2) Laboratory for Marine Drugs and Bioproducts, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3) Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao 266003, China
Gorgonian corals are recognized as the main source of natural products like steroids, acetogenins, prostanoids, diterpenoids, and sesquiterpenoids (Chung et al., 2018; Su et al., 2020; Tin et al., 2020; Xu et al., 2020). Until now, many guaiane sesquiterpenoids have been obtained from gorgonians and other marine sources. Gorgonian corals contain the majority (60%) of marine-derived guaiane sesquiterpenoids (Diep et al., 2015; Lyakhova et al., 2016), while the remaining guaiane analogs have been mainly obtained from soft corals (Liang and Guo, 2013) and sponges (Hlrota et al., 1998).
To search for novel bioactive metabolites from marine fauna and flora, a batch of gorgonian Echinomuricea indomalaccensis was collected from the South China Sea. In our previous study, we reported two new steroids isolated from this gorgonian (Cao et al., 2012). To discover more bioactive metabolites, our group recently performed further chemical studies on this species. From the organic extract of the title animal, five compounds were isolated, including one highly oxygenated guaiane-type sesquiterpene lactone, namely, 5-epi-menverin C (1), together with a sesquiterpene (2) and three steroids (3 – 5).
2 Materials and Methods 2.1 General MethodsOptical rotations were performed using a JASCO P-1020 digital polarimeter. ECD spectra were obtained with a Jasco J-815-150S circular dichroism spectrometer. IR spectra were obtained using a Nicolet-Nexus-470 spectrometer. UV spectra were obtained using a Beckman DU 640 spectrophotometer. NMR spectra were obtained using a JEOL Eclips-600 spectrometer at 600 MHz for 1H and 150 MHz for 13C, with TMS as an internal standard. ESIMS was performed using a Micromass Q-TOF spectrometer. HRESIMS spectra were obtained using a Thermo Scientific LTQ Orbitrap XL spectrometer. Semi-preparative HPLC was performed on a Waters 1525 system using a semi-preparative C18 (Kromasil, 5 μm, 10 mm × 250 mm) column coupled with a Waters 2996 photodiode array detector. CC silica gel (200 – 300 mesh) was obtained from the Qingdao Ocean Chemical Group Co. Analytical TLC was performed using precoated silica gel plates (G60, F-254) from Yantai Zifu Chemical Group Co.
2.2 Computational SectionThe OPLS_2005 force field was employed for conformational searches using the torsional sampling (MCMM) method. At the B3LYP/6-311G(d, p) level, conformers above 1% population were re-optimized with a PCM solvent model for acetonitrile. ECD spectra were obtained by TDDFT calculations, performed with the same basis set, solvent model, and function as the energy optimization. The Boltzmann-averaged ECD spectrum of (1S, 5S, 10S)-1 was then obtained with SpecDis1.62.
2.3 Animal MaterialThe gorgonian E. indomalaccensis (GXWZ-25) was collected from the Xisha Islands in the South China Sea in September 2018. The gorgonian E. indomalaccensis was identified by Prof. H. Huang from the South China Sea Institute of Oceanology, Chinese Academy of Sciences. The gorgonian coral was deposited in the Key Laboratory of Marine Drugs, the Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao, P. R. China.
2.4 Extraction and IsolationThe frozen specimens (457 g, dried weight) were crushed mechanically and extracted by EtOAc (5 L × 2.0 L) until completion. The EtOAc extract (48.3 g) was isolated by CC on silica gel and eluted using petroleum ether (PE)EtOAc (10%, 20%, 30%, 50%, and 100%) to obtain five fractions (Fr.1–Fr.5). Through CC over silica gel, Fr.3 was isolated with PE-EtOAc (2:1) to give 1 (1.5 mg), and Fr.4 was isolated under PE-EtOAc (1:1) to provide 2 (3.2 mg). Fraction 5 was eluted using PE-EtOAc (1:2) to obtain two sub-fractions SFr.5-1 and SFr.5-2. SFr.5-1 was subjected to HPLC (MeOH: H2O, 4:1; 2.0 mL min−1) to give 3 (13.5 mg, tR = 13.6 min), and SFr.5-2 was also purified on HPLC (MeOH: H2O, 3:1; 2.0 mL min−1) to give 4 (21.9 mg, tR = 10 min) and 5 (24.7 mg, tR = 22.3 min).
5-epi-menverin C (1): colorless oil; [α]D25 = −34.6 (c 0.15, MeOH); UV (MeOH): λmax (log ε) 276 (2.09) nm; IR (KBr) vmax: 3749, 2361, 1747, 1651 cm−1; 1H and 13C NMR, Table 1; HRESIMS: 263.1281 [M+H]+ (calculated for C15H19O4, 263.1278).
|
|
Table 1 1D NMR data of 5-epi-menverin C and menverin C in CDCl3 |
The anti-HSV-1 activity of compounds (1–5) was examined using the MTT test (Grela et al., 2018) to inhibit virus-induced CPE on Vero cells in vitro. Acyclovir was used as the positive control, with an HSV-1 virus inhibition rate found to be equal to 94.7% (c = 25 μmol L−1).
3 Results and DiscussionThe frozen specimens of gorgonian E. indomalaccensis were crushed mechanically and extracted by ethyl acetate (EtOAc) until exhaustion. The EtOAc extract was repeatedly isolated by column chromatography (CC) and high performance liquid chromatography (HPLC) to afford one new guaiane-type sesquiterpene lactone 5-epi-menverin C (1), together with four reported compounds (2 – 5) (Fig.1). The four known compounds (2 – 5) were identified as menverin B (2) (Zhang et al., 2004), cerevisterol (3) (Qin et al., 2009), cholesterol (4) (Gao et al., 2011), and 1β, 3β, 5α, 6β-tetrahydroxycholestane (5) (Parameswaran et al., 2002) through comparison with related spectroscopic information.

|
Fig. 1 Structures of compounds 1 − 5 and menverin C. |
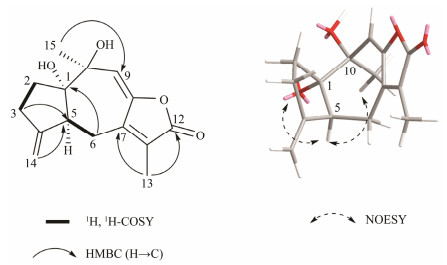
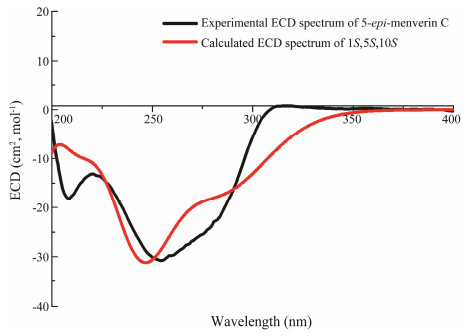
5-epi-menverin C (1) was determined as C15H18O4 through high resolution electrospray ionization mass spectroscopy (HRESIMS), implying seven degrees of unsaturation. A guaiane-type sesquiterpene lactone framework involving two OH at C-1 and C-10, and an exocyclic olefin moiety group between C-4 and C-14 through the analysis of its NMR spectroscopy (Table 1, Fig.2) was identified. The above-mentioned structural characteristics of compound 1 were strongly similar to those of menverin C (Zhang et al., 2004). In fact, compound 1 differed from menverin C only in the configuration of its stereogenic center C-5. Two hydroxyl groups at C-1 (OH-1 δH 4.74) and C-10 (OH-10 δH 5.05) were revealed by 1H-NMR spectroscopy recorded in DMSO-d6. The presence of the NOESY cross-peaks of OH-1/H-5 and Me-15/H-5 indicated that H-5, OH-1, and Me-15 were α-oriented, while OH-10 was β-oriented (Fig.2). The absolute stereochemistry of 5-epi-menverin C (1) was confirmed through the TDDFT-ECD calculation (Nugroho and Morita, 2014). The ECD spectrum of (1S, 5S, 10S)-1 was found to be identical to its experimental one, implying that the absolute stereochemistry of 5-epi-menverin C (1) should be 1S, 5S, 10S (Fig.3). Therefore, compound 1 was the 5-epimer of menverin C.
|
Fig. 2 2D NMR correlations for 5-epi-menverin C. |
|
Fig. 3 ECD spectra of 5-epi-menverin C. |
The anti-HSV-1 activity of compounds (1 – 5) was examined using the MTT test to inhibit virus-induced CPE on Vero cells in vitro. 5-epi-menverin C (1) displayed antiviral activity against the HSV-1 virus with an inhibition rate of 69.2% (c = 25 μmol L−1).
4 ConclusionsIn the course of our research on novel bioactive natural products from the South China Sea, one new guaiane sesquiterpenoid 5-epi-menverin C (1) and four reported compounds (2 – 5) were obtained from the gorgonian E. indomalaccensis. The absolute configurations of 5-epi-menverin C (1) were successfully confirmed by the TDDFT-ECD calculation. The discovery of antiviral guaiane sesquiterpenoid has added to the complex, diverse, and rapidly expanding range of marine terpenoids that exhibit antiviral properties.
AcknowledgementsOur project was supported by the National Key Research and Development Program of China (No. 2018YF C0310900), the National Natural Science Foundation of China (No. 41830535), the Fundamental Research Funds for the Central Universities of China (No. 201962002), and the Taishan Scholars Program, China.
Cao, F., Wang, C. Y., and Shao, C. L., 2012. Studies on chemical constituents of gorgonian Echinomuricea indomalaccensis from the South China Sea. The 2012 Chinese Pharmaceutical Congress and the Twelfth Chinese Journal of Pharmacology. Nanjing, China, 1460-1466 (in Chinese).
(  0) 0) |
Chung, H. M., Chen, N. F., Tseng, C. C., Wu, Y. C., Wen, Z. H., Wang, Y. C., et al., 2018. Natural product chemistry of gorgonian corals of genus Junceella-Part III. Marine Drugs, 16: 339. DOI:10.3390/md16090339 (  0) 0) |
Diep, C. N., Berdyshev, D. V., Minh, C. V., Tu, V. A., Cuong, N. X., Kalinovsky, A. I., et al., 2015. Structures and absolute stereochemistry of guaiane sesquiterpenoids from the gorgonian Menella woodin. Tetrahedron Letters, 56: 7001-7004. DOI:10.1016/j.tetlet.2015.10.102 (  0) 0) |
Gao, C. H., Wang, P., Luo, X. M., Qi, S. H., and Zhang, S., 2011. Sterols from the South China Sea gorgonian Echinogorgia pseudossapo. Natural Product Research and Development, 23: 1006-1010 (in Chinese with English abstract). DOI:10.16333/j.1001-6880.2011.06.002 (  0) 0) |
Grela, E., Grabowiecka, A., and Kozłowskab, J., 2018. Current methodology of MTT assay in bacteria. Acta Histochemica, 120: 303-311. DOI:10.1016/j.acthis.2018.03.007 (  0) 0) |
Hlrota, H., Fusetani, N., Yoshimura, E., and Okino, T., 1998. Five new antifouling sesquiterpenes from two marine sponges of the genus Axinyssa and the nudibranch Phyllidia pustulosa. Tetrahedron, 54: 13971-13980. DOI:10.1016/S0040-4020(98)00867-9 (  0) 0) |
Liang, L. F., and Guo, Y. W., 2013. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chemistry & Biodiversity, 10(12): 2161-2196. (  0) 0) |
Lyakhova, E. G., Kolesnikova, S. A., Diep, C. N., Stonika, V. A., Berdyshev, D. N., Dmitrenok, P. S., et al., 2016. Guaiane sesquiterpenoids from the gorgonian Menella woodin. Natural Product Communications, 11(7): 913-916. (  0) 0) |
Nugroho, A. E., and Morita, H., 2014. Circular dichroism calculation for natural products. Journal of Natural Medicines, 68(1): 1-10. DOI:10.1007/s11418-013-0768-x (  0) 0) |
Parameswaran, P. S., Hegde, V. R., Govenkar, M., and Naik, C. G., 2002. Secondary metabolites from the gorgonian Echinomuracea esplendens (Thomson & Simon). Indian Journal of Chemietry, 41B(5): 1093-1096. (  0) 0) |
Qin, J. C., Laatsch, H., Ma, Y. T., Bai, M. S., Yang, S. X., Zhang, Y. M., et al., 2009. Polyhydroxylated steroids from an endophytic fungus, Chaetomium globosum ZY-22 isolated from Ginkgo biloba. Steroids, 74: 786-790. DOI:10.1016/j.steroids.2009.04.011 (  0) 0) |
Su, T. P., Sung, P. J., Chen, J. J., Lee, G. H., Wang, Y. C., Hwang, T. L., et al., 2020. 11β, 20β-Epoxybriaranes from the gorgonian coral Junceella fragilis (Ellisellidae). Marine Drugs, 18: 183. DOI:10.3390/md18040183 (  0) 0) |
Tin, N. N., Van, M. C., Hoai, N. N., Xuan, C. N., Van, T. N., Cong, T. D., et al., 2020. Briarane-type diterpenoids from the vietnamese gorgonian Junceella fragilis. Natural Product Research, 34(3): 385-389. DOI:10.1080/14786419.2018.1534850 (  0) 0) |
Xu, C. C., Zhang, W., Tang, H., Su, L., and Li, J., 2020. Osteoclastogenesis modulatory steroids from the South China Sea gorgonian coral Iciligorgia sp. Chemistry & Biodiversity, 17: e2000266. DOI:10.1002/cbdv.202000266 (  0) 0) |
Zhang, W., Guo, Y. W., Mollob, E., and Ciminob, G., 2004. Menverins A-D, new highly oxygenated guaiane lactones from Hainan gorgonian Menella verrucosa (BRUNDIN). Helvetica Chimica Acta, 87: 2919-2925. DOI:10.1002/hlca.200490263 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


