2) School of Materials Science and Engineering, Ocean University of China, Qingdao 266100, China
The detection of electric field in marine environments is an important method for marine oil and gas exploration, in which the electric field electrodes are the key equipment. There are several electric field electrode types, mainly including Cu/CuSO4, Pb/PbCl2 and Ag/AgCl. They are utilized in electrode pair and have the same working principle which depends on chemical reverse reaction (Neuman, 2000). When the applied electric field signal occurs between a couple of identical electrode, the positive and negative ions in seawater will migrate in an opposite direction with each other due to the electric field forces, which will break the reversible reaction equilibrium and then causes the electric potential difference between the identical electrode pair according to the Nernst equation. So, the electric field changes can be detected by measuring the potential variation (ΔE) between a pair of identical electrodes. The Ag/AgCl electric field electrodes are commonly used for low-frequency electric field measurements in the ocean because of its stability, precision and excellent response capability to the low frequency and extremely weak electric signal (Constable, 2013; Wang et al., 2013). The Ag/AgCl electric field electrodes, however, have some drawbacks, such as complicated manufacturing process, short service life, inconvenient transportation and high price. So it is necessary to develop new electric field electrodes to meet the relevant technical demands.
Carbon fiber has procured great attention for its special texture and structure, high specific surface area, good electrical conductivity and high chemical stability (Hwang, 2016). Our project team once used the carbon fiber as the positive and negative electrode material to construct marine sediment microbial fuel cell to harvest bioenergy for long term on ocean floor (Fu et al., 2014). Japan Panasonic Electronic Industrial Co., Ltd ever developed the super electric double layer capacitors (EDLC) using activated carbon fiber to substitute for memory storage device and small batteries, which accelerates the miniaturization of electronic products (Yoshida et al., 1987). L Crona and A Brage developed a new type of electrode based on carbon fibers and carried out sea trial for measuring the induced electrical field caused by moving sea water across a narrow channel (Crona et al., 1997). Polyacrylonitrile-based carbon fiber (noted as PAN in the paper) is a kind of common and high modulus material, whose physical and chemical properties can make great influences on composite mechanical performance, thus we can adjust the composite properties by its surface modification (Gao et al., 2016; Ma et al., 2016; Zheng et al., 2016). The common modification methods of PAN include silane coupling agent, chemical oxidation, coating, heat treatments, etc. The purpose of modifications is to improve its interfacial adhesion strength between the carbon fiber and epoxy resin in composite materials. PAN is also widely used electrode material for energy storage (Im et al., 2008; Hu et al., 2009; Lee et al., 2015).
Some researchers ever modified the electric field response performances of carbon fiber electrode through heat treatment under different temperatures and concentrated nitric acid (Shen et al., 2017; Wang et al., 2017), however, its electrode pair still has a high self-noise, low potential stability and cannot work well in low frequency (1mHz, for example). Electro-chemical oxidation treatment in acidic solution of carbon fiber will greatly improve its uniform electric activity due to its stronger oxidation ability and mild conditions, which will be possible to enhance its electric field response sensitivity (Liu et al., 2019).
Therefore, in order to verify the feasibility of this type of oxidation modified electrode used as detecting electric field in seawater, this paper utilized the PAN (before and after electrochemical oxidative modification) to design and prepare marine electric field electrodes to analyze their electrochemical and electric field properties, reaching a conclusion that the modified electrode pair have much smaller electric potential difference drifts, higher response sensitivity to low frequency and weaker electric field and lower self-noise level. This paper also proposed a new capacitor model of its working mechanism to explain these excellent performances.
2 Materials and Methods 2.1 MaterialsTORAY T700, with a diameter of 7 μm, Copper sulfate (Tianjin Yong Da Chemical Reagent Production Development Center), NaOH (Tianjin Bo Di Chemical Co., Ltd.), H3PO4, AgNO3, HCl, SnCl2·2H2O, NH3·H2O, HCHO, KNaC4H4O6·4H2O, H2SO4 (Sinopharm) are all analytic reagent (AR) and used directly.
2.2 Electrochemical Oxidative TreatmentElectrochemical oxidation was carried out in a conventional two-electrode cell using PAN as anode, graphite as cathode, 0.5 mol L-1 H3PO4 as electrolyte and with a constant current density of 5 A/g for 5min. After modification, the PAN was washed thoroughly with distilled water and then dried at 80℃.
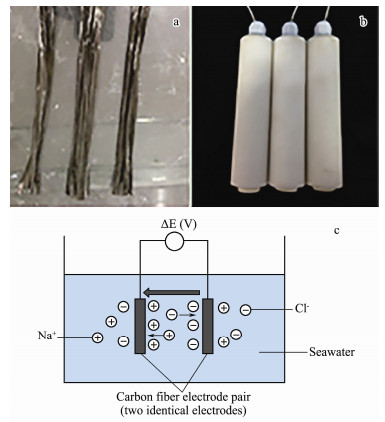
2.3 Electrode DesignThe electrode consisted of carbon fibers, each with a length of 15 cm. One end of the fiber tufts was fixed and then impregnated with epoxy. After epoxy is polished, copper plating procedure was conducted and copper lead welded. The contact area was sealed by epoxy to protect it from seawater corrosion. Fig. 1 shows the carbon fibers (a) and the prepared electrodes (b) as well as the schematic sketch of the electric field testing by the electrode pair (c).
|
Fig. 1 Carbon electric field electrodes: (a) Carbon fiber bundle, (b) prepared electric field electrodes and (c) the schematic sketch of the electric field testing. |
Their surface appearances (before and after modification) were characterized by Hitachi S-4800 scanning electron microscope (SEM). The nitrogen isotherms of the materials were measured at 77 K using ASAP2020 Accelerated Surface Area and Porosimetry System and surface areas of the samples were evaluated with the application of the Brunner-Emmet-Teller (BET). The surface function group of the samples was determined by Fourier Transform Infrared Spectroscopy (FTIR) by using an AVATAR 360 FTIR and their contact angles were measured by the Dynamic Contact Angle Analyzer (JC2000C1).
Electro-chemical tests were performed using a threeelectrode configuration on an electro-chemical workstation (LK 2005B) in seawater electrolyte. Carbon fiber electrode was used as working electrode; a Pt electrode and Ag/AgCl electrode were used as the counter and reference electrode respectively. All the electro-chemical measurements were then performed at ambient temperature. The potential range for CV measurements was -0.6 V to +0.6 V and its scanning rate was 5 mV s-1. The Tafel curves were obtained from -0.5 V to +0.5 V at a rate of 5 mV s-1.
2.5 Electric Field Properties MeasurementsTo get the potential difference drifts, the identical electrode pair was put into seawater in parallel and connected with Data Recorder (Aligent 34972A). Data recording frequency was 0.1 Hz and total recording time was about 55 h. For the steady data accuracy, we started data collection after soaking 1 hr. For electric field response testing, an Agilent 33500B signal generator was applied as a source (sine wave) at a frequency of 1 mHz and amplitude of 10 mVpp. Two vertical parallel carbon plates (30 cm× 20 cm×1 cm, spacing 35.5 cm) connected to the signal generator as emission electrodes. Vertical parallel testing electrodes (spacing 16 cm) connected to the Aligent 34972 data recorder were on a straight line with the emission electrodes. In order to obtain a further study of the response to a weaker electric field, we adjusted the amplitude to 5 mVpp and then measured their response.
For linearity testing, the applied electric field frequency was kept at 10 mHz and amplitudes at 1, 5, 10, 20, 30, 40, 50, 60 and 70 mVpp respectively. Circuit current and calculating signal peak values of each group were recorded according to the measurement data. The linearity was calculated according to the fitting line.
Their self-noise measurements were as follows: putting the identical electrode pair in parallel into a plastic container containing NaCl solution (mass fraction 3.5%) and connecting them to low Noise Amplifier and Data Recorder. The whole noise testing system was in an electromagnetic shielding room. Collector started to measure electrodes, open circuit voltage after 24 h at the frequency of 50 Hz and voltage range of -10 mV to +10 mV. We analyzed the signal power spectrum and obtained the total noise data and the inherent noise data of measurement system respectively. Finally, the electrode pair self-noise data was calculated according to the Root Mean Square relationships:
| $ {N_{({\rm{Total}}\;{\rm{noise}})}}^2 = {N_{({\rm{Equipment}}\;{\rm{noise}})}}^2 + {N_{({\rm{Electrode}}\;{\rm{pair}}\;{\rm{noise}})}}^2. $ |
In our experiment design, more electrodes have been prepared and three parallel measurements have been performed for each experimental condition to estimate its repeatability.
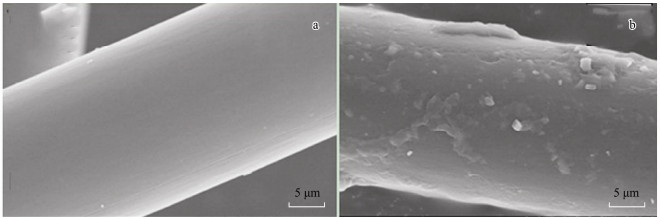
3 Results and Discussion 3.1 Carbon Fiber Surface CharacteristicsAs shown in Fig. 2, the surface of the plain carbon fiber (Fig. 2a) is very smooth, however, after modified (Fig. 2b), its surface become rough, appearing clearly etched, which will increase its surface area. Thus it is more advantageous for the carbon fiber to contact with the seawater in larger area.
|
Fig. 2 Surface characteristics of the two carbon fibers: (a) before modification and (b) after modification. |
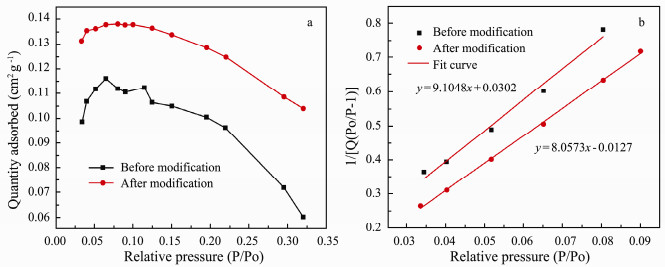
The N2 adsorption isotherms (Relative Pressure: P/P0= 0.05 to 0.35) of the carbon fibers (before and after modification) was shown in Fig. 3a. Applying the BET model (Fig. 3b) to these isotherms, specific surface areas were determined for the samples are 0.4766 (before modification) and 0.5412 m2g-1 (after modification). The increase in specific surface area after modification is due to the rough surface caused by electro-chemical etching. This is consistent with the SEM results (Fig. 2b).
|
Fig. 3 Specific surface areas: (a) N2 adsorption isotherms and (b) BET model. |
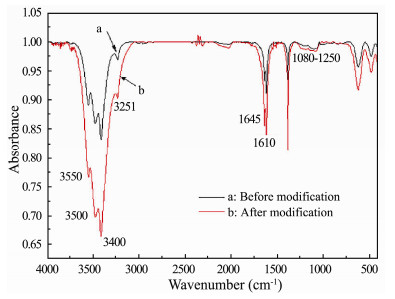
The FTIR spectrum of the PAN carbon fibers before and after modification is shown in Fig. 4. The peak shape of the FTIR spectrum of the samples is similar, but the intensity of the characteristic absorption peak was markedly increased after modification (Fig. 4b). The 3476 cm-1 and 3413 cm-1 band in the FTIR can be linked to the presence of hydroxyl groups. The presence of bands at 3530 cm-1 and 3251 cm-1 can be attributed to the stretching vibrations of O-H moieties in carboxylic group. The 1645 cm-1 and 1610 cm-1 bands are the stretching vibrations of C=O moieties in quinone and carboxylate respectively. The absorption bands at 1250 cm-1-1080 cm-1 are ascribed to -C-O stretching and -OH bending modes in carboxyl and phenolic structures. These characteristic peaks confirm that there exist a greater amount of carboxyl group and hydroxyl groups after electro-chemical modification.
|
Fig. 4 FTIR spectrum of the carbon fibers before and after modification. |
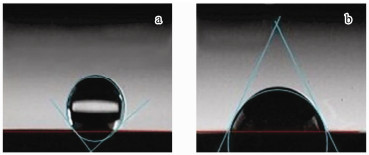
The contact angle change is presented in Fig. 5. Before modification (Fig. 5a), its contact angle is 142°, then it changes to 52° after modification (Fig. 5b), which indicates its improved wettability. The reason is that electrochemical oxidation can make fiber form oxygen-containing functional groups (carboxyl, hydroxyl and carbonyl group etc.), which are hydrophilic groups to decrease its contact angle (Kim et al., 2013).
|
Fig. 5 The contact angle of the two carbon fibers: (a) before modification and (b) after modification. |
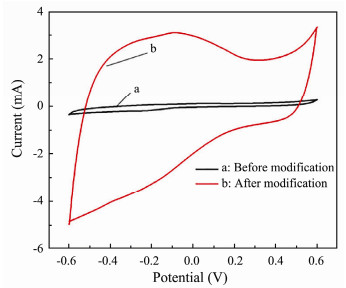
CV curves (Fig. 6) indicate that its surrounded area of the modified electrode is obviously enlarged, which is 24 times bigger than the plain one, indicating the enhancement of its integrated capacitive property (both electric double layer capacitance and pseudo-capacitance). The increased oxygen-containing functional groups and surface area (after modification) can promote the adsorption ions and increase its electric double layer capacitance. Carboxyl functional groups has redox capacitance activity which can increase the apparent capacitance. The hydroxy groups with weak polarity will greatly promote the formation of electric double layer capacitance as shown in Eq. (1), among them, the symbol '∥' stands for the electric double layer (Oda et al., 2006).
|
Fig. 6 Cyclic voltammetry curves of PAN electrodes before and after modification (potential V vs. Ag/AgCl). |
| $ {\rm{C}} - {\rm{O}}{{\rm{H}}^{{\rm{ \mathsf{ δ} }} - }} + {\rm{N}}{{\rm{a}}^ + }\left({{{\rm{H}}^ + }} \right) \leftrightarrow {\rm{C}} - {\rm{O}}{{\rm{H}}^{{\rm{ \mathsf{ δ} }} - }}//{\rm{N}}{{\rm{a}}^ + }\left({{{\rm{H}}^ + }} \right). $ | (1) |
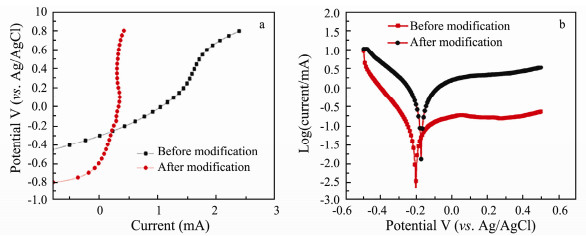
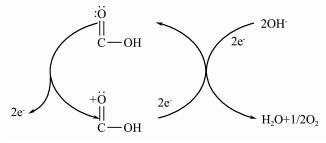
It can be seen in Fig. 7a that the potential value changes much faster for the unmodified electrode with the current shift, which indicates it has weak dynamic anti-polarization. According to the Tafel's equation: η = a + blgi and Tafel extrapolation methods, their exchange current densities (i0) are 3.45 mA g-1 (before modification) and 29.4 mA g-1 (after modification) respectively according to their Tafel curves in Fig. 7b, the latter is eight times larger than the former. This result can also prove the modified electrode has better dynamic anti-polarization. This may be attributed to the active groups (carboxyl) on the carbon fiber surface that would cause redox reaction, leading to the larger exchange density. Furthermore, carboxyl group has catalytic performance (Nian et al., 2003), which decreases the reaction activation energy, thus accelerates electron transfer efficiency (i0). Possible reaction equations are shown in Fig. 8.
|
Fig. 7 Anti-polarization analysis diagrams of before and after modified carbon fiber electrodes: (a) Linear sweep voltammetry and (b) Tafel curves (vs. Ag/AgCl). |
|
Fig. 8 Catalysis of the carboxyl group. |
If the electrodes are easily polarized, the potential difference between the two identical electrodes will drift greater, which would decrease its response sensitivity to applied electric field and augment itself electro-chemical noise.
3.3 Electric Potential Difference and Electric FieldResponse Properties of the Electrode Pair 3.3.1 Electric potential difference stability
The electric potential difference stability between a pair of identical electrodes is one of the important properties, which will influence that whether they can be used as electric field sensor or not. If the potential difference drifts greatly, it will not accurately reflect the external electric field signal. As shown in Fig. 9, their potential differences have a larger variation in the first 24 h, which are -0.31 mV to 0.75 mV (before modification) and 0.70 mV to 0.87 mV (after modification) respectively, then it gets relatively steady in the second 24 h, which are 0.34 mV (before modification) and 0.06 mV (after modification) respectively.
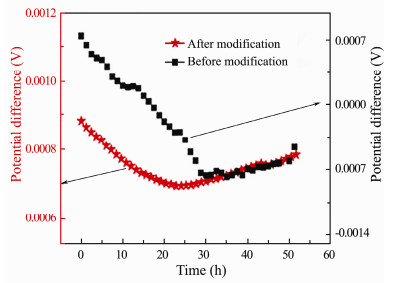
|
Fig. 9 Potential variations of the electrode pair before and after modification over time. |
There are lots of oxygen-containing functional groups for the modified carbon fiber, which can enhance wettability and promote ions adsorption, thus leading to the increase in the stability of the electric double layer and the stronger dynamic anti-polarization, so that their potential difference is getting much more steady.
3.3.2 Electric field response propertyAs is shown in Fig. 10a, both of the electrode pair (before and after modification) can respond to the electric field signal at 1 mHz and 10 mVpp, however, when the amplitude changes to 5 mVpp, only the modified electrodes can respond to the external signal (Fig. 10b). The results indicate that the modified electrode pair has better response ability to the applied electric field than the plain one pair.
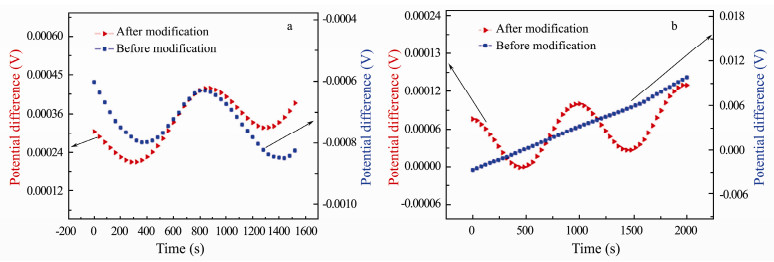
|
Fig. 10 Responses of carbon fiber electrode pair before and after modification to the applied electric field: (a) 0.001 Hz, 10 mV and (b) 0.001 Hz, 5 mV. |
As shown in Table 1, nine groups of current peaks with different amplitudes and the corresponding potential differences of each electrode pair (before and after modification) are used to draw fitting line to analyze its linearity.
|
|
Table 1 Summary of peak potentials of the carbon fiber electrode pair response to applied electric field |
According to the above data, its head-end fitting line can be obtained, as shown in Fig. 11. Their linearities are 1.47% (before modification) and 1.34% (after modification) respectively. The lower the linearity is, the higher the response accuracy is. The result is consistent with its potential difference stability and anti-polarization after its modification.
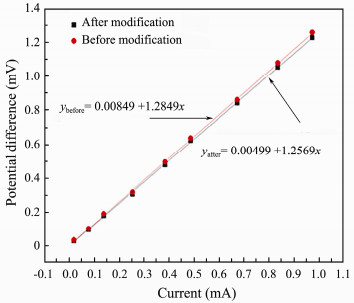
|
Fig. 11 Linearity testing result of carbon fiber electrode pair before and after modification. |
The electrode pair noise level is a key factor for detection electric field signal. If their noise overtops the equipment's inherent noise level, they will interfere the equipment's precision, and then the result will be inaccurate. The testing result of their noises is shown in Fig. 12.
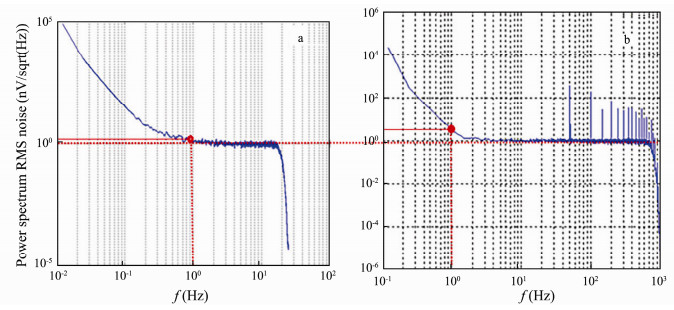
|
Fig. 12 Total noise testing result of carbon fiber electrode pair and equipment: (a) after modification and (b) before modification. |
The noise level is characterized by the power spectrum RMS value (Mills et al., 2014) at 1 Hz frequency point. The system short circuit noise (equipment noise) value is 0.89 and their total noise values are 2.97
(before modification) and 1.39
(after modification). According to the square root relationship between the total noise value and the system short circuit noise value, each electrode pair self-noise value is 2.83
Results indicate that the plain electrode pair has higher noise level at 1 Hz than the modified one. It may be attributed to the following aspects: for one thing, the higher electric double layer capacitance will make itself capacitance change less bit under the external electric field; for another, the increased exchange current density after modification enhances the reversible reaction balance, which would be beneficial to lower its self-noise.
3.4 Mechanism Analysis of Carbon Fiber Electrode Pair Response to Applied Electric FieldThe response mechanism of carbon fiber to applied electric field is mainly attributed to the electric double layer capacitance effect. When the electrode is immersed into the seawater, the electric double layer with different polarity will form at its interface due to the electrostatic force between electrode and seawater. There are less charges in the electric double layer when there is no external electric field. The identical electrode pair in parallel are used for detecting electric field (Fig. 1c), and they should ideally have the identical potential which displays no potential difference. But once the external electric field occurs, the positive and negative ions in solution will move in opposite direction and their ions rearrangement occurs at the each electrode interface respectively. Novel electric double layers eventually form with opposite polarity, which leads to the potential change between the electrodes. Therefore, it is the electric double layer capacitance variation that causes the potential difference (ΔE) and reflects external electric field.
The sketch map of its mechanism analysis is shown in Fig. 13. The more ions the electrode absorb, the larger the electric double layer capacitance is. Thus when there is applied electric field, the quantity of the ions at the interface changes more (Fig. 13a and 13a') then the potential difference value(ΔE) between the electrodes gets larger, indicating a better response ability to external electric field. The modified electrode pair can respond better, which has a direct relationship with its higher surface area and more oxygen-containing functional groups (especially the hydroxyl groups Figs. 13b and 13b').
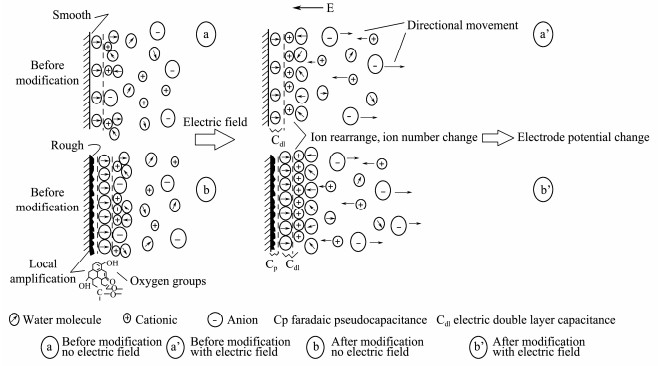
|
Fig. 13 Mechanism model of carbon fiber electrodes surface electric double layer and its response to applied electric field. |
A new PAN carbon fiber electric field electrode pair by electro-chemical oxidation has been fabricated and its electro-chemical properties can be greatly improved. Its surface appearance is rougher and wettability improved. Meanwhile, its capacitance and dynamic anti-polarization property have a greater enhancement. The modified electrode pair has a smaller potential difference drift (60 μV-170 μV d−1), more sensitive electric field response (1 mHz, 5 mV), lower linearity (1.34%) and much lower self-noise level (1.07
This study was supported by the National Defense Science and Technology Innovation Zone Project (No. 18-H863-05-ZT-001-018-09).
Constable, S., 2013. Review paper: Instrumentation for marine magnetotelluric and controlled source electromagnetic sounding. Geophysical Prospecting, 61(s1): 505-532. DOI:10.1111/j.1365-2478.2012.01117.x (  0) 0) |
Crona, L. and Brage, A., 1997. Carbon fiber electrodes and their qualities in salt water. Proc. MARELEC 97. International Conference Marine Electromagn: 1-5. (  0) 0) |
Fu, Y. B., Liu, Y. Y., Xu, Q., Lu, Z. K. and Zhang, Y. L., 2014. Comparative study of two carbon fiber cathodes and theoretical analysis in microbial fuel cells on ocean floor. Journal of Ocean University of China, 13(2): 257-261. DOI:10.1007/s11802-014-2162-z (  0) 0) |
Gao, B., Du, W. T., Zhang, R. L., Lu, F. and Zhang, J., 2016. Propagation of generation 1-3 dendritic poly(amidoamine) on carbon fiber surface and its effect on the mechanical properties of fiber composites. Materials Letters, 179: 16-19. DOI:10.1016/j.matlet.2016.05.037 (  0) 0) |
Hu, J. L., Huang, J. H., Chih, Y. K., Chuang, C. C., Chen, J. P., Cheng, S. H. and Horng, J. L., 2009. Effects of thermal treatments on the supercapacitive performances of PAN-based carbon fiber electrodes. Diamond and Related Materials, 18(2): 511-515. DOI:10.1016/j.diamond.2008.10.025 (  0) 0) |
Hwang, S. S., 2016. Tensile, electrical conductivity and EMI shielding properties of solid and foamed PBT/carbon fiber composites. Composites Part B: Engineering, 98: 1-8. DOI:10.1016/j.compositesb.2016.05.028 (  0) 0) |
Im, J. S., Woo, S. W., Jung, M. J. and Lee, Y. S., 2008. Improved capacitance characteristics of electrospun ACFs by pore size control and vanadium catalyst. Journal of Colloid and Interface Science, 327(1): 115-119. DOI:10.1016/j.jcis.2008.08.030 (  0) 0) |
Kim, H., Lee, Y. J., Lee, D. C., Park, G. G. and Yoo, Y., 2013. Fabrication of the carbon paper by wet-laying of ozonetreated carbon fibers with hydrophilic functional groups. Carbon, 60: 429-436. DOI:10.1016/j.carbon.2013.04.057 (  0) 0) |
Lee, H. M., Kim, H. G., Kang, S. J., Park, S. J., An, K. H. and Kim, B. J., 2015. Effects of pore structures on electrochemical behaviors of polyacrylonitrile (PAN)-based activated carbon nanofibers. Journal of Industrial and Engineering Chemistry, 21: 736-740. DOI:10.1016/j.jiec.2014.04.004 (  0) 0) |
Liu, A., Fu, Y. B., Zai, J. Z., Duan, Z. W. and Zai, X. R., 2019. Electrochemical and electric field response properties of highly sensitive electrodes based on carbon fiber with oxygen and nitrogen surface groups. IEEE Sensors Journal, 19(11): 3966-3974. DOI:10.1109/JSEN.2019.2891905 (  0) 0) |
Ma, Q. S., Gu, Y. Z., Li, M., Wang, S. K. and Zhang, Z. G., 2016. Effects of surface treating methods of high-strength carbon fibers on interfacial properties of epoxy resin matrix composite. Applied Surface Science, 379: 199-205. DOI:10.1016/j.apsusc.2016.04.075 (  0) 0) |
Mills, D., Picton, P. and Mularczyk, L., 2014. Developments in the electrochemical noise method (ENM) to make it more practical for assessment of anti-corrosive coatings. Electrochimica Acta, 124: 199-205. DOI:10.1016/j.electacta.2013.09.067 (  0) 0) |
Neuman, M. R., 2000. Biopotential Electrodes, The Biomedical Engineering Handbook. CRC Press LLC, 189-240.
(  0) 0) |
Nian, Y. R. and Teng, H., 2003. Influence of surface oxides on the impedance behavior of carbon-based electrochemical capacitors. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 540: 119-128. DOI:10.1016/S0022-0728(02)01299-8 (  0) 0) |
Oda, H., Yamashita, A., Minoura, S., Okamoto, M. and Morimoto, T., 2006. Modification of the oxygen-containing functional group on activated carbon fiber in electrodes of an electric double-layer capacitor. Journal of Power Sources, 158(2): 1510-1516. DOI:10.1016/j.jpowsour.2005.10.061 (  0) 0) |
Shen, Z., Song, Y. S. and Zhang, L., 2017. The impact of heat treatment on the performance of carbon fiber electrode. Journal of Functional Materials, 48(3): 3214-3217 (in Chinese with English abstract). DOI:10.3969/j.issn.1001-9731.2017.03.040 (  0) 0) |
Wang, Y. M., Song, Y. S. and Shen, Z., 2017. The underwater electric field response characteristics of carbon fiber electrodes oxidated by concentrated nitric acid. Journal of Functional Materials, 48(12): 12204-12208 (in Chinese with English abstract). DOI:10.3969/j.issn.1001-9731.2017.12.037 (  0) 0) |
Wang, Z., Deng, M., Chen, K. and Wang, M., 2013. An ultralow-noise Ag/AgCl electric field sensor with good stability for marine EM applications. International Conference on Sensing Technology (ICST): 747-750. DOI:10.1109/ICSensT.2013.6727752 (  0) 0) |
Yoshida, A., Tanahashi, I., Takeuchi, Y. and Nishino, A., 1987. An electric double-layer capacitor with activated carbon fiber electrodes. IEEE Transactions on Components, Hybrids, and Manufacturing Technology, 10: 100-102. DOI:10.1109/TCHMT.1987.1134717 (  0) 0) |
Zheng, N., He, J. M., Zhao, D., Huang, Y. D., Gao, J. F. and Mai, Y. W., 2016. Improvement of atomic oxygen erosion resistance of carbon fiber and carbon fiber/epoxy composite interface with a silane coupling agent. Materials Design, 109: 171-178. DOI:10.1016/j.matdes.2016.07.004 (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


