2) Research Center of Analysis and Test, Jinan University, Guangzhou 510632, China;
3) Key Laboratory of Water/Soil Toxic Pollutants Control and Bioremediation of Guangdong Higher Education Institutes, Department of Environmental Engineering, Jinan University, Guangzhou 510632, China
Recently, nanoparticles have received much attention due to their toxic effects to human and other organism. TiO2 nanoparticle (nano-TiO2) is widely used in various areas of the economy, such as textiles, cosmetics, pharmaceuticals, electronics and environmental remediation (Patra et al., 2008; Chong et al., 2010). Annual global commercial production of nano-TiO2 was more than 10000 metric tons per year, and approximately 2.5 million metric tons by 2025 (Robichaud et al., 2009).
Considering the widespread use of nano-TiO2, which may lead to a large amount of nano-TiO2 released into the environment. Tian et al. (2014) reported that the annual input concentration of nano-TiO2 in the sediment can reach 1.9 mg L-1 in the U.S., Switzerland and Europe. Nano-TiO2 concentration in the environmental media, including sediment, surface water, natural and urbansoils, was estimated to be in the range of mg kg-1 to low mg kg-1, which is much higher than other nanoparticles (Gottschalk et al., 2009; Tian et al., 2014). Therefore, it is necessary to determine the potential toxicity of nano- TiO2. The ecotoxicity of nano-TiO2 to algae has been described elsewhere (Menard et al., 2009). Some studies have indicated that nano-TiO2 decreased the photosynthetic abilities, induced DNA damage and altered enzymatic activity in algal (Aruoja et al., 2009; Li et al., 2015). Hund-Rinke and Simon (2006) also reported that nano- TiO2 may inhibit the growth of D. subspicatus with the EC50 value of 32 mg L-1 (72 h).
Gracilaria lemaneiformis (Gracilariales, Rhodophyta), a kind of macroalgae, played a pivotal role in ecosystems. It was discovered that G. lemaneiformis was a potential algicide for the harmful algae (Yang et al., 2014). Simultaneously, G. lemaneiformis plays the role of bioremediation in eutrophic waters to improve water quality by absorbing nutrients (Marinho-Soriano et al., 2009). Macroalgae contain a great deal of biologically active compounds that have been regarded economically important. So far, more than 2400 marine natural products have been isolated from tropical and subtropical seaweeds (Manilal et al., 2009). Some of the important potential antitumor biologically active compounds have been extracted from macroalgae, and they are expected to become new antitumor drugs for cancer treatment (Fan et al., 2012). Various studies proved that ethyl acetate, ethanol, methanol or aqueous extracts from macroalgae has a cell proliferation inhibitory effect in cancer (Zandi et al., 2010; Sundaram et al., 2012). However, environmental changes, such as temperature, salinity, solar radiation and harmful substances, will affect the physiological and biochemical reactions of algae (Kursar et al., 1983; Schmidt et al., 2010; Zandi et al., 2010; Liu et al., 2016). The influences of environmental pollutants on the anticancer activity of macroalgae have rarely been reported.
Recently, some studies concerning G. lemaneiformis have being focused on the effects of changes in environmental conditions and phytotoxic contaminants, such as environmental hormone, metal, and ultraviolet radiation (Schmidt et al., 2010; Huang et al., 2013; Wang and Yue, 2015). Studies have shown that heavy metals can accumulate in G. lemaneiformis and increase oxidative damage in seaweed (Huang et al., 2013). Also, other studies revealed that macroalgae is an important aquatic organism to evaluate the toxicity of pollutants (Azman et al., 2014). However, less study about the potential toxicity of nanoparticles on G. lemaneiformis have been reported (Han et al., 2012).
In the present study, changes in the photosynthetic pigment concentration and antioxidantive enzyme defense system (activities of SOD and CAT) and the lipid peroxidation, were measured by culturing G. lemaneiformis under the different concentrations of nano-TiO2 in the indoor seawater culture systems. Moreover, the negative impact of nano-TiO2 on anti-tumor activities were investigated after G. lemaneiformis exposed to nano-TiO2 for 15 d.
2 Materials and Methods 2.1 Algal Culture and TreatmentG. lemaneiformis used in this study was collected from its farming place at Nanao Island (116.6°E, 23.3°N), Guangdong Province, China. After harvesting, the healthy algae were selected and rinsed with sterile artificial seawater to remove contamination. Then the thalli were acclimatized continuously in Von Stosch enriched (VSE) medium for a week under the following conditions: salinity of 30‰, temperature of 20 ± 1℃, illumination of 2000 lx, light/dark cycles of 12 h:12 h. The culture medium was renewed every other day. After pre-culturing, about 4 g fresh weight (FW) of healthy thalli were moved to a conical flask containing 1 L fresh VSE medium and adding different concentrations of nano-TiO2 ranging from 0, 5, 10, 20 and 40 mg L-1 (all assay contain three replicates) for 3, 7, and 15 days. In addition, other conditions were consistent with the conditions of the acclimatization period. Nano-TiO2 (Aladdin, anatase 5-10 nm, hydrophilic purity-99.8%) was prepared with distilled water as the stock solutions (0.5 g L-1). The preparation of nano-TiO2 stock solution and the selection of concentration were described in previous research (Li et al., 2015).
2.2 Pigment DeterminationChlorophyll a (Chl a) and phycobilins are important photosynthetic pigments in G. lemaneiformis. To determine the content of Chl a, about 0.3 g FW of algae was grinded with 3 mL of 90% acetone at 4℃. After, the homogenates was centrifuged at 5000 rmin-1 for 15 min at 4℃. The content of Chl a was determined according to Wellburn (1994). To determine phycobilins, about 0.2 g FW of algal were grinded with 5 mL phosphate buffer (50 mmol L-1, pH=5.7) at 4℃. The extracts were then centrifuged at 10000 rmin-1 for 10 min at 4℃ and the contents of phycoerythrin (PE), phycocyanin (PC), and allophycocyanin (APC) in the supernatant were then calculated according to the method of Kursar and Alberte (1983).
2.3 Lipid Peroxidation, Superoxide Dismutase, and Catalase DeterminationExtracts for measuring lipid peroxidation (LPO), superoxide dismutase (SOD), and catalase (CAT) were ground with ice-cold phosphate buffer (pH 7.8) under ice bath at the ratio of 1:9 (w/v). Then the homogenate was centrifuged (10000 rmin-1, 10 min), and the supernatant was used for the assessment of LPO, and SOD, CAT.
The LPO was detected by thiobarbituric acid reactive substance (TBARS). A 2 mL extract was loaded in each test tube with 2 mL of 5% thiobarbituric acid (TCA) containing 0.5% thiobarbituric acid and 1 mL of TCA (0.6%) solution. The mixture was put in the boiling water for 30 min and cooled quickly in an ice bath. After centrifuging at 10000 rmin-1 for 10 min, the supernatant was collected. Then, the absorbance of the supernatant was measured at 450, 532, and 600 nm. The contents malondialdehyde (MDA) were calculated according to Fu et al. (2014).
The activity of SOD was assayed by detecting the inhibition of total nitroblue tetrazolium (NBT) oxidation, a method described by Bradford (1976). Briefly, 100 μL extract was loaded in each test tube followed by 2.55 mL phosphate buffer (pH 7.8), methionine (300 μL 130 mmol L-1), NBT (300 μL 0.75 mmol L-1), EDTA·2Na (300 μL 0.1 mmol L-1) and riboflavin (300 μL of 0.02 mmol L-1). Immediately, the tubes were shaken and light-exposed for 20 min at 35℃. A tube containing enzyme extract was kept in the dark preserved as the blank, and a tube without enzyme was kept in the light preserved as a control. Finally, the absorbance of the reactant solution was detected at 560 nm.
The activities of CAT were determined according to the manufacturer's instructions of CAT assay kit (No. A007-1) purchased from Nanjing Jiancheng Bioengineering Institute, China.
2.4 Preparation of Algal Aqueous ExtractG. lemaneiformis were cultured in different concentrations of nano-TiO2 for 15 d. Then the algae was rinsed with ultrapure water, dried and ground into powder. AEGL was prepared according to Zandi et al. (2010). 1.0 g powder was extracted in ultrapure water at the ratio of 1:100 (w/v). Then the algae homogenate was centrifuged at 7500 rmin-1 for 15 min. The supernatant was filtered by Whatman paper No.1 filter paper and sterilized by the Millipore filter with 0.22-μm pore size and stored at -20℃. The crude extract was diluted with Dulbecco's Modified Eagle Medium (DMEM) to 1 mg mL-1 prior to use.
2.5 Determination of Antitumor Activity 2.5.1 HepG2 cell cultureThe human hepatoma cell line HepG2 cells were cultured in 25 cm2 flasks with DMEM which contain 1% penicillin-streptomycin solution and 10% fetal bovine serum (FBS). The cells were maintained at 37℃ in a humidified atmosphere with 5% CO2.
2.5.2 Measurement of cell viabilityThe inhibitory effects of AEGL on growth of HepG2 cells were conducted in vitro by methyl thiazolyl tetrazolium (MTT) assay (Nwagbara et al., 2007). Briefly, HepG2 cells were incubated in 96-well plates for 24 h, and then the culture medium was replaced with 100 μL fresh medium containing AEGL (1 mg mL-1). All samples were tested in triplicate. Solvent control (DMEM) was also assayed. After 24 and 48 h treatment, each well added 10 μL MTT (5 mg mL-1) solution and then the plates was incubated for 4 h, after that the supernatant was replaced with 100 μL DMSO. Finally, the plates were shaken for 10 min in the dark and the absorbance was measured at 490 nm by microplate reader (SYNERGY H1). Cell viability compared solvent control was calculated from the mean absorbance values of three replicates.
2.5.3 Determination of reactive oxygen speciesReactive oxygen species (ROS) levels in HepG2 cells were assessed with 2.7-dichlorofluorescein diacetate (DCFH-DA) method using ROS Assay Kit (Beyotime Biotechnology, China) purchased from Beyotime Biotechnology. HepG2 cells were treated with AEGL (1 mg mL-1) in 6-well plate for 24 h. Solvent control (DMEM) was also conducted. After 24 h incubation, cells were collected and washed in phosphate buffered saline (PBS) and stained with DCFH-DA. ROS levels were analyzed by flow cytometry (Gallios, USA). The relative fluorescence intensity (%) compared with solvent control was determined.
2.5.4 Analysis of cell apoptosisApoptosis was measured with an Annexin V-FITC kit (BD Biosciences, USA) purchased from BD Biosciences according to the manufacturer's protocol (Wu et al., 2012). After adherence, HepG2 cells were treated with AEGL (1mg mL-1) in 6-well plate for 48 h incubation. Solvent control (DMEM) was also assayed. Then cells were collected and re-suspended in binding buffer consisting of PI and Annexin V-FITC. The stained cells were analyzed by flow cytometry.
2.5.5 Analysis of cell cycleEffects of AEGL on cell-cycle phase distribution were also assessed by flow cytometry (Wang et al., 2016). HepG2 cells were treated with AEGL (1 mg mL-1) and harvested in 6-well plate for 48 h. Solvent control (DMEM) was also assayed. Then cells were collected and re-suspended in 300 μL cold PBS, and then injected to 700 μL pre-cooling absolute alcohol at 4℃. After centrifugation, the cells were washed with PBS and then stained with PI for 15 min at 37℃ in the dark. Finally, the proportion of cell-cycle was measured by flow cytometry (Gallios, USA).
2.6 Statistical AnalysesAll the experimental dates were represented as means ± SD. Statistical differences were performed using one-way analysis of variance followed by Tukey test. *P < 0.05 and **P < 0.01 were expressed significant and very significant differences between control and the tested samples, respectively.
3 Results 3.1 Pigment ContentThe influences of different nano-TiO2 concentration on Chl a and phycobilins content of G. lemaneiformis are summarized in Table 1. After 3 d exposure to nano-TiO2, the Chl a level of G. lemaneiformis had no obvious change. However, when G. lemaneiformis treated with nano-TiO2 for 7 or 15 days, the contents of Chl a decreased significantly (P < 0.05). Compared with control, the level of Chl a reached a minimum on 40 mg L-1 of 15 days which was decreased by 59.3%. The contents of PE, PC and APC were also significantly reduced in 7 and 15 days' treated groups, and the trend was similar to Chl a when the concentration and time were same. In the 15 days, the PE, PC and APC content of the 40 mg L-1 group were decreased by 59.8%, 60.4%, 72.4% respectively compared with the control.
|
|
Table 1 The photosynthetic pigment content (μg g-1 FW) of G. lemaneiformis after the treatment of nano-TiO2 for 3, 7, 15 d |
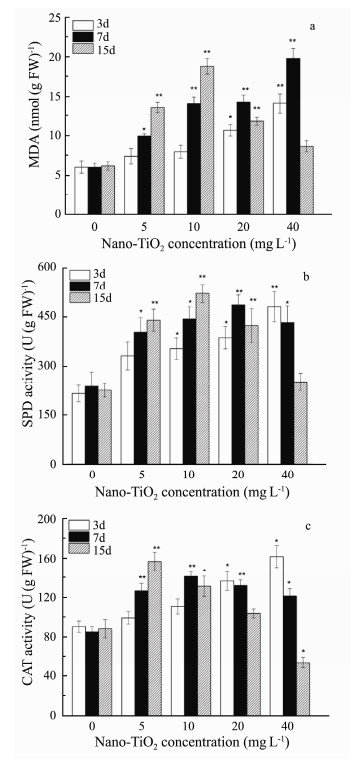
In order to assess the oxidative stress induced by nano- TiO2, LPO was expressed as malondialdehyde (MDA) level, CAT and SOD activities were detected after 3, 7, and 15 day treatment. MDA levels and antioxidant enzyme activities of G. lemaneiformis were shown in Fig. 1.
|
Fig. 1 (a) MDA level, (b) SOD activity and (c) CAT activity in G. lemaneiformis exposed to different concentrations of nano-TiO2. Values were indicated as means ± SD of triplicates. *P < 0.05, **P < 0.01. |
From Fig. 1a, the MDA contents have significantly increased by 1.65-3.3 folds in all groups when compared with control group. It indicated that nano-TiO2 induced oxidative damage to G. lemaneiformis. After the G. lemaneiformis exposed to nano-TiO2 for 3 and 7 days, the MDA level increased linearly as exposure concentration increased and showed the highest level with the increase of 3.3 fold at concentration of 40 mg L-1 on the 7th day (P < 0.05). On the 15th day, the level of MDA was increased first and then decreased. The high concentration reduction trend may be due to the decay of the algae leading to the corresponding enzyme inactivation.
3.2.2 Superoxide dismutaseEffect of nano-TiO2 on SOD activity in G. lemaneiformis was summarized in Fig. 1b. The activity of SOD in G. lemaneiformis was higher than the controls, and the trend of variations was similar to MDA when the concentration and time were the same. Moreover, the values of SOD activity were 1.54-2.24 folds larger than that control group, which were detected after 3 and 7 days culture. And a slightly decrease in SOD activity was observed at 20 mg L-1 in the 15 days. Furthermore, there is a peak value at 10 mg L-1 of 15 days with the increase of 2.32- fold (P < 0.01).
3.2.3 CatalaseIt can be observed from Fig. 1c that the activity of CAT has no obvious change in the concentration of 5 and 10 mg L-1 for 3 days exposure, while, CAT activity varied obviously in the concentration of 20 and 40 mg L-1. For 7 days culture, the CAT activity increased first and then decreased with the increase of exposure concentration. For 15 days culture, the CAT activity was 1.77-fold and reached a maximum in the concentration of 5 mg L-1. Though, the CAT level was gradually decreased at the concentration of 10 mg L-1, and the values in the concentration of 20 and 40 mg L-1 decreased obviously, especially at the concentration of 40 mg L-1 which was significantly lower than the control group.
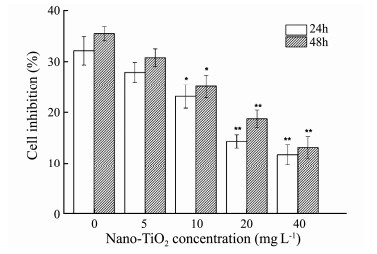
3.3 Effect of Nano-TiO2 on Antitumor Activity of G. lemaneiformis 3.3.1 Cell viability in vitroThe activity of cells was investigated by MTT assay text in order to evaluate the effect of nano-TiO2 on against HepG-2 cells of algal extract (Fig. 2). The results were expressed as a percentage compared with corresponding solvent control. It showed that AEGL could inhibit HepG2 cell viability effectively. In addition, with the extension of treatment time, the cell inhibition of AEGL on HepG2 cells increased slightly. However, when G. lemaneiformis was exposed to nano-TiO2, it may reduce the inhibitory effect of AEGL on cell proliferation and show significant dose effect connection to a certain extent. When the concentration of nano-TiO2 was 0 and 40 mg L-1, the inhibition were 35% and 13%, respectively.
|
Fig. 2 The effect of AEGL on HepG2 cells proliferation when G. lemaneiformis was treated with different concentrations of nano-TiO2. Values were indicated as means ± SD of triplicates. *P < 0.05, **P < 0.01. |
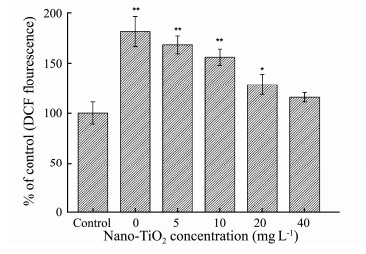
ROS plays a significant role in the regulation of cellular function, such as cell apoptosis and the oxidative signal transduction (Shi et al., 2013). The generation of intracellular ROS was investigated after cells were treated with AEGL for 24 h. As shown in Fig. 3, the production of ROS level visibly increased. The result revealed that AEGL could lead to the accumulation of ROS in cells. The production of ROS increased 1.8-fold compared with controls. However, AEGL showed lower effects on the level of intracellular ROS when G. lemaneiformis were exposed to nano-TiO2 for 15 d.
|
Fig. 3 The effect of AEGL on intracellular ROS generation of HepG2 cells after 24 h treatment when G. lemaneiformis was cultured in different concentrations of nano- TiO2. Values were indicated as means ± SD of triplicates. *P < 0.05, **P < 0.01. |
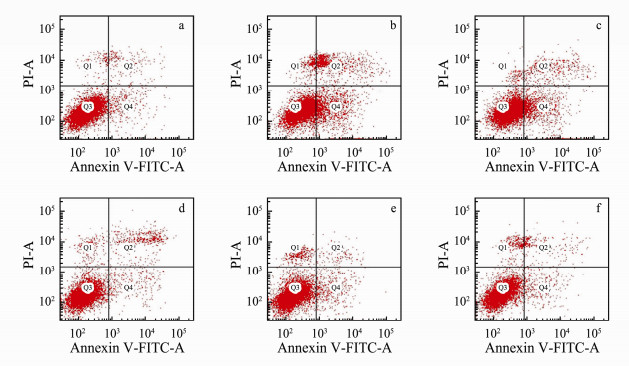
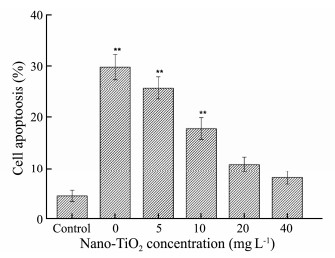
The active compounds which were extracted from marine macroalgae have the function of antitumor and can inducing cell apoptosis (Rengarajan et al., 2013). To research the toxic effect of nano-TiO2 on antitumor activity of G. lemaneiformis, we utilized flow cytometry to determine apoptosis in HepG2 cells after AEGL treatment. HepG-2 cells were induced to undergo apoptosis significantly by treatment with EEGL compared with the control group (Figs. 4 and 5). In Fig. 4, Q1, Q2, Q3 and Q4 represent living cells, early apoptosis, late apoptosis, and dead cells, respectively. Interestingly, the extract of G. lemaneiformis which were not exposed to nano-TiO2 showed a higher rate of apoptosis on HepG2 cells (Fig. 4b). Nevertheless, the apoptosis induced by AEGL decreased visibly with the increase of exposure concentrations of nano-TiO2 to G. lemaneiformis (Figs. 4c-f). Those were also consistent with the MTT result, indicating that AEGL inhibited apoptosis and nano-TiO2 may reduce the antitumor effect of G. lemaneiformis extracts.
|
Fig. 4 AEGL-induced apoptosis after 48-h incubation of HepG-2 cells when G. lemaneiformis were cultured in different concentrations of nano-TiO2 determined by flow cytometry. a, control; b, 0 mg L-1 nano-TiO2; c, 5 mg L-1 nano-TiO2; d, 10 mg L-1 nano-TiO2; e, 20 mg L-1 nano-TiO2; f, 40 mg L-1 nano-TiO2. |
|
Fig. 5 The effect of AEGL on apoptosis of HepG2 cells after 48 h treatment when G. lemaneiformis were cultured in different concentrations of nano-TiO2. Values were indicated as means ± SD of triplicates. *P < 0.05, **P < 0.01. |
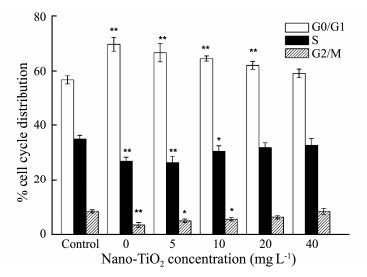
In order to confirm the mechanism which AEGL inducing apoptosis in HepG2 cells, the effects of AEGL on cell cycle were detected by flow cytometry. As shown in Fig. 6, AEGL incubation induced a strong G0/G1 phase arrest. After being treated with the extract of G. lemaneiformis without the addition of nano-TiO2, the proportion of G0/G1 phase in HepG2 cells markedly increased from 56.7% to 69.7%. However, when G. lemaneiformis were exposed to high concentrations of nano-TiO2, the percentage of G0/G1 phase increase was relatively slow. Therefore, the present results demonstrated that the extract of G. lemaneiformis was able to induce cell cycle arrest at G0/G1 phase and nano-TiO2 may reduce the antitumor effect of AEGL.
|
Fig. 6 The effect of AEGL on cell-cycle of HepG2 cells after 48 h treatment when G. lemaneiformis was cultured in different concentrations of nano-TiO2 determined by flow cytometry. Data are presented as the mean ± SD of three replicates. *P < 0.05, **P < 0.01. |
Recently, macroalgae has received wide attention for their positive influences on nutrient cycling, energy transformation and medicine (Raize et al., 2010; Zandi et al., 2010; Mendes et al., 2013). This result indicated that nano-TiO2 may inhibit the growth, increase oxidant damage and weak the antitumor activity of G. lemaneiformis. Accordingly, a decreased in cell inhibition, intracellular reactive oxygen species (ROS) and cell apoptosis were observed in HepG-2 cells treated with AEGL after G. lemaneiformis exposing to different concentrations of nano-TiO2 for 15 days, and which were linearly associated with nano-TiO2 exposure concentration.
Chl a and phycobilins, the key pigments in red algae, play significant roles in converting light energy into chemical energy. Their contents were the important physiological index of photosynthesis in the red alga (Yu and Yang, 2008). In this work, the content of Chl a and phycobilins declined dramatically when G. lemaneiformis was exposed to nano-TiO2, it may be the reason that nano-TiO2 interfered with the synthesis and normal metabolism of photosynthetic protein and pigments. Similar results were confirmed in studies such as Simioni and Xu(1900, 2012). The result of this study indicated that, when the culture time delayed and nano-TiO2 concentrations increased, the content of PE, PC, APC and Chl a went down and the color of G. lemaneiformis was gradually changing from red to yellow. There is a same result studied by Peng et al., (2007). This showed that nano-TiO2 could have a negative impact on pigmentation and growth of the seaweed. The disappearance of the algae color may be due to the metabolic effects on phycobiliprotein as a nitrogen source. In other words, the pigments could be synthesized by those proteins (Ryder et al., 2004). Many studies indicated that the phycobiliprotein is positively correlated with nitrogen availability in Gracilariales (Jones et al., 1997; Zubia et al., 2014).
Generally, ROS could be easily induced by polluted environment. Antioxidant system plays an important role in preventing the overproduction of free radical, mitigating membrane lipid peroxidation (Bradford, 1976; Dummermuth et al., 2003). SOD is the key enzyme in catalyzing superoxide radical to H2O2, which could be decomposed into water and oxygen molecules by CAT (Alscher et al., 2002). The result of this study showed that the activity of SOD increased significantly compared with the controls though there was a slight drop when G. lemaneiformis was exposed to 20 and 40 mg L-1 of nano-TiO2 for 15 days. For CAT, there was no obvious variation in the 3 days when the G. lemaneiformis were exposed to 5 and 10 mg L-1. Subsequently, it presented a significant increase initially and then decreased with the increase of time and exposure dose. The level of SOD and CAT increased in G. lemaneiformis, which can be regarded as a self protection of G. lemaneiformis against the impact of nano-TiO2 toxicity and oxidative stress (Reddy et al., 2005). Therefore, SOD and CAT activity decreases showed that long time stress and high nano-TiO2 concentrations could restrain the resistance ability of algae, which might lead to algae's death (Yu and Yang, 2008). MDA, one of the important indicators of membrane lipid peroxidation, is a product of lipid peroxidation when organisms are in adversity. Hence, the level of MDA in G. lemaneiformis can reflect the peroxidation degree. The result of this study indicated that the content of MDA increased obviously in the most experimental groups compared with the control during the experiment of nano-TiO2 exposure to G. lemaneiformis. This showed that nano-TiO2 have oxidative damage to G. lemaneiformis. However, the MDA level decreased visibly with the 20 or 40 mg L-1 nano-TiO2 exposure to algae for 15 days; it was probably due to the decay of algae which resulted in relevant enzymes inactive.
Emerging published papers has proved that macroalgae are promising natural materials to inhibit tumor cell proliferation and induce apoptosis (Sundaram et al., 2012). The bioactive ingredients of Gracilaria have been studied and they mainly included ethanol/methanol, ethyl acetate or aqueous extract (Sundaram et al., 2012). The different extracted solvents or nature products may show different cytotoxicity to cancerous cells. Zandi et al. (2010) described that the water crude extract of red alga Gracilaria corticata had significant cytotoxicity against human cancer cell lines. Huang et al. (2005) also reported that the ethyl acetate extract of Galaxaura oblongata, Halimeda discoidae, and Colpomenia sinuosa showed strongly cytotoxic effect against liver cancer (HuH-7) with the IC50 of 123.54, 230.53, and 112.38 μg mL-1 (72 h), respectively. And the IC50 value of the ethanol extract of Gracilaria edulis for ehrlich ascites tumor (EAT) cells was 45 μg mL-1 (72 h) (Patra and Muthuraman, 2013). In consideration of the toxic effects of nano-TiO2 on G. lemaneiformis, nano-TiO2 may reduce the antitumor activity of AEGL.
The effects of AEGL on cell proliferative were affirmed by MTT cytotoxic assay in HepG2 cells. This study showed that AEGL has a chemoprophylactic role in HepG2 cell proliferation. By comparing to those without exposed to nano-TiO2, G. lemaneiformis exposed to nano-TiO2 showed lower antitumor activity in HepG2 cells. Patra and Muthuraman (2013) proved that the bioactive extract of seaweeds induced apoptosis in EAT cells mainly correlated with the rise of ROS. This study showed that production of intracellular ROS may mediate oxidative stress, and eventually induce cell apoptosis. Nevertheless, with the increasing the exposure concentration of nano-TiO2 to G. lemaneiformis, cell apoptosis and ROS generation decreased significantly. Accordingly, it demonstrated that nano-TiO2 can cause inescapable damage to the anticancer activity of G. lemaneiformis. In order to further detect the negative effects of apoptosis, cell cycle was also examined. The cell cycle plays an important role in the growth and proliferation of cells. For example, many anticancer drugs can arrest the cell cycle at the G0/G1, S or G2/M phase and then induce apoptosis (Zhao et al., 2015). A noticeably cell cycle arrest noted in this work was positive correlated with decreased ROS and apoptosis following nano-TiO2 treated algae. These demonstrated that AEGL may lead to cell apoptosis by G0/G1 phase arrest and nano-TiO2 can reduce the G1 phase arrest of G. lemaneiformis. In a word, the results proved that the nano-TiO2 exposure caused oxidative damage to G. lemaneiformis and visibly weakened its latent against HepG2 cells.
5 ConclusionsThe results in this study indicated that nano-TiO2 is toxic to G. lemaneiformis which can cause oxidative stress and decreasing the contents of pigment. During the experimental period, both the Chl a and phycobilins contents in G. lemaneiformis were influenced by nano-TiO2 concentrations and culture time. The activities of SOD and CAT enhanced significantly and then diminished under high concentrations and prolonged exposure. Furthermore, the AEGL has antitumor activity and can induce HepG2 cell apoptosis and induce cell cycle disruption in the G0/G1 phases. But the toxicity of nano-TiO2 would inhibit the anticancer activity of AEGL. A decreased in cell inhibition, ROS and cell apoptosis were also observed in HepG-2 cells treated with AEGL after G. lemaneiformis exposing to different concentrations of nano-TiO2 for 15 day, and which were linearly associated with nano-TiO2 exposure concentration. Experimental data, based on toxicity tests of nano-TiO2 in this research, can contribute to understanding the ecological effects of oxide nanoparticles.
AcknowledgementsThis work was supported by the NSFC-Guangdong Province Joint Key Project (No. U1301235), the National Natural Science Foundation of China (No. 41676110), the Industry-University-Research Combination Project of Guangdong Province (No. 2013B090600009), and the Science and Technology Program of Guangdong, China (No. 2014A020217007).
Alscher, R. G., Erturk, N. and Heath, L. S., 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany, 53: 1331. DOI:10.1093/jexbot/53.372.1331 (  0) 0) |
Aruoja, V., Dubourguier, H. C., Kasemets, K. and Kahru, A., 2009. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Science of the Total Environment, 407: 1461-1468. DOI:10.1016/j.scitotenv.2008.10.053 (  0) 0) |
Azman, S., Said, M. I. M., Ahmad, F. and Mohamad, M., 2014. Biofiltration potential of macroalgae for ammonium removal in outdoor tank shrimp wastewater recirculation system. Biomass and Bioenergy, 66: 103-109. DOI:10.1016/j.biombioe.2014.02.031 (  0) 0) |
Bradford, M. M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Analytical Biochemistry, 6: 3177-3188. (  0) 0) |
Chong, M. N., Jin, B., Chow, C. W. and Saint, C., 2010. Recent developments in photocatalytic water treatment technology: A review. Water Research, 44: 2997-3027. DOI:10.1016/j.watres.2010.02.039 (  0) 0) |
Dummermuth, A. L., Karsten, U., Fisch, K. M., König, G. M. and Wiencke, C., 2003. Responses of marine macroalgae to hydrogen-peroxide stress. Journal of Experimental Marine Biology & Ecology, 289: 103-121. (  0) 0) |
Fan, Y., Wang, W., Song, W., Chen, H., Teng, A. and Liu, A., 2012. Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydrate Polymers, 88: 1313-1318. DOI:10.1016/j.carbpol.2012.02.014 (  0) 0) |
Fu, F., Sui, Z. H., Zhou, W., Wang, J. G., Chang, L. P. and Ci, S. F., 2014. UV-irradiation mutation of tetraspores of Gracilariopsis lemaneiformis and screening of thermotolerant strains. Periodical of Ocean University of China, 26: 647-656. (  0) 0) |
Gottschalk, F., Sonderer, T., Scholz, R. W. and Nowack, B., 2009. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environmental Science & Technology, 43: 9216. (  0) 0) |
Han, Z. X., Zhang, M. and Lv, C. X., 2012. Bioaccumulation and toxicity of NiO nanoparticles in Gracilaria lemaneiformis. Advanced Materials Research, 518-523: 942-945. DOI:10.4028/www.scientific.net/AMR.518-523.942 (  0) 0) |
Huang, H., Liang, J., Wu, X., Zhang, H., Li, Q. and Zhang, Q., 2013. Comparison in copper accumulation and physiological responses of Gracilaria lemaneiformis and G. lichenoides (Rhodophyceae). Chinese Journal of Oceanology and Limnology, 31: 803-812. DOI:10.1007/s00343-013-2261-5 (  0) 0) |
Huang, H. L., Wu, S. L., Liao, H. F., Jiang, C. M., Huang, R. L., Chen, Y. Y., Yang, Y. C. and Chen, Y. J., 2005. Induction of apoptosis by three marine algae through generation of reactive oxygen species in human leukemic cell lines. Journal of Agricultural and Food Chemistry, 53: 1776. DOI:10.1021/jf049445n (  0) 0) |
Hund-Rinke, K. and Simon, M., 2006. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environmental Science & Pollution Research International, 13: 225. (  0) 0) |
Jones, A., Dennison, W. and Stewart, G., 1997. Macroalgal responses to nitrogen source and availability: Amino acid metabolic profiling as a bioindicator using Gracilaria edulis (Rhodophyta). Oceanographic Literature Review, 5: 443. (  0) 0) |
Kumar, R. S., Rajkapoor, B. and Perumal, P., 2011. Antitumor and cytotoxic activities of methanol extract of Indigofera linnaei Ali. Asian Pacific Journal of Cancer Prevention, 12: 613. (  0) 0) |
Kursar, T. A., Van, D. M. J. and Alberte, R. S., 1983. Lightharvesting system of the red alga Gracilaria tikvahiae: I. Biochemical analyses of pigment mutations. Plant Physiology, 73: 361-369. DOI:10.1104/pp.73.2.361 (  0) 0) |
Li, F., Liang, Z., Zheng, X., Zhao, W., Wu, M. and Wang, Z., 2015. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquatic Toxicology, 158: 1-13. DOI:10.1016/j.aquatox.2014.10.014 (  0) 0) |
Liu, C., Zou, D., Yang, Y., Chen, B. and Jiang, H., 2016. Temperature responses of pigment contents, chlorophyll fluorescence characteristics, and antioxidant defenses in Gracilariopsis lemaneiformis (Gracilariales, Rhodophyta) under different CO2 levels. Journal of Applied Phycology, 29: 983-991. (  0) 0) |
Manilal, A., Sujith, S., Sabarathnam, B., Kiran, G. S., Selvin, J., Shakir, C. and Lipton, A. P., 2009. Antifouling potentials of seaweeds collected from the southwest coast of India. Journal of Marine Science & Technology, 17: 67-73. (  0) 0) |
Marinho-Soriano, E., Nunes, S. O., Carneiro, M. A. A. and Pereira, D. C., 2009. Nutrients' removal from aquaculture wastewater using the macroalgae Gracilaria birdiae. Biomass and Bioenergy, 33: 327-331. DOI:10.1016/j.biombioe.2008.07.002 (  0) 0) |
Menard, A., Drobne, D. and Jemec, A., 2011. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environmental Pollution, 159: 677. (  0) 0) |
Mendes, L., Zambotti-Villela, F., Colepicolo, L., Marinho-Soriano, E. and Stevani, V. C., 2013. Metal cation toxicity in the alga Gracilaria domingensis as evaluated by the daily growth rates in synthetic seawater. Journal of Applied Phycology, 25: 1939-1947. DOI:10.1007/s10811-013-0036-1 (  0) 0) |
Nwagbara, O., Darling-Reed, S. F., Tucker, A., Harris, C., Abazinge, M., Thomas, R. D. and Gragg, R. D., 2007. Induction of cell death, DNA strand breaks, and cell cycle arrest in DU145 human prostate carcinoma cell line by benzo[a]pyrene and benzo[a]pyrene-7, 8-diol-9, 10-epoxide. International Journal of Environmental Research & Public Health, 4: 10-14. (  0) 0) |
Patra, J. K., Rath, S. K., Jena, K. B., Rathod, V. K. and Thatoi, H., 2008. Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: A study on inhibition of glutathione-s-transferase activity. Turkish Journal of Biology: 119-125. (  0) 0) |
Patra, S. and Muthuraman, M. S., 2013. Gracilaria edulis extract induces apoptosis and inhibits tumor in Ehrlich ascites tumor cells in vivo. BMC Complementary and Alternative Medicine, 13: 1-12. DOI:10.1186/1472-6882-13-1 (  0) 0) |
Peng, C. L., Xue, W., Lin, Z. F., Zhou, H. C. and Chen, S. W., 2007. Response of Gracilaria lemaneiformis to nitrogen and phosphorus eutrophic seawater. Journal of Plant Ecology, 31: 505-512. DOI:10.17521/cjpe.2007.0063 (  0) 0) |
Raize, O., Argaman, Y. and Yannai, S., 2010. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnology & Bioengineering, 87: 451-458. (  0) 0) |
Reddy, M. K., Alexanderlindo, R. L. and Nair, M. G., 2005. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. Journal of Agricultural & Food Chemistry, 53: 9268. (  0) 0) |
Rengarajan, T., Rajendran, P., Nandakumar, N. and Nishigaki, I., 2013. Cancer preventive efficacy of marine carotenoid fucoxanthin: Cell cycle arrest and apoptosis. Nutrients, 5: 4978. DOI:10.3390/nu5124978 (  0) 0) |
Robichaud, C. O., Uyar, A. E., Darby, M. R., Zucker, L. G. and Wiesner, M. R., 2009. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environmental Science & Technology, 43: 4227-4233. (  0) 0) |
Ryder, E., Nelson, S. G., Mckeon, C., Glenn, E. P., Fitzsimmons, K. and Napolean, S., 2004. Effect of water motion on the cultivation of the economic seaweed Gracilaria parvispora (Rhodophyta) on Molokai, Hawaii. Aquaculture, 238: 207-219. DOI:10.1016/j.aquaculture.2004.05.019 (  0) 0) |
Schmidt, E. C., dos Santos, R., Horta, P. A., Maraschin, M. and Bouzon, Z. L., 2010. Effects of UVB radiation on the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales): Changes in cell organization, growth and photosynthetic performance. Micron, 41: 919-930. DOI:10.1016/j.micron.2010.07.010 (  0) 0) |
Shi, L., Yu, X., Yang, H. and Wu, X., 2013. Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. PLoS One, 8: e66781. DOI:10.1371/journal.pone.0066781 (  0) 0) |
Simioni, C., Schmidt, É. C., Felix, M. R. D. L., Polo, L. K., Rover, T., Kreusch, M., Pereira, D. T., Chow, F., Ramlov, F. and Maraschin, M., 1900. Effects of ultraviolet radiation (UVA+UVB) on young gametophytes of Gelidium floridanum: Growth rate, photosynthetic pigments, carotenoids, photosynthetic performance, and ultrastructure. Photochemistry & Photobiology, 90: 1050-1060. (  0) 0) |
Sundaram, M., Patra, S. and Maniarasu, G., 2012. Antitumor activity of ethanol extract of Gracilaria edulis (Gmelin) Silva on Ehrlich ascites carcinoma-bearing mice. Journal of Chinese Integrative Medicine, 10: 430-435. DOI:10.3736/jcim20120412 (  0) 0) |
Tian, Y. S., Gottschalk, F., Hungerbühler, K. and Nowack, B., 2014. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environmental Pollution, 185: 69. DOI:10.1016/j.envpol.2013.10.004 (  0) 0) |
Wang, G. D., Zhu, L., Yu, B., Chen, K., Liu, B. and Liu, J., 2016. Exopoly-saccharide from trichoderma pseudokoningii induces the apoptosis of mcf-7 cells through an intrinsic mitochondrial pathway. Carbohydrate Polymers, 136: 1065-1073. DOI:10.1016/j.carbpol.2015.09.108 (  0) 0) |
Wang, Z. and Yue, W., 2015. Removal of cypermethrin with seaweed Gracilaria lemaneiformis. Journal of Ocean University of China, 14: 858-864. DOI:10.1007/s11802-015-2567-3 (  0) 0) |
Wellburn, A. R., 1994. The spectral determination of Chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144: 307-313. DOI:10.1016/S0176-1617(11)81192-2 (  0) 0) |
Wu, J., Zhou, J., Lang, Y. and Yao, L., 2012. A polysaccharide from Armillaria mellea, exhibits strong in vitro anticancer activity via apoptosis-involved mechanisms. International Journal of Biological Macromolecules, 51: 663-667. DOI:10.1016/j.ijbiomac.2012.06.040 (  0) 0) |
Xu, Z. and Gao, K., 2012. NH4+ enrichment and UV radiation interact to affect the photosynthesis and nitrogen uptake of Gracilaria lemaneiformis (Rhodophyta). Marine Pollution Bulletin, 64: 99. DOI:10.1016/j.marpolbul.2011.10.016 (  0) 0) |
Yang, Y., Liu, Q., Chai, Z. and Tang, Y., 2014. Inhibition of marine coastal bloom-forming phytoplankton by commercially cultivated Gracilaria lemaneiformis (Rhodophyta). Journal of Applied Phycology, 27: 2341-2352. (  0) 0) |
Yu, J. and Yang, Y. F., 2008. Physiological and biochemical response of seaweed Gracilaria lemaneiformis to concentration changes of N and P. Journal of Experimental Marine Biology and Ecology, 367: 142-148. DOI:10.1016/j.jembe.2008.09.009 (  0) 0) |
Zandi, K., Tajbakhsh, S., Nabipour, I., Rastian, Z., Yousefi, F., Sharafian, S. and Sartavi, K., 2010. In vitro antitumor activity of Gracilaria corticata (a red alga) against Jurkat and molt-4 human cancer cell lines. African Journal of Biotechnology, 9: 6787-6790. (  0) 0) |
Zhao, K., Jin, M., Chen, Q. and Zheng, P. S., 2015. Polysaccharides produced by Enterobacter cloacae induce apoptosis in cervical cancer cells. International Journal of Biological Macromolecules, 72: 960-964. DOI:10.1016/j.ijbiomac.2014.09.047 (  0) 0) |
Zubia, M., Freile-Pelegrín, Y. and Robledo, D., 2014. Photosynthesis, pigment composition and antioxidant defences in the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta) under environmental stress. Journal of Applied Phycology, 26: 2001-2010. DOI:10.1007/s10811-014-0325-3 (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


