fig. 4d). This is consistent with the predominance of C3 plants in north China, with a previous study having shown that δ13C values of n-alkanes in aerosols near the north China coast have terrestrial C3 plant origins with the C4 contribution being negligible (Guo et al., 2006). Moreover, soil organic matter δ13C values in a N–S section (34–52˚N) through central and eastern Asia indicate that vegetation in the area comprises mainly C3 plants (Feng et al., 2008). Records of δ13C values in surface soils of northeast China indicate that the abundance of C4 plants is relatively high in warm periods and almost exclusively C3 plants exist in cold periods (Sun et al., 2018b). Previous studies have shown that δ13C values of dominant C3 plants in the Chinese Loess Plateau range from −30.7‰ to −22.6‰, with average value of 27.2‰ (Zheng and Shangguan, 2007) and −27.1% ± 2.4‰ (n = 39; Liu et al., 2005). Both δ13C values of total organic carbon and long-chain n-alkanes derived from terrestrial higher plants show minor variations among surface soil samples from northern China, indicating the major contributor is from local grasses with a uniform C3 photosynthetic pathway (Rao et al., 2011). It is likely, therefore, that long-chain n-alkanes in BS and NYS surface sediments are mainly derived from terrestrial higher plants, particularly C3 plants.
Furthermore, recent studies have also shown that δ13C values of n-alkanes in gymnosperms are heavier than those in angiosperms (Diefendorf et al., 2011; Lane, 2017; Zhao et al., 2018). And angiosperm δ13C values generally decrease with increasing chain length of n-alkanes, while gymnosperm values increase (Bush and McInerney, 2009). It is clear here that δ13C values of long-chain n-alkanes decrease with increasing chain length (Fig. 3). Average δ13C values of C29 and C31 n-alkanes are −31.9% ± 0.6‰ and −32.1% ± 1.0‰, respectively, similar to values for herbaceous plants in the modern Yellow River drainage basin (−31.1‰ to −31.5‰ for C29 n-alkanes, and −31.3‰ to −32.6‰ for C31 n-alkanes in dust episode periods, Guo et al., 2006). This suggests that the contribution of C3 angiosperms to the sedimentary long-chain n-alkanes is greater. This is consistent with the predominance of angiosperms in the last glacial period and Holocene on the Chinese Loess Plateau (Li et al., 2016).
4.2 Mid-Chain n-AlkanesC21, C23, and C25 n-alkanes are mainly contributed by aquatic plants. Previous studies have shown that the δ13C values of mid-chain n-alkanes in aquatic emergent macrophytes range from −28.6‰ to −31.2‰ (Chikaraishi and Naraoka, 2003; Mead et al., 2005). Although non-emergent marine macrophytes can also produce mid-chain n-alkanes, their δ13C values are relatively heavy, ranging from −13.0‰ to −22.0‰ (Ficken et al., 2000; Jaffé et al., 2001). In the NYS, there was little difference between stable carbon isotopic compositions of samples from muddy and non-muddy areas: average δ13C values of mid-chain n-alkanes (C21, C23, and C25) in non-muddy areas were −29.8‰, −30.3‰, and −30.2‰, respectively, and those in muddy areas were −29.7‰, −30.3‰, and −30.2‰, respectively. This also applied to the BS, where average δ13C values were −29.7‰, −30.4‰, and −30.2‰, respectively, indicating that stable carbon isotope compositions of mid-chain n-alkanes in the BS and NYS were similar. The narrow range of these values may be due to there being a common source for BS and NYS sediments, namely the Yellow River (Bi et al., 2010). The δ13C values of C21, C23, and C25 n-alkanes fell within the range of values for the corresponding n-alkanes in aquatic emergent macrophytes, with sediment mid-chain n-alkanes in the study area thus being mainly derived from such plants. Furthermore, the Pmar-aq index provides a measure of the relative contributions of aquatic non-emergent/emergent plants and terrestrial vegetation, with values of < 0.25 corresponding to terrigenous plants, 0.3–0.6 to aquatic emergent plants, and > 0.6 to aquatic non-emergent macrophytes in coastal marine environments (Ficken et al., 2000; Mead et al., 2005). The Pmar-aq values ranged from 0.2 to 0.7 (average = 0.4) in the study area (Fig. 4c). We concluded, therefore, that mid-chain n-alkanes were mainly derived from aquatic emergent macrophytes in the BS and NYS.
4.3 Short-Chain n-AlkanesShort-chain n-alkanes are generally considered as being derived from microorganisms and marine algae. Those produced by marine planktonic algae are mainly C15, C17, and C19 n-alkanes with odd carbon predominance, while even-carbon-numbered short-chain n-alkanes (C16, C18, and C20) are derived from marine bacteria or petroleum hydrocarbons (Gogou et al., 2000; Wang and Fingas, 2006). Short-chain n-alkanes in marine sediments are predominantly C17, indicating the major contribution of algae and photosynthetic bacteria (Han and Calvin, 1969), while even-carbon-numbered (C16–22) n-alkanes in marine sediments are mainly attributable to non-photosynthetic bacteria (Jeng and Huh, 2008). Most of sediments in the BS and NYS exhibited an even-carbon-number preference in the range of n-C16 to n-C22 (Fig. 3), indicating that these short-chain n-alkanes could be from non-photosynthetic bacterial sources. The values of n-C18/n-C17 can be used to compare the relative contributions of n-alkanes from petroleum-derived n-alkanes and natural n-alkanes from algae and photosynthetic bacteria. Here, the calculated n-C18/n-C17 values of surface sediments are higher than 1 at all stations, indicating that short-chain n-alkanes are affected by petroleum pollution to some degree. Extremely depleted △14C values (−932‰ to −979‰) for short-chain n-alkanes (C16 and C18) were found in BS and YS sediments, suggesting a predominant input from sedimentary rocks (organic carbon) or petroleum products (Tao et al., 2016). The average δ13C value of short-chain n-alkanes, δ13C17, δ13C18, and δ13C19, is −30.1% ± 0.5‰, −28.7% ± 0.4‰, and −29.8% ± 0.6‰, respectively (Fig. 3). Previous studies show that the δ13CC17 values of cyanobacteria vary from −34.0‰ to −36.0‰ (Kristen et al., 2010), while δ13C17 and δ13C19 values of petroleum hydrocarbons are about −30.6‰ and −31.0‰, respectively (Li et al., 2009). The average δ13C value of algae in Laizhou Bay is −20.5‰ (Cai and Cai, 1993). Our results showed δ13C values of short-chain n-alkanes were relatively lighter than those of algae, possibly due to biodegradation of bacteria and input of petroleum hydrocarbons or other sources.
5 ConclusionsThe relative inputs of terrestrial and marine organic matter were assessed using n-alkane. Terrigenous plants are the main source of n-alkanes in BS and NYS sediments. Long-chain n-alkanes in sediments were mostly derived from terrestrial sources with some contribution from biogenic and/or petroleum sources. The average δ13C values of long-chain n-C27, n-C29, and n-C31 alkanes are −30.8% ± 0.5‰, −31.9% ± 0.6‰, and −32.1% ± 1.0‰, respectively, within the range of n-alkanes δ13C values of terrestrial C3 plants. A hydrocarbon source distribution derived using a binary end-number mixing model based on δ13C values of long-chain n-alkanes indicates that organic matter in BS and NYS sediments is mainly sourced from C3 plants, particularly angiosperms. The relative contribution of C3 plants decreases from estuary to ocean. δ13C values of mid-chain n-alkanes in surface sediments indicate that mid-chain n-alkanes are mainly of aquatic emergent macrophyte origin. δ13C17, δ13C18 and δ13C19 values, n-C18/n-C17 ratios indicate that short-chain n-alkanes in BS and NYS sediments have complex sources including petroleum pollution and bacterial action.
AcknowledgementsThis work was financially supported by the Ministry of Science and Technology of People's Republic of China (No. 2016YFA0600904), and the National Natural Science Foundation of China (No. 41476058).
Alexander, C.R., De Master, D.J. and Nittrouer, C.A., 1991. Sediment accumulation in a modern epicontinental-shelf setting: The Yellow Sea. Marine Geology, 98: 51-72. DOI:10.1016/0025-3227(91)90035-3 (  0) 0) |
Ankit, Y., Mishra, P. K., Kumar, P., Jha, D. K., Kumar, V. V., Ambili, V. and Anoop, A., 2017. Molecular distribution and carbon isotope of n-alkanes from Ashtamudi Estuary, South India: Assessment of organic matter sources and paleoclimatic implications. Marine Chemistry, 196: 62-70. DOI:10.1016/j.marchem.2017.08.002 (  0) 0) |
Bi, N., Yang, Z., Wang, H., Hu, B. and Ji, Y., 2010. Sediment dispersion pattern off the present Huanghe (Yellow River)subdelta and its dynamic mechanism during normal river discharge period. Estuarine, Coastal and Shelf Science, 86: 352-362. DOI:10.1016/j.ecss.2009.06.005 (  0) 0) |
Bourbonniere, R. A. and Meyers, P. A., 1996. Sedimentary geolipid records of historical changes in the watersheds and productivities of Lakes Ontario and Erie. Limnology Oceanography, 41: 352-359. DOI:10.4319/lo.1996.41.2.0352 (  0) 0) |
Bray, E. E. and Evans, E. D., 1961. Distribution of n-paraffins as a clue to recognition of source beds. Geochimica et Cosmochimica Acta, 22: 2-15. DOI:10.1016/0016-7037(61)90069-2 (  0) 0) |
Bush, R. T. and McInerney, F. A., 2013. Leaf wax n-alkane distributions in and across modern plants: Implications for paleoecology and chemotaxonomy. Geochimica et Cosmochimica Acta, 117: 161-179. DOI:10.1016/j.gca.2013.04.016 (  0) 0) |
Bush, R. T. , and McInerney, F. A. , 2009. Re-evaluating the isotopic divide between angiosperms and gymnosperms using n-alkane δ13C values. AGU Fall Meeting Abstracts. Washington D. C. , 1-9.
(  0) 0) |
Cai, D. and Cai, A., 1993. The organic carbon isotope geochemistry study of Yellow River Mouth. Science in China Series B-Chemistry, Life Sciences & Earth Sciences, 23(10): 1105-1113. DOI:10.1360/zb1993-23-10-1105 (  0) 0) |
Cao, Y., Xing, L., Wang, X. and Zhao, M., 2018. Study on the indication of n-alkanes in surface sediments from the Bohai Sea and the North Yellow Sea. Periodical of Ocean University of China, 48: 104-113 (in Chinese with English abstract). DOI:10.16441/j.cnki.hdxb.20160341 (  0) 0) |
Chikaraishi, Y. and Naraoka, H., 2003. Compound-specificδD-δ13C analyses of n-alkanes extracted from terrestrial and aquatic plants. Phytochemistry, 63: 361-371. DOI:10.1016/S0031-9422(02)00749-5 (  0) 0) |
Collister, J. W., Rieley, G., Stern, B., Eglinton, G. and Fry, B., 1994. Compound-specificδ13C analyses of leaf lipids from plants with differing carbon dioxide metabolisms. Organic geochemistry, 21: 619-627. DOI:10.1016/0146-6380(94)90008-6 (  0) 0) |
Cranwell, P. A., Eglinton, G. and Robinson, N., 1987. Lipids of aquatic organisms as potential contributors to lacustrine sediments-Ⅱ. Organic Geochemistry, 11: 513-527. DOI:10.1016/0146-6380(87)90007-6 (  0) 0) |
Cui, B. L. and Li, X. Y., 2011. Coastline change of the Yellow River estuary and its response to the sediment and runoff(1976-2005). Geomorphology, 127: 32-40. DOI:10.1016/j.geomorph.2010.12.001 (  0) 0) |
Diefendorf, A. F., Freeman, K. H., Wing, S. L. and Graham, H. V., 2011. Production of n-alkyl lipids in living plants and implications for the geologic past. Geochimica et Cosmochimica Acta, 75: 7472-7485. DOI:10.1016/j.gca.2011.09.028 (  0) 0) |
Eglinton, G. , 1969. Organic geochemistry the organic chemist's approach. In: Organic Geochemistry. Eglinton, G. , Murphy, M. T. J. eds. , Springer, Berlin, Heidelberg, 20-73, https://doi.org/10.1007/978-3-642-87734-6_2.
(  0) 0) |
Feng, Z. D., Wang, L. X., Ji, Y. H., Guo, L. L., Lee, X. Q. and Dworkin, S. I., 2008. Climatic dependency of soil organic carbon isotopic composition along the S-N Transect from34?N to 52?N in central-east Asia. Palaeogeography, Palaeoclimatology, Palaeoecology, 257: 335-343. DOI:10.1016/j.palaeo.2007.10.026 (  0) 0) |
Ficken, K. J., Li, B., Swain, D. L. and Eglinton, G., 2000. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Organic Geochemistry, 31: 745-749. DOI:10.1016/S0146-6380(00)00081-4 (  0) 0) |
Gao, S., Park, Y. A., Zhao, Y. Y., and Qin, Y. S., 1996. Transport and resuspension of fine-grained sediments over the southeastern Yellow Sea. Proceedings of the Korean-China International Seminar on Holocene and Late Pleistocene Environments in the Yellow Sea Basin. Seoul National University Seoul, Korea, 83-98.
(  0) 0) |
Garcin, Y., Schefuẞ, E., Schwab, V. F., Garreta, V., Gleixner, G., Vincens, A., Todou, G., Séné, O., Onana, J. M., Achoundong, G. and Sachse, D., 2014. Reconstructing C3 and C4 vegetation cover using n-alkane carbon isotope ratios in recent lake sediments from Cameroon, Western Central Africa. Geochimica et Cosmochimica Acta, 142: 482-500. DOI:10.1016/j.gca.2014.07.004 (  0) 0) |
Gogou, A., Bouloubassi, I. and Stephanou, E. G., 2000. Marine organic geochemistry of the eastern Mediterranean: 1. Aliphatic and polyaromatic hydrocarbons in Cretan Sea surficial sediments. Marine Chemistry, 68: 265-282. DOI:10.1016/S0304-4203(99)00082-1 (  0) 0) |
Guo, Z., Li, J., Feng, J., Fang, M. and Yang, Z., 2006. Compound-specific carbon isotope compositions of individual long-chain n-alkanes in severe Asian dust episodes in the North China coast in 2002. Chinese Science Bulletin, 51: 2133-2140. DOI:10.1007/s11434-006-2071-7 (  0) 0) |
Han, J. and Calvin, M., 1969. Hydrocarbon distribution of algae and bacteria, microbiological activity in sediments. Proceedings of the National Academy of Sciences, 64(2): 436-443. DOI:10.1073/pnas.64.2.436 (  0) 0) |
Hayes, J. M., Freeman, K. H., Popp, B. N. and Hoham, C. H., 1990. Compound-specific isotopic analyses: A novel tool for reconstruction of ancient biogeochemical processes. Organic Geochemistry, 16: 1115-1128. DOI:10.1016/0146-6380(90)90147-R (  0) 0) |
Hedges, J. I., Keil, R. G. and Benner, R., 1997. What happens to terrestrial organic matter in the ocean?. Organic GeoChemistry, 27: 195-212. DOI:10.1016/S0146-6380(97)00066-1 (  0) 0) |
Hostettler, F. D., Pereira, W. E., Kvenvolden, K. A., Van Geen, A., Luoma, S. N., Fuller, C. C. and Anima, R., 1999. Arecord of hydrocarbon input to San Francisco Bay as traced by biomarker profiles in surface sediment and sediment cores. Marine Chemistry, 64: 115-127. DOI:10.1016/S0304-4203(98)00088-7 (  0) 0) |
Hu, L., Shi, X., Guo, Z., Wang, H. and Yang, Z., 2013. Sources, dispersal and preservation of sedimentary organic matter in the Yellow Sea: The importance of depositional hydrodynamic forcing. Marine Geology, 335: 52-63. DOI:10.1016/j.margeo.2012.10.008 (  0) 0) |
Jaffé, R., Mead, R., Hernandez, M. E., Peralba, M. C. and Di, Guida O. A., 2001. Origin and transport of sedimentary organic matter in two subtropical estuaries: A comparative, biomarker-based study. Organic Geochemistry, 32: 507-526. DOI:10.1016/S0146-6380(00)00192-3 (  0) 0) |
Jeng, W. L. and Huh, C. A., 2008. A comparison of sedimentary aliphatic hydrocarbon distribution between East China Sea and southern Okinawa Trough. Continental Shelf Research, 28: 582-592. DOI:10.1016/j.csr.2007.11.009 (  0) 0) |
Kristen, I., Wilkes, H., Vieth, A., Zink, K. G., Plessen, B., Thorpe, J., Partridge, T. C. and Oberhänsli, H., 2010. Biomarker and stable carbon isotope analyses of sedimentary organic matter from Lake Tswaing: Evidence for deglacial wetness and early Holocene drought from South Africa. Journal of Paleolimnology, 44: 143-160. DOI:10.1007/s10933-009-9393-9 (  0) 0) |
Kuang, H., Zhou, H., Hu, J., Yang, X., Peng, P. and Yang, H., 2013. Variations of n-alkanes and compound specific carbon isotopes in sedments from Huguanyan Maar lake during the last glacial maximum and holoceneoptimum: Implications for paleovegetation. Quaternary Sciences, 33(6): 1222-1233. DOI:10.3969/j.issn.1001-7410.2013.06.18 (  0) 0) |
Lane, C. S., 2017. Modern n-alkane abundances and isotopic composition of vegetation in a gymnosperm-dominated ecosystem of the southeastern U. S. coastal plain. Organic Geochemistry, 105: 33-36. DOI:10.1016/j.orggeochem.2016.12.003 (  0) 0) |
Li, Y., Xiong, Y., Yang, W., Xie, Y., Li, S. and Sun, Y., 2009. Compound-specific stable carbon isotopic composition of petroleum hydrocarbons as a tool for tracing the source of oil spills. Marine Pollution Bulletin, 58: 114-117. DOI:10.1016/j.marpolbul.2008.08.012 (  0) 0) |
Li, Y., Yang, S., Wang, X., Hu, J., Cui, L., Huang, X. and Jiang, W., 2016. Leaf wax n-alkane distributions in Chinese loess since the Last Glacial Maximum and implications for paleoclimate. Quaternary International, 399: 190-197. DOI:10.1016/j.quaint.2015.04.029 (  0) 0) |
Lim, D. I., Choi, J. Y., Jung, H. S., Rho, K. C. and Ahn, K. S., 2007. Recent sediment accumulation and origin of shelf mud deposits in the Yellow and East China Seas. Progress in Oceanography, 73: 145-159. DOI:10.1016/j.pocean.2007.02.004 (  0) 0) |
Liu, J., Yu, Z., Zang, J., Sun, T., Zhao, C. and Ran, X., 2015a. Distribution and budget of organic carbon in the Bohai and Yellow Seas. Advances in Earth Science, 30: 564-578 (in Chinese with English abstract). DOI:10.11867/j.issn.1001-8166.2015.05.0564 (  0) 0) |
Liu, L. Y., Wang, J. Z., Guan, Y. F. and Zeng, E. Y., 2012. Use of aliphatic hydrocarbons to infer terrestrial organic matter in coastal marine sediments off China. Marine Pollution Bulletin, 64: 1940-1946. DOI:10.1016/j.marpolbul.2012.04.023 (  0) 0) |
Liu, W., Ning, Y., An, Z., Wu, Z., Lu, H. and Cao, Y., 2005. Carbon isotopic composition of modern soil and paleosol as a response to vegetation change on the Chinese Loess Plateau. Science in China, 48(1): 93-99. DOI:10.1360/02YD0148 (  0) 0) |
Liu, W., Yang, H., Wang, H., An, Z., Wang, Z. and Leng, Q., 2015b. Carbon isotope composition of long chain leaf wax n-alkanes in lake sediments: A dual indicator of paleoenvironment in the Qinghai-Tibet Plateau. Organic Geochemistry, 83-84: 190-201. DOI:10.1016/j.orggeochem.2015.03.017 (  0) 0) |
Lu, X., Chen, Y., Huang, G., Liu, D., Tang, J., Li, J. and Zhang, G., 2011. Distribution and sources of lipid biomakers in surface sediments of the Yellow Sea and Bohai Sea. Ecology and Environmental Sciences, 20: 1117-1122. DOI:10.16258/j.cnki.1674-5906.2011.z1.013 (  0) 0) |
Mead, R., Xu, Y., Chong, J. and Jaffé, R., 2005. Sediment and soil organic matter source assessment as revealed by the molecular distribution and carbon isotopic composition of n-alkanes. Organic Geochemistry, 36: 363-370. DOI:10.1016/j.orggeochem.2004.10.003 (  0) 0) |
Meyers, P. A. and Ishiwatari, R., 1993. The early diagenesis of organic matter in lacustrine sediments, Organic Geochemistry. Springer: 185-209. DOI:10.1007/978-1-4615-2890-6-8 (  0) 0) |
Mügler, I., Sachse, D., Werner, M., Xu, B., Wu, G., Yao, T. and Gleixner, G., 2008. Effect of lake evaporation onδD values of lacustrine n-alkanes: A comparison of Nam Co (Tibetan Plateau) and Holzmaar (Germany). Organic Geochemistry, 39: 711-729. DOI:10.1016/j.orggeochem.2008.02.008 (  0) 0) |
Pancost, R. D., Baas, M., Van Geel, B., Sinninghe, Damsté and J., S., 2002. Biomarkers as proxies for plant inputs to peats: An example from a sub-boreal ombrotrophic bog. Organic Geochemistry, 33: 675-690. DOI:10.1016/S0146-6380(02)00048-7 (  0) 0) |
Qiao, S., Shi, X., Wang, G., Zhou, L., Hu, B., Hu, L., Yang, G., Liu, Y., Yao, Z. and Liu, S., 2017. Sediment accumulation and budget in the Bohai Sea, Yellow Sea and East China Sea. Marine Geology, 390: 270-281. DOI:10.1016/j.margeo.2017.06.004 (  0) 0) |
Rao, Z., Jia, G., Zhu, Z., Wu, Y. and Zhang, J., 2008. Comparative study and significance on carbon isotopes of total organic matter and long-chain n-alkanes in topsoil of eastern China. Chinese Science Bulletin, 53: 2077-2084. DOI:10.1360/csb2008-53-17-2077 (  0) 0) |
Rao, Z., Zhu, Z., Jia, G., Zhang, X. and Wang, S., 2011. Compound-specific hydrogen isotopes of long-chain n-alkanes extracted from topsoil under a grassland ecosystem in northern China. Science China Earth Sciences, 54: 1902-1911. DOI:10.1007/s11430-011-4252-8 (  0) 0) |
Schefuẞ, E., Ratmeyer, V., Stuut, J. B. W., Jansen, J. H. F., Sinninghe, Damsté and J., S., 2003. Carbon isotope analyses of n-alkanes in dust from the lower atmosphere over the central eastern Atlantic. Geochimica et Cosmochimica Acta, 67: 1757-1767. DOI:10.1016/S0016-7037(02)01414-X (  0) 0) |
Sikes, E. L., Uhle, M. E., Nodder, S. D. and Howard, M. E., 2009. Sources of organic matter in a coastal marine environment: Evidence from n-alkanes and theirδ13C distributions in the Hauraki Gulf, New Zealand. Marine Chemistry, 113: 149-163. DOI:10.1016/j.marchem.2008.12.003 (  0) 0) |
Silliman, J. E. and Schelske, C. L., 2003. Saturated hydrocarbons in the sediments of Lake Apopka, Florida. Organic Geochemistry, 34: 253-260. DOI:10.1016/S0146-6380(02)00169-9 (  0) 0) |
Sun, D., Tang, J., He, Y., Liao, W. and Sun, Y., 2018a. Sources, distributions, burial efficiency of terrigenous organic matter in surface sediments from the Yellow River Mouth, Northeast China. Organic Geochemistry, 118: 89-102. DOI:10.1016/j.orggeochem.2017.12.009 (  0) 0) |
Sun, W., Zhang, E., Liu, E., Chang, J., Chen, R. and Shen, J., 2018b. Glacial-interglacial vegetation changes in Northeast China inferred from isotopic composition of pyrogenic carbon from Lake Xingkai sediments. Organic Geochemistry, 121: 80-88. DOI:10.1016/j.orggeochem.2018.03.004 (  0) 0) |
Tao, S., Eglinton, T. I., Montluçon, D. B., McIntyre, C. and Zhao, M., 2016. Diverse origins and pre-depositional histories of organic matter in contemporary Chinese marginal sea sediments. Geochimica et Cosmochimica Acta, 191: 70-88. DOI:10.1016/j.gca.2016.07.019 (  0) 0) |
Tao, S., Eglinton, T. I., Montluçon, D. B., McIntyre, C. and Zhao, M., 2015. Pre-aged soil organic carbon as a major component of the Yellow River suspended load: Regional significance and global relevance. Earth and Planetary Science Letters, 414: 77-86. DOI:10.1016/j.epsl.2015.01.004 (  0) 0) |
Wang, S., Liu, G., Yuan, Z. and Da, C., 2018. n-Alkanes in sediments from the Yellow River Estuary, China: Occurrence, sources and historical sedimentary record. Ecotoxicology and Environmental Safety, 150: 199-206. DOI:10.1016/j.ecoenv.2017.12.016 (  0) 0) |
Wang, Y., Liu, D., Richard, P. and Li, X., 2013. A geochemical record of environmental changes in sediments from Sishili Bay, northern Yellow Sea, China: Anthropogenic influence on organic matter sources and composition over the last 100years. Marine Pollution Bulletin, 77: 227-236. DOI:10.1016/j.marpolbul.2013.10.001 (  0) 0) |
Wang, Z. and Fingas, M., 2006. Oil and petroleum product fingerprinting analysis by gas chromatographic techniques. Chromatographic Science Series, 93: 1027. (  0) 0) |
Xing, L., Zhang, H., Yuan, Z., Sun, Y. and Zhao, M., 2011. Terrestrial and marine biomarker estimates of organic matter sources and distributions in surface sediments from the East China Sea shelf. Continental Shelf Research, 31: 1106-1115. DOI:10.1016/j.csr.2011.04.003 (  0) 0) |
Yang, Z.S. and Liu, J.P., 2007. A unique Yellow River-derived distal subaqueous delta in the Yellow Sea. Marine Geology, 240: 169-176. DOI:10.1016/j.margeo.2007.02.008 (  0) 0) |
Zhang, S., Li, S., Dong, H., Zhao, Q., Lu, X. and Shi, J., 2014. An analysis of organic matter sources for surface sediments in the central South Yellow Sea, China: Evidence based on macroelements and n-alkanes. Marine Pollution Bulletin, 88: 389-397. DOI:10.1016/j.marpolbul.2014.07.064 (  0) 0) |
Zhang, Z., Zhao, M., Lu, H. and Faiia, A.M., 2003. Lower temperature as the main cause of C4 plant declines during the glacial periods on the Chinese Loess Plateau. Earth and Planetary Science Letters, 214: 467-481. DOI:10.1016/S0012-821X(03)00387-X (  0) 0) |
Zhao, B., Zhang, Y., Huang, X., Qiu, R., Zhang, Z. and Meyers, P.A., 2018. Comparison of n-alkane molecular, carbon and hydrogen isotope compositions of different types of plants in the Dajiuhu peatland, central China. Organic Geochemistry, 124: 1-11. DOI:10.1016/j.orggeochem.2018.07.008 (  0) 0) |
Zheng, S.X. and Shangguan, Z.P., 2007. Foliarδ13C values of nine dominant species in the Loess Plateau of China. Photosynthetica, 45(1): 110-119. DOI:10.1007/s11099-007-0017-1 (  0) 0) |
 2021, Vol. 20
2021, Vol. 20


