2) School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
The G-protein-coupled receptor (GPCR), GPR103, also known as SP9155 or AQ27 (Lee et al., 2001; Fukusumi et al., 2003; Jiang et al., 2003) was recently renamed QRFPR (for the neuropeptide pyroglutamilated RFamide peptide receptor) by the HUGO Gene Nomenclature Committee (Bonner et al., 2016). QRFPR was firstly identified as an orphan GPCR from the human hypothalamus cDNA (Lee et al., 2001). Later, QRFPR was deorphanized and proved to be specifically activated by two endogenous ligands generated by QRFP precursor-encoding gene, 26RFa (also referred to as QRFP) and its N-terminal extended form, 43RFa (Chartrel et al., 2003; Fukusumi et al., 2003; Jiang et al., 2003).
QRFPR belongs to the class A GPCR superfamily that contains five distinct groups (Ⅰ – Ⅴ) (Alexander et al., 2015; Yun et al., 2015). Group Ⅰ has several members, such as receptors for RFamide neuropeptide family, neuropeptide Y receptor (NPYR), and orexin receptor (also called hypocretin receptor, HCRTR) (Yun et al., 2015). As one of the receptors for RFamide neuropeptide family, QRFPR shares approximately 50% sequence homology with other RFamide neuropeptide receptors, including the receptors of neuropeptide FF 1 (NPFF1), neuropeptide FF 2 (NPFF2), prolactin-releasing peptide (PrRP), and kisspeptin (Lee et al., 2001; Jiang et al., 2003; Ayachi and Simonin, 2014). Additionally, QRFPR was reported to show sequence identity with other GPCR groups, such as 37% and 47% to the neuropeptide Y receptor (NPYR) and orexin receptor, respectively (Lee et al., 2001; Jiang et al., 2003). QRFPR and its counterpart have been largely described in vertebrates, including human, rat, mouse, birds and fish (Lee et al., 2001; Fukusumi et al., 2003; Jiang et al., 2003; Baribault et al., 2006; Kampe et al., 2006; Takayasu et al., 2006; Bruzzone et al., 2007; Chen et al., 2016). In human and birds, only a single QRFPR-encoding gene was identified, while two isoforms of the receptor, designated as QRFPR1/2 (also called GPR103a/b) were described in rat and mouse (Kampe et al., 2006; Takayasu et al., 2006). In zebrafish (Danio rerio), although three Qrfpr genes (including one orthologous and two paralogous genes) were included in the genome database, only Gpr103a and Gpr103b were investigated (Chen et al., 2016; Wang et al., 2020). Additionally, one QRFPR gene was identified from an invertebrate chordate amphioxus (Branchiostoma floridae) (Mirabeau and Joly, 2013; Xu et al., 2015). Two articles published in 2014 independently proposed the presence of three and four QRFPR clades in vertebrate QRFPR genes (Larhammar et al., 2014; Ukena et al., 2014).
The distribution pattern, structure, and biological actions of QRFPR and its ligand (QRFP) have been relatively conserved across mammals and non-mammalian species (Ukena et al., 2014). In all vertebrate studied, from fish to mammals, QRFP and QRFPR mRNA are predominantly detected in the brain, especially in specific regions of the hypothalamus (Lee et al., 2001; Chartrel et al., 2003; Fukusumi et al., 2003; Jiang et al., 2003; Baribault et al., 2006; Bruzzone et al., 2006, 2007; Kampe et al., 2006; Takayasu et al., 2006; Liu et al., 2009; Ukena et al., 2010; Tobari et al., 2011; Chen et al., 2016). Those areas are thought to be responsible for regulating food intake and/or energy homeostasis (Bruzzone et al., 2006). Results showed that QRFP/QRFPR system usually functions as an orexigenic signaling and regulates feeding behavior, which is partially mediated by one of the positive regulators of food intake, neuropeptide Y (NPY) (Takayasu et al., 2006; Lectez et al., 2009; Georgsson et al., 2014). The mRNA of this peptide-receptor pair is detected in the extrahypothalamic regions of the brain and multiple peripheral tissues, such as the pituitary, thyroid gland, parathyroid gland, prostate, bladder, larger intestine, coronary artery, cortex, adrenal gland, spinal cord, testis, eye, bone, heart, and kidney in human and/or rodents (Lee et al., 2001; Fukusumi et al., 2003; Jiang et al., 2003; Baribault et al., 2006; Takayasu et al., 2006; Bruzzone et al., 2007; Ramanjaneya et al., 2013; Prevost et al., 2015). Therefore, it is reported that this peptidereceptor system regulates other physiological processes than feeding behavior, like bone formation (Baribault et al., 2006), blood pressure regulation (Takayasu et al., 2006), pituitary hormone secretion (Patel et al., 2008; Liu et al., 2009), nociceptive transmission (Yamamoto et al., 2008, 2011), and steroidogenesis regulation (Fukusumi et al., 2003; Ramanjaneya et al., 2013).
In invertebrates, QRFPR and its ligands has been only reported in amphioxus (Mirabeau and Joly, 2013; Xu et al., 2015). Although homologous sequences of QRFPR have been found in several other invertebrate species, they were in silico identified or predicted, and none of them was cloned or validated. Therefore, it is very necessary to investigate the characterization and function of this peptide-receptor system in invertebrates.
Cephalopods have been regarded as the pinnacle of invertebrate nervous system evolution since they have the largest brains of all invertebrate and across molluscs show the highest degree of centralization (Shigeno and Yamamoto, 2002; Polese et al., 2015). Various behaviors of the cephalopod, such as feeding and reproductive behavior are under the control of a complex of neuropeptide-receptor systems and molecules (Ukena et al., 2014; Polese et al., 2015; Yan et al., 2016; Song et al., 2021; Qiu et al., 2022). As one of the representative species in the cephalopod, cuttlefish, Sepiella japonica, is an excellent model to study the peptidergic regulation since it possesses numerous neuropeptide-receptor systems, GnRH-like peptide/receptor (Yan et al., 2016; Wu et al., 2021), and FMRFamide-like peptide/receptor system (Li et al., 2018). In this study, we identified and characterized the first non-chordates QRFP-like peptide receptor gene in the cephalopod S. japonica, termed Sj_QRFPLR. Furthermore, functional analysis of Sj_QRFPLR was performed by a food deprivation and refeeding experiment and a Vibrio harveyi infection assay.
2 Materials and Methods 2.1 Experimental Cuttlefish and Tissues CollectionS. japonica adults and some ready-to-hatch eggs were obtained from a local cuttlefish breeding base (Xixuan island, Zhoushan, Zhejiang Province). Cuttlefish adults are classified to stage Ⅰ – Ⅱ to Ⅴ based on growth time and gonad maturity as previously reported (Jiang et al., 2007; Luo et al., 2014), with their weight ranging from 17.3 g ± 4.7 g to 70.0 g ± 9.4 g. Cuttlefish eggs were reared in the laboratory at 22℃ in artificial seawater until hatching, and juveniles were fed daily with the rotifer Brachionus plicatilis.
Cuttlefish were anesthetized with magnesium chloride solution (MgCl2, 17 g L−1) before dissection. A total of fifteen tissues (the brain, optic lobe, heart, liver, intestine, skin, muscle, stomach, pancreas, gill, ovary, nidamental gland, accessory nidamental gland, testis and spermatophore) from female and/or male were sampled and stored at −80℃ immediately for further analyses. The brains of stage Ⅰ – Ⅱ to Ⅴ were fixed in 4% paraformaldehyde (PFA) overnight at 4℃. The fixation was washed by 1 × PBST, followed by gradient methanol dehydration (75% – 85% – 95% – 100%). The dehydrated brains were put into 100% methanol and stored at −20℃ for future use. All experimental protocols were approved by the Institutional Animal Care and Use Committees (IACUC) of Zhejiang Ocean University.
2.2 RNA Extraction, cDNA Synthesis and cDNA CloningRNA extraction was conducted as previously reported by Qiu et al. (2022). Briefly, total RNA from dissected tissues was isolated using Trizol reagent (Takara, Kusatsu, Japan). The quantity and quality of the RNA were examined using Nanodrop 2000 (Thermo Scientific, USA) and 2.0% agarose gel electrophoresis, respectively. 1.0 µg total RNA was used to synthesize cDNA by using PrimerScriptTM RT reagent kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Japan) according to the manufacturer's protocol. The cDNA was stored in −20℃ for future use.
To amplify Sj_QRFPLR, gene specific primers (Table 1) were designed based on the unigene annotated in S. japonica transcription (Lü et al., 2016). The open reading frame (ORF) of Sj_QRFPLR was amplified with QRFPLR-F/R in a 25 μL PCR reaction using 2 × San Taq PCR Mix (with Blue Dye) (Sangon Biotech, Shanghai, China). The PCR fragment was extracted from the gel purified, and inserted into the pMD19-T vector (Takara Bio Inc., Kyodo, Japan). The insert was confirmed by sequencing (Sangon Biotech, Shanghai, China).
|
|
Table 1 Primers and their sequences |
To obtain full-length Sj_QRFPLR cDNA, 5'-RACE and 3'-RACE were performed following the instructions of SMARTerTM RACE cDNA Amplification Kit (Clontech, MA, USA). Briefly, 5'-QRFPLR-RACE-R, 5'-QRFPLR-R1 and 5'-QRFPLR-R2 designed based on Sj_QRFPLR ORF obtained together with UPM (10 × Universal Primer A Mix, Table 1) and NUP primers supplied with the kit were used for 5'-RACE amplification. Likewise, 3'-RACE amplification was conducted by combing three specifically designed primers (3'-QRFPLR-RACE-F, 3'-QRFPLR-F1 and 3'-QRFPLR-F2) with UPM and NUP primers supplied. Amplification fragments were gel purified and cloned into the pMD19-T vector and sequenced. All primers are listed in Table 1.
2.3 RT-PCR, Quantitative PCR and In Situ HybridizationThe Sj_QRFPLR expression pattern was examined by reverse transcriptase-PCR (RT-PCR), quantitative PCR (qPCR) and in situ hybridization (ISH). RT-PCR and qPCR were carried out using specific primers for Sj_QRFPLR, β-actin, and GAPDH (Table 1). The amplified products of RT-PCR were visualized and separated on 2% agarose gel. qPCR was performed on a CFX Connect Real-time PCR amplifier (Bio-Rad, VA, USA) with TB Green® Premix Ex TaqTM II (Tli RNaseH Plus) (Takara, Kyodo, Japan). The relative mRNA expression levels of target genes were calculated by the 2−ΔΔCt method (Livak and Schmittgen, 2001). The 18S rRNA, β-actin, and/or GAPDH were used as the reference genes.
For ISH, specific primers ISH-QRFPLR-F/R (Table 1) and brain cDNA were used to amplify templates for sense and antisense probes. The probes were synthesized with the DIG RNA Labeling kit (Roche Diagnostics, Mannheim, Germany) as described previously (Qiu et al., 2022). ISH was performed following the method of Qiu et al. (2022). Briefly, the cuttlefish brains were dehydrated, paraffin-embedded, sectioned at 7 μm and mounted on slides. Next, the brain sections were dewaxed, gradient ethanol-rehydrated (100% – 95% – 80% – 70% – 50%), fixed in 4% PFA for 10 min and protease K-permeabilized (1 μg mL−1) for 8 min, followed by 2h-prehybridization at 42℃ to reduce non-specific signals. The slices were then incubated for 16 h with the probes at 55℃, followed by several rounds of rinse before blocking. In the end, the slices were incubated overnight in anti-DIG-AP antibody (1:1000, Roche Diagnostics, Mannheim, Germany) at 4℃, followed by incubation with NBT/BCIP (Roche Diagnostics, Mannheim, Germany). Signals were checked every 30 min and images were taken using a microscope (Nikon, Tokyo, Japan).
2.4 Recombinant Plasmid Construction and TransfectionSubcellular localization of Sj_QRFPLR was examined in vitro in human embryonic kidney (HEK293) cells. The CDS of Sj_QRFPLR was amplified using primers QRFPLR-EGFP-F/R containing restriction sites KpnI in F and BamH I in R. Expression construct for Sj_QRFPLR (Sj_QRFPLR-EGFP) were generated in pEGFP-N1 vector. The HEK293 cells were gifted by Dr Tianming Wang at Zhejiang Ocean University and maintained in an incubator at 37℃ with 5% CO2. The recombinant construct Sj_QRFPLR-EGFP and Lipo6000TM Transfection Reagent (Beyotime Biotechnology, Shanghai, China) were co-transfected into HEK293 cells. After 6h incubation, cell medium was removed and fresh DEMD medium was added for another 24 h culture. Cells were then fixed in 4% PFA overnight. The cytomembrane and nucleus were visualized by DiI (7 mmol L−1) (Beyotime Biotechnology, Shanghai, China) and DAPI (5 mg mL−1) (Beyotime Biotechnology, Shanghai, China) staining. Representative images were taken using the TCS SP5II laser scanning confocal microscope (Leica, Wetzlar, Germany).
2.5 Food Deprivation and Refeeding ExperimentTotally 55 cuttlefish females at stage V were acclimatized and randomly divided into control group (C group, 30 individuals) and food deprivation and refeeding group (F group, 25 individuals) (Fig.6A). Five individuals were cultured in each tank to avoid food competition and cannibalism. Cuttlefish in the C group were daily fed. For the F group, two stages were set up, namely food deprivation stage (day 1 to day 5) and refeeding stage (day 6 to day 8). Cuttlefish were sampled on days 1, 3, 5, 6 and 8. For each time point, at least 3 individuals were sampled for each group. The brain, optic lobe, liver, intestine, gill, stomach, pancreas, and ovary were dissected and stored at −80℃ for RNA extraction.
2.6 V. harveyi InfectionV. harveyi were separated and identified from sick S. japonica naturally infected with bacterial diseases on Xixuan Island (Zhoushan) (Zhou et al., 2023). They were cultured in LB medium and incubated at 28℃ overnight. 3-day posthatching cuttlefish juveniles were co-cultured with V. harveyi at a concentration of 2×107 CFU mL−1 in the artificial seawater. A group of cuttlefish juveniles without bacterial infection were set up as the control. Cuttlefish were randomly sampled at 3, 6, 12, 24, 48 and 72 h post treatment. Three independent duplications were conducted for each time point, and two individuals were grouped in each duplication. Total RNA was extracted from the whole juveniles and applied for qPCR analysis.
2.7 Bioinformatics Analysis of Sj_QRFPLRTo confirm the identity of Sj_QRFPLR cDNA sequnence, the ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/) and the BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi) were applied for predicting and checking the ORF of Sj_QRFPLR cDNA, respectively. Signal peptide was predicted by the online tool signal 6.0 (https://services.healthtech.dtu.dk/services/SignalP-6.0/). Online software NetNGly 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/) was used to predict glycosylation sites. ExpasyProtParam (http://web.expasy.org/protparam/) was used to predict the molecular weight (MW) and isoelectric point (pI). The online tools DeepTMHMM (1.0.24) (https://dtu.biolib.com/DeepTMHMM) and PROSITE (https://prosite.expasy.org/) were used to predict the transmembrane domains and the disulfide bond formation sites, respectively. The three-dimensional (3D) structure of Sj_QRFPLR was deduced using SWISS-MODEL (https://swissmodel.expasy.org/). DNAMAN (9.0.116) (Lynnon biosoft, San Ramon, CA, United states) was used to do multiple alignments and homologous analysis. The Bayesian phylogenetic tree (JTT+I+G model) was conducted in the software PhyloSuite v1.2.2 (Zhang et al., 2020) and edited using iTOL (https://itol.embl.de/).
2.8 Statistical AnalysisStatistical analysis was performed by GraphPad Prism 9.0 software (GraphPad Software, Fl, Boston, MA, USA). Difference between experimental groups were analyzed using unpaired two-tailed t-test. Values are shown as means ± SD/SEM. Statistical significances were accepted at P < 0.05.
3 Results 3.1 Cloning and Characterization of Sj_QRFPLR Sequences with Full-LengthOne putative S. japonica QRFP-like peptide receptor precursor gene was identified in silico from transcriptome data and cloned from cuttlefish brain cDNA. The nucleotide and deduced amino acid sequences of Sj_QRFPLR (GenBank number OR065176) are shown in Fig.1. The full-length of Sj_QRFPLR was 1284 bp, containing 75 bp 5'-untranslated region (UTR), 153 bp 3'-UTR, and 1056 bp ORF encoding 351 amino acids. No signal peptide was predicted. The MW and pI of Sj_QRFPLR were 40.5 kDa and 9.63, respectively. Two Cys (C) residues that are likely to form the disulfide bridge were predicted. Seven transmembrane domains (TMDs) were detected in Sj_QRFPLR by bioinformatics analysis (Fig.1).
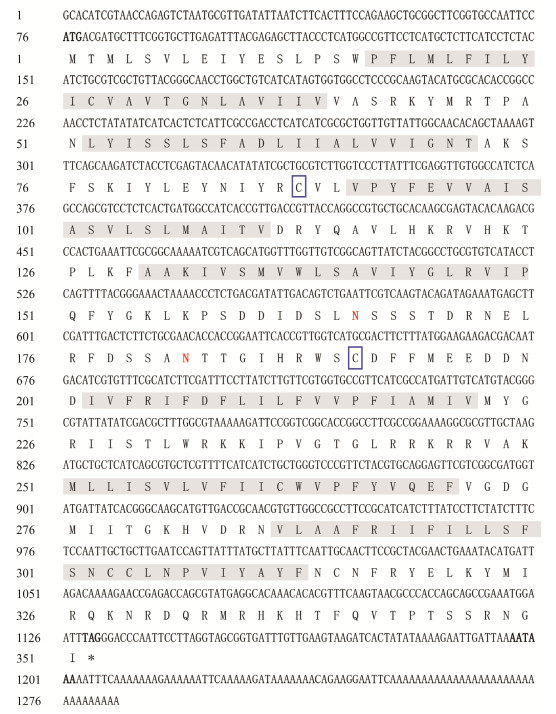
|
Fig. 1 The nucleotide and deduced amino acid sequences of Sj_QRFPLR. Seven putative transmembrane domains (TMDs) are shaded in gray. Two Cys (C) residues that are likely to form the disulfide bridge are boxed by blue frames. Red highlights the expected glycosylation sites (N). The start codon (ATG), termination codon (TAG), and polyadenylation signal (AATAAA) are bolded. Asterisk denotes the stop codon. |
To confirm the identity and characterize the primary structure of Sj_QRFPLR, multiple alignments were performed (Fig.2). Overall, Sj_QRFPLR exhibited an amino acid identity to the verified vertebrate QRFPRs and amphioxus QRFPR, with the highest sequence homology to amphioxus QRFPR (22%) and the lowest to chicken QRFPR (17%) (Table 2). Next, the primary structure of Sj_QRFPLR was examined. Seven TMDs of Sj_QRFPLR are conserved and highly overlapped with those of chordate species (Fig.2).
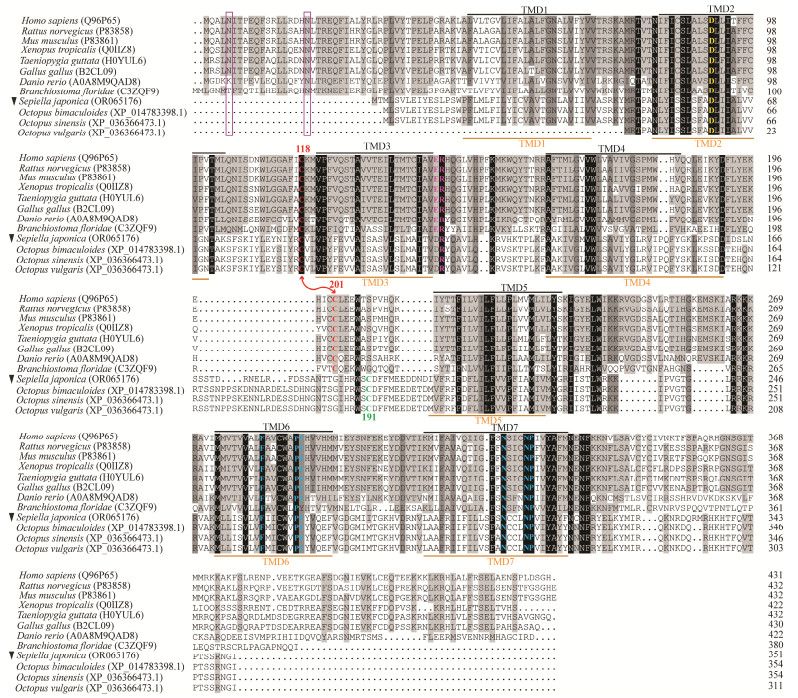
|
Fig. 2 Multiple alignments of QRFPLR amino acid sequences. Amino acid sequence alignments of Sj_QRFPLR (indicated by black inverted triangle) and QRFPR from different species. Fully conserved and highly conserved residues are shaded in black and dark gray, respectively. Identity with at least one of the twelve sequences is indicated by light grey shading. Seven TMDs of vertebrate QRFPRs and Sj_QRFPLR are overlined in black and underlined in orange, respectively. The Asp (D) residue in TMD2, which implicated in G protein coupling, is highlighted in yellow. Two Cys (C) residues situated in the first and second extracellular loops are highlighted by red letters, respectively. The disulfide bridge between two C is marked by red lines. The conserved Arg (R) residue in the second intracellular loop, and the conserved Phe (F), Pro (P), and Asn (N) residues in TMD6 and TMD7 are represented by colored letters. Two N-linked glycosylation sites in the N-terminus of chordates QRFPR are boxed by purple frames. Representative QRFPR sequences from different species are downloaded from Uniprot (https://www.uniprot.org/). |
|
|
Table 2 Amino acids identities of QRFPR among different species |
Meanwhile, Sj_QRFPLR displays typical characteristic features of class A GPCRs. For example, there is a disulfide bridge between the two Cys (C) residues located in the first and second extracellular loops (EL); there is an Asp (D) residue within TMD2; and three residues that are crucial for receptor activation are conserved within TMD6 and TMD7, i.e., Phe (F), Pro (P) and Asn (N) (Fig.2). Compared with chordates QRFPR, an N-terminus extension that harbors one/two N-linked glycosylation sites were not detected in Sj_QRFPLR (Fig.2).
To briefly compare QRFPR-like peptide receptors in cephalopods, available sequences in silico identified from three octopuses were aligned with Sj_QRFPLR. Sj_QRFPLR exhibited 88%, 86% and 76% sequence identities with the genes in Octopus sinensis, O. bimaculoides, and O. vulgaris respectively (Table 2). Meanwhile, the structural features of class A GPCRs that Sj_QRFPLR possess were fully conserved in the three sequences except TMD1 was not identified in O. vulgaris. In addition, the N-terminus extension was not detected in them (Fig.2).
3.3 Phylogenetic AnalysisTo further determine the identity of Sj_QRFPLR, a Bayesian phylogenetic tree was constructed. Amino acids from the whole group Ⅰ members in the class A GPCR superfamily were downloaded and included. Here, amphioxus KissR1 was used to root the tree since it is the only member of the RFamide family located in group Ⅱ of class A GPCRs. As shown in Fig.3, different members of class A GPCRs were distinctly separated. Sj_QRFPLR (highlighted in red) together with other three cephalopods QRFP-like peptide receptors were located in QRFPR group. Within the QRFPR family, four groups were clearly detected in vertebrate QRFPRs. Four non-chordates QRFP-like peptide receptors (including Sj_QRFPLR) were first clustered with amphioxus QRFPR, and further grouped with vertebrate QRFPRs.
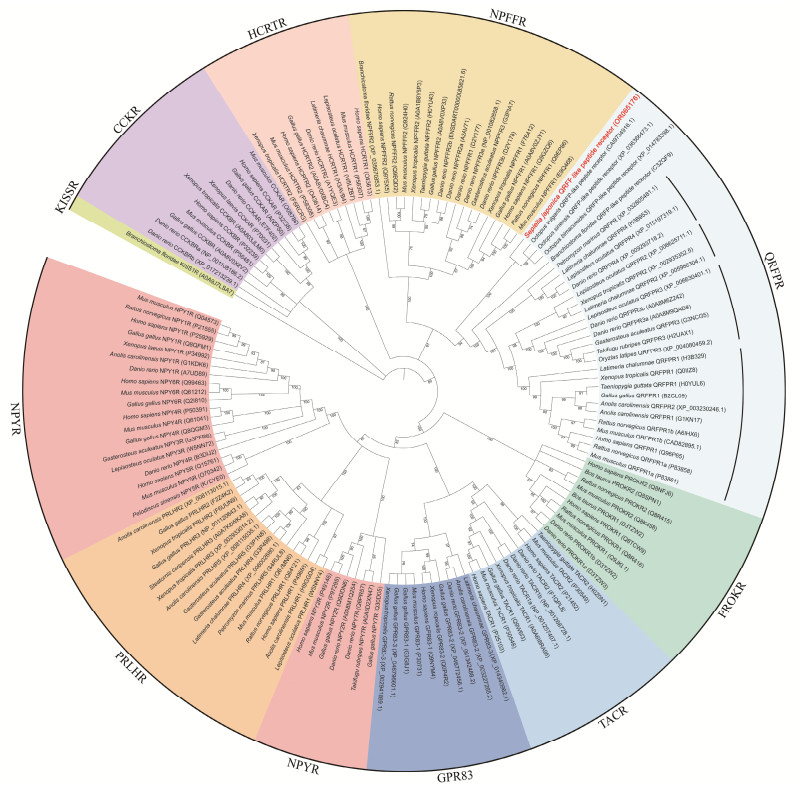
|
Fig. 3 Bayesian phylogenetic tree of the class A GPCR superfamily. Amphioxus KISSR1 is used to root the tree. Repre-sentative sequences of various class A GPCR members from different species are downloaded from Uniprot (https://www.uniprot.org/) and NCBI (https://www.ncbi.nlm.nih.gov). Sj_QRFPLR is highlighted by red in the QRFPR group. |
GPCRs are a group of proteins located in the cell membrane. To study the localization of Sj_QRFPLR in the cell, an in vitro subcellular localization assay was conducted. Sj_QRFPLR, along with a fluorescent marker EGFP (Sj_QRFPLR-EGFP), was expressed in HEK 293 cells. As shown in Fig.4, Sj_QRFPLR showed clearly cell membrane localization, suggesting that Sj_QRFPLR is a cell membrane receptor.
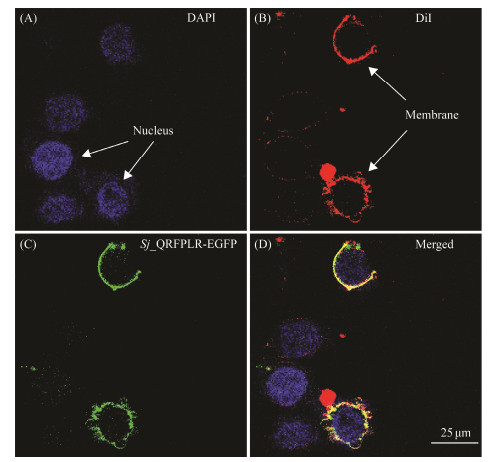
|
Fig. 4 In vitro subcellular localization of Sj_QRFPLR in HEK293 cells. Arrowheads in different panels mark the nucleus (visualized by DAPI staining, blue in A) and cytomembrane (visualized by DiI staining, red in B), respectively. |
The tissue expression profile of Sj_QRFPLR was performed by RT-PCR. As shown in Figs.5A and B, Sj_QRFPLR mRNA was detected in thirteen and twelve different tissues in female and male cuttlefish, respectively. In females, Sj_QRFPLR mRNA was highly expressed in the optic lobe, gill, liver, muscle, skin, ovary, as well as the accessary nidamental gland (Fig.5A). In males, Sj_QRFPLR mRNA was primarily detected in the brain, gill, intestine, muscle, skin, heart, testis, and spermatophore (Fig.5B).
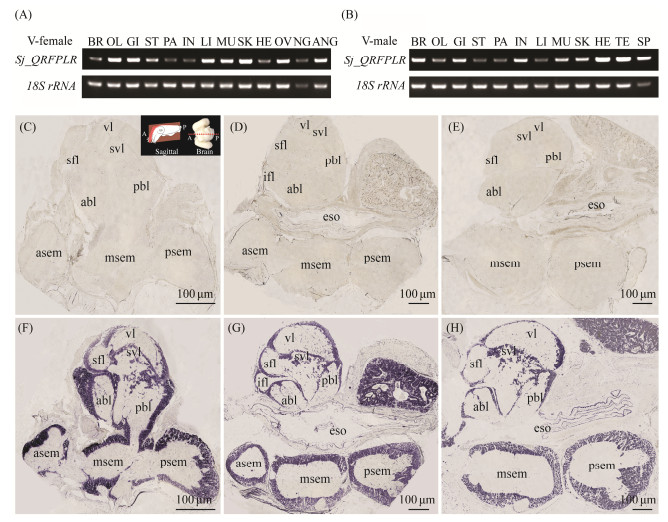
|
Fig. 5 Tissue distribution pattern of Sj_QRFPLR in cuttlefish. (A, B) RT-PCR results of various tissues in female (A) and male (B) cuttlefish. BR, brain; OL, optic lobe; GI, gill; ST, stomach; PA, pancreas; IN, intestine; LI, liver; MU, muscle; SK, skin; HE, heart; OV, ovary; NG, nidamental gland; ANG, accessory nidamental gland; TE, testis; SP, spermatophore. (C – H) In situ hybridization analysis of Sj_QRFPLR mRNA in cuttlefish brain at stages Ⅰ – Ⅱ (C, F), Ⅳ (D, G), and Ⅴ (E, H). The cuttlefish brain was sectioned in the sagittal plane. A, anterior; P, posterior. The schematic of the section orientation was modified from Montague et al. (2023). The brain sections were stained with Sj_QRFPLR sense probe (C – E) and antisense probe (F – H), respectively. abl, anterior basal lobe; asem, anterior suboesophageal mass; eso, esophagus; ifl, inferior frontal lobe; msem, middle suboesophageal mass; psem, posterior suboesophageal mass; sfl, superior frontal lobe; svl, subvertical lobe; vl, vertical lobe; pbl, posterior basal lobe. |
The mRNA localization of Sj_QRFPLR was further characterized in female cuttlefish brain at stage Ⅰ – Ⅱ to Ⅴ by in situ hybridization. As a neuropeptide receptor, Sj_QRFPLR mRNA was ubiquitously distributed in both supraesophageal mass and suboesophageal mass of cuttlefish brain at all stages, and the distribution pattern at stages Ⅳ and Ⅴ are more identical, as shown in Fig.5D. In the supraesophageal mass, strong signals were observed in the function lobes of the vertical lobe complex, including the vertical lobe (vl), the subvertical lobe (svl), the anterior basal lobe (abl), the posterior basal lobe (pbl), the superior frontal lobe (sfl), and the inferior frontal lobe (ifl) (Figs.5F – H). In the suboesophageal mass, positive signals were evident in almost all regions, including peripheral cells of the anterior suboesophageal mass (asem), the middle suboesophageal mass (msem), and the posterior suboesophageal mass (psem) (Figs.5F – H). Moreover, a strong staining was detected in an area that has not been reported yet, as shown in Figs. 5G and H.
3.6 Sj_QRFPLR Expression Levels After Food Deprivation and RefeedingThe QRFP/QRFPR system sparked initial interest partially due to its resemblance to the orexin system. To explore the putative function of Sj_QRFPLR in feeding behavior, a food deprivation and refeeding experiment was carried out (Fig.6A). Sj_QRFPLR mRNA was greatly elevated in the brain five days after food deprivation, and it was significantly restored to the same level as in control group once feeding resumed (Fig.6B). Meanwhile, the mRNA expression levels of Sj_QRFPLR in the liver (Fig.6C) and gill (Fig. 6D) were significantly enhanced on day one at the food deprivation stage, and subsequent starvation or refeeding seems to have little effect on it (Figs.6C – D). In contrast, food deprivation or refeeding had no effect on Sj_QRFPLR mRNA expression levels in the optic lobe, intestine, stomach, pancreas, and ovary (Figs.6E – I).
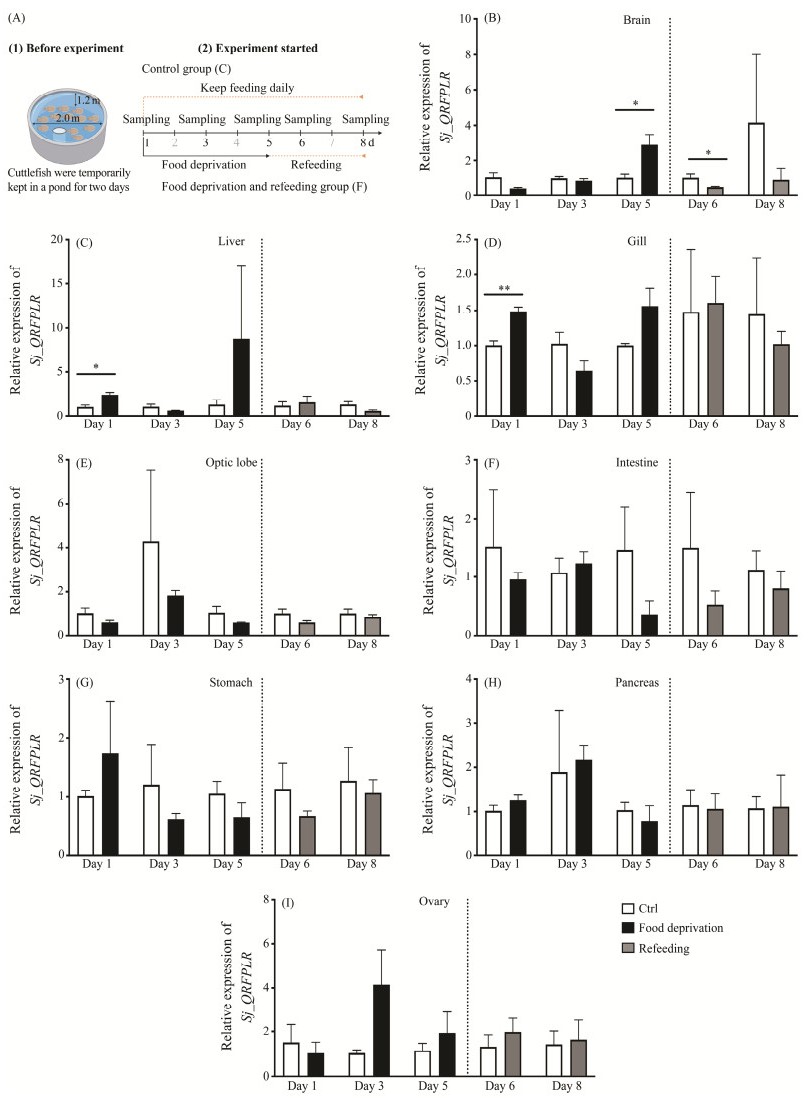
|
Fig. 6 Time-course mRNA expression of Sj_QRFPLR in different tissues after food deprivation and refeeding. (A) Schematic of the experimental design for food deprivation and refeeding. (B – I) The expression level of Sj_QRFPLR mRNA in cuttlefish brain (B), liver (C), gill (D), optic lobe (E), intestine (F), stomach (G), pancreas (H), and ovary (I) after food deprivation and feeding resumed. The expression level of Sj_QRFPLR mRNA was normalized by β-actin and GAPDH. Data shown are from three individuals. Values are shown as means ± SD. * P < 0.05, ** P < 0.01. |
Next, a V. harveyi infection was conducted to investigate a possible role of Sj_QRFPLR in immune response in cuttlefish. As shown in Fig.7, 72 h treatment has dramatically boosted the expression level of Sj_QRFPLR mRNA in cuttlefish juveniles.
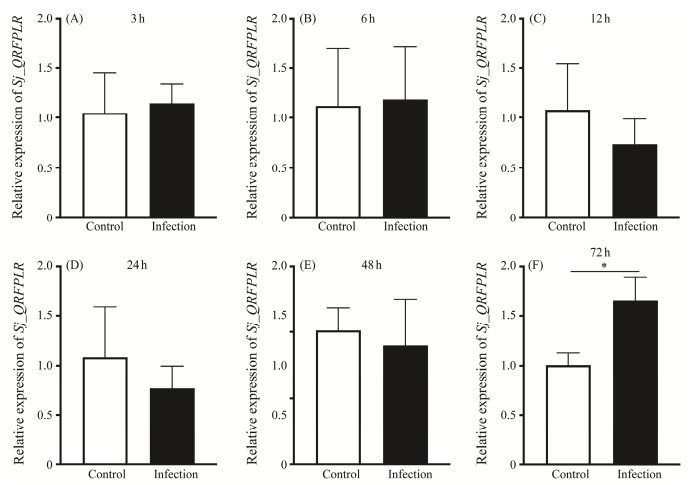
|
Fig. 7 Relative mRNA expression of Sj_QRFPLR after V. harveyi infection. (A – F) Relative expression levels of Sj_QRFPLR at 3 h (A), 6 h (B), 12 h (C), 24 h (D), 48 h (E) and 72 h (F) after V. harveyi infection. The expression level of Sj_QRFPLR mRNA was normalized by β-actin and GAPDH. Data are from three independent experiments. Values are shown as means ± SD. * P < 0.05. |
The novel identified receptor, QRFPR is the endogenous receptor for the neuropeptide QRFP that is a member of RFamide neuropeptide family. QRFPR and its ligand have been studied for more than two decades; however, the knowledge is limited in vertebrates and amphioxus. Therefore, the identification, characterization and functional analysis of QRFP-like receptor gene in cuttlefish are helpful to understand the function of QRFPR in cephalopod, and further understand the peptidergic regulation of QRFP/QRFPR system in invertebrates.
4.1 Sj_QRFPLR Displays Typical Characteristic Features of Class A GPCRsIn this study, a non-chordates QRFP-like peptide receptor gene was identified and cloned from cuttlefish S. japonica. QRFPR belongs to class A GPCRs family including seven transmembrane proteins located in the cell membrane (Leprince et al., 2017; Yang et al., 2021). Sj_QRFPLR displays seven TMDs that are highly overlapping with those of mammals, frog, bird, chicken and fish. In vitro subcellular localization analysis supports the fact that Sj_QRFPLR is a cell membrane receptor.
Furthermore, although only 17% – 22% sequence identities are shared between Sj_QRFPLR and verified vertebrates as well as amphioxus QRFPR, the structural hallmarks of class A GPCRs are conserved in Sj_QRFPLR. For example, a disulfide bridge between the two C residues located in the EL1 and EL2. Interestingly, the first C located in EL1 (C118 in vertebrates QRFPR, which corresponding to C88 in Sj_QRFPLR) is conserved, whereas the second C located in EL2 (C201 in vertebrates QRFPR but C191 in Sj_QRFPLR) shows six amino acids shift in cuttlefish. Notably, the second C predicted in vertebrates QRFPR in this paper is C201, while it is C200 (the C in front of C201 in our analysis) in the review paper published by Ukena et al. (2014). A possible explanation for this inconsistency is that the software and/or algorithm used may be different. Additionally, a D residue within TMD2 that seems to be essential for G protein coupling is conserved, and three residues that are crucial for receptor activation are conserved within TMD6 and TMD7, i.e., F, P and N. Although the conserved Glu (E)-Arg (R) doublet sequence at the N-terminus of IL2 in vertebrates QRFPR is replaced by D-R in Sj_QRFPLR, the R is conserved. Whether this replacement is crucial for the Sj_QRFPLR function needs to be further studied. Thus, like QRFPR in vertebrates, Sj_QRFPLR displays typical characteristic features of class A GPCRs.
Compared with chordates QRFPR, Sj_QRFPLR shared a relatively high degree of identity with other three cephalopods sequences. In contrast, the lowest identity (75%) was observed between Sj_QRFPLR and O. vulgaris QRFP-like peptide receptor, because the latter lacks TMD1. Among cephalopod species, the structural features of class A GPCRs that Sj_QRFPLR possess were fully conserved in the rest three sequences except for the one in O. vulgaris lacking of TMD1, suggesting that this receptor is likely to be structurally conserved in cephalopod species. Notably, unlike the chordates QRFPR, four non-chordates QRFPR-like peptide receptors do not show a N-terminus extension. One reason might be that in cephalopod species analyzed, no extra N-terminus extension exists in these receptors. Whether it exists in other invertebrates or not still needs to be further studied. Another reason might be that the N-terminus of this receptor failed to be identified in these species. Further genome searches are necessary to answer this question.
4.2 Sj_QRFPLR Belongs to QRFPR FamilyTo further confirm the identity of Sj_QRFPLR, a phylogenetic analysis was performed. According to the previous study, class A GPCR superfamily is divided into five groups (Ⅰ – Ⅴ). Group Ⅰ contains nine members, QRFPR, NPYR, PRLHR, PROKR, TACR, NPFFR, HCRTR, CCKR, and GPR83, which is supported by the Bayesian analysis (Yun et al., 2015). Previous study on amphioxus QRFPR detected four major clades (Larhammar et al., 2014). Consistently, four groups of vertebrates QRFPR were detected in the QRFPR branch in our study. Meanwhile, Sj_QRFPLR together with other three cephalopods QRFP-like peptide receptors were clearly grouped to QRFPR family. Compared with invertebrates QRFPR, as with amphioxus QRFPR, four non-chordates QRFP-like peptide receptors are located in a relatively basal position, indicating a relatively close relationship among invertebrates QRFPR, which supported the results of multiple alignments analysis. Considering these results, Sj_QRFPLR obtained belongs QRFPR family.
4.3 Wide Distribution of Sj_QRFPLR in Cuttlefish Implies Its Functional DiversityPrevious studies reported that QRFPR was highly distributed in the brain and could be widely detected in multiple peripheral tissues of mammals and non-mammalian vertebrates (Lee et al., 2001; Fukusumi et al., 2003; Jiang et al., 2003; Baribault et al., 2006; Kampe et al., 2006; Takayasu et al., 2006; Bruzzone et al., 2007; Chen et al., 2016). In this study, Sj_QRFPLR mRNA is highly detected in cuttlefish brain. Cuttlefish brain is composed of the supraesophageal mass, the suboesophageal mass, and the optic lobe (Boycott, 1961; Shigeno and Yamamoto, 2002; Montague et al., 2023). Each mass is subdivided into several different lobe complexes or regions with different functions (Boycott, 1961; Shigeno and Yamamoto, 2002; Montague et al., 2023). The supraesophageal mass is consist of vertical lobe complex and basal lobe complex (Montague et al., 2023). The suboesophageal mass is divided into three regions, including asem, msem and psem (Boycott, 1961; Shigeno and Yamamoto, 2002). In situ hybridization results reveal wide distribution of Sj_QRFPLR mRNA in the vertical lobe complex of the supraesophageal mass, where the functional lobes form this complex have been reported to be involved in learning and memory (Boycott, 1961; Montague et al., 2023). Similarly, Sj_QRFPLR mRNA is strongly expressed in all peripheral cells of all three regions of the suboesophageal mass. The asem (also described as brachial lobe) is a part of brachial lobe complex and is responsible for the motor control of arms and feeding, especially for the biting movements of the buccal mass (Montague et al., 2023). The rest msem and psem were documented to be implicated in ⅰ) intermediate and lower motor control of movement, like movement of arms, tentacles, funnels, and eyes, and control of chromatophores and papillae on the head and arms), ⅱ) giant fiber response (escape movements), and ⅲ) lower motor control of locomotion, like control of escape movements, ink ejection, chromatophores and papillae on mantle (Boycott, 1961; Montague et al., 2023). Additionally, a strong staining region next to the supraesophageal mass was observed. However, no such a region has been reported in cuttlefish (Boycott, 1961; Shigeno and Yamamoto, 2002; Chung et al., 2023; Montague et al., 2023). Therefore, it's difficult to name it at this point. Future research on the fine structure of cuttlefish and even cephalopod brains will help to confirm the identity.
Sj_QRFPLR mRNA is also detected in various peripheral tissues in both male and female cuttlefish, which is similar with the widespread pattern of QRFPR in vertebrate species. Interestingly, RT-PCR performed in various tissues showed a tissue-dependent variation in Sj_QRFPLR expression levels between males and females. Further studies are needed to elucidate the meaningfulness underneath the variations. Sj_QRFPLR mRNA is generally highly expressed in the optic lobe, gill, liver, muscle, skin, and gonads. Those evidences suggest that, as with vertebrates QRFPR, Sj_QRFPLR seems to function in diverse physiological processes, such as learning and memory, chromatophore activity, locomotor activity, feeding, energy homeostasis, and reproduction regulation.
4.4 Sj_QRFPLR is Likely to Stimulate Food Intake in CuttlefishPrevious study on mice indicated that, intracerebroventricular administration of 26RFa after 18 h food deprived lead to a dose-dependent increase in food intake, indicating that 26RFa possesses orexigenic properties (Chartrel et al., 2003). Subsequently, research reported that QRFP exerts its orexigenic impact through activating QRFPR, which is partially mediated by orexigenic molecule NPY (Takayasu et al., 2006; Lectez et al., 2009; Georgsson et al., 2014). Results from our RT-PCR revealed a strong expression of Sj_QRFPLR in the asem of cuttlefish brain, suggesting a possible role of Sj_QRFPLR in controlling feeding activity. This idea was tested by a food deprivation and refeeding experiment. The qPCR data show that, five-day food deprivation significantly elevated the mRNA level of Sj_QRFPLR in cuttlefish brain. This result is consistent with previous study showing that four-day food deprivation prominently upregulated hypothalamic QRFP level in goldfish (Liu et al., 2009). Following that, Sj_QRFPLR mRNA is immediately restored to the control group level at the first day after food resumed. Given those findings and the fact that QRFPR is a receptor for a peptide with orexigenic activity (Chartrel et al., 2003), Sj_QRFPLR is expected to have an evolutionary ancient role in stimulating food intake in cuttlefish. Is this ancient action of Sj_QRFPLR mediated by QRFP-like peptide? Future studies on genome analysis and/or in-depth in silico searches for S. japonica transcriptome data in combination with more molecular assays will be helpful to answer the question.
It is interesting to note that the expression of Sj_QRFPLR mRNA was significantly upregulated in the liver and gill on the first day of food restriction, while no upregulation was observed in subsequent days. Since these two organs are sensitive to external conditions, it is possible that Sj_QRFPLR in them is the 'rapid responder' with the abrupt food shortage. The liver serves as a hub for organizing glucose metabolism and energy regulation (Wu et al., 2010; Mitra and Metcalf, 2012). For cuttlefish, the gill is crucial for respiration and blood circulation (Dong, 1987). The fast reaction of Sj_QRFPLR mRNA levels in the liver and gill raises the possibility that cuttlefish maintains its energy supply during short-term food shortages by coordinating and balancing energy metabolism, respiration, and blood circulation. Further research on other species is required to examine this possibility.
4.5 A Possible Link Between Sj_QRFPLR and Immune Response in CuttlefishAlthough multiple functions were documented for QRFP-QRFPR system, no immune response was reported. To identify the potential role of Sj_QRFPLR in cuttlefish immune response, a V. harveyi infection was set up. The results show that Sj_QRFPLR mRNA expression levels are much higher in juvenile cuttlefish 72 hours after infection, pointing to a possible link between Sj_QRFPLR and cuttlefish immune response. However, a late increase in receptor expression after infection might be caused by other physiological changes, which needs to be further validated.
5 ConclusionsIn summary, the first non-chordates QRFP-like peptide receptor gene was identified and characterized in the cephalopod S. japonica. Evidence from multiple alignments, phylogenetic analysis, and in vitro subcellular localization analysis indicated that Sj_QRFPLR is a class A GPCR and it belongs to the QRFPR family. Meanwhile, QRFPR is likely to be structurally conserved in cephalopod species. In situ hybridization and RT-PCR data revealed a widespread distribution pattern of Sj_QRFPLR in multiple function lobes of female brain and numerous peripheral tissues in both male and female cuttlefish, suggesting a functional diversity of Sj_QRFPLR. Functional analysis revealed that Sj_QRFPLR is likely to stimulate food intake in cuttlefish. Additionally, a possible link between Sj_QRFPLR and immune response is suggested in cuttlefish.
AcknowledgementsThe current study was supported by the National Natural Science Foundation of China (No. 31872547), the Natural Science Foundation of Zhejiang Province, China (No. LTGN24C190015), and the Excellent Postdoctoral Program of Jiangsu Province (No. 314865). We thank Dr. Tianming Wang for providing us the cells as a gift. We also thank Mr. Huilai Shi and Mr. Hongling Ping for their help in providing cuttlefish.
Author Contributions
Conceptualization, Changfeng Chi; methodology, Shuang Li, Qing Hao and Jiayin Qiu; investigation, Shuang Li, Qing Hao and Jiayin Qiu; software, Shuang Li, Qing Hao and Jiayin Qiu; writing-original draft preparation, Shuang Li; writing-review and editing, Shuang Li, Xu Zhou, Libing Zheng and Changfeng Chi; funding support, Xu Zhou, Libing Zheng and Changfeng Chi; supervision, Libing Zheng and Changfeng Chi. All authors approved the final version of the manuscript.
Data Availability
All data generated and analyzed during this study are included in this published article and its additional files.
Declarations
Ethics Approval and Consent to Participate
The experiments were conducted according to the guidelines approved by the ethics committee of Zhejiang Ocean University (permit number 2021031) and the Academy of Experimental Animal Center of Zhejiang Ocean University.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests.
Alexander, S. P., Davenport, A. P., Kelly, E., Marrion, N., Peters, J. A., Benson, H. E., et al., 2015. The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. British Journal of Pharmacology, 172: 5744-5869. DOI:10.1111/bph.13348 (  0) 0) |
Ayachi, S., and Simonin, F., 2014. Involvement of mammalian RF-amide peptides and their receptors in the modulation of nociception in rodents. Frontiers in Endocrinology, 5: 158. DOI:10.3389/fendo.2014.00158 (  0) 0) |
Baribault, H., Danao, J., Gupte, J., Yang, L., Sun, B., Richards, W., et al., 2006. The G-protein-coupled receptor GPR103 regulates bone formation. Molecular and Cellular Biology, 26(2): 709-717. DOI:10.1128/MCB.26.2.709-717 (  0) 0) |
Bonner, T., Carlebur, M., Davenport, A., Foord, S., and Maguire, J., 2016. QRFP receptor. IUPHAR/BPS Guide to Pharmacology. Available at: http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward.
(  0) 0) |
Boycott, B. B., 1961. The functional organization of the brain of the cuttlefish Sepia officinalis. Proceedings of the Royal Society B: Biological Sciences, 153: 503-534. DOI:10.1098/rspb.1961.0015 (  0) 0) |
Bruzzone, F., Lectez, B., Alexandre, D., Jégou, S., Mounien, L., Tollemer, H., et al., 2007. Distribution of 26RFa binding sites and GPR103 mRNA in the central nervous system of the rat. Journal of Comparative Neurology, 503: 573-591. DOI:10.1002/cne.21400 (  0) 0) |
Bruzzone, F., Lectez, B., Tollemer, H., Leprince, J., Dujardin, C., Rachidi, W., et al., 2006. Anatomical distribution and biochemical characterization of the novel RFamide peptide 26RFa in the human hypothalamus and spinal cord. Journal of Neurochemistry, 99: 616-627. DOI:10.1111/j.1471-4159.2006.04090.x (  0) 0) |
Chartrel, N., Dujardin, C., Anouar, Y., Leprince, J., Decker, A., Clerens, S., et al., 2003. Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proceedings of the National Academy of Sciences, 100: 15247-15252. DOI:10.1073/pnas.2434676100 (  0) 0) |
Chen, A., Chiu, C. N., Mosser, E. A., Khan, S., Spence, R., and Prober, D. A., 2016. QRFP and its receptors regulate locomotor activity and sleep in zebrafish. Journal of Neuroscience, 36: 1823-1840. DOI:10.1523/JNEUROSCI.2579-15.2016 (  0) 0) |
Chung, W. S., López-Galán, A., Kurniawan, N. D., and Marshall, N. J., 2023. The brain structure and the neural network features of the diurnal cuttlefish Sepia plangon. Iscience, 26: 1. DOI:10.1016/j.isci.2022.105846 (  0) 0) |
Dong, Z., 1987. Fauna Sinica, Phylum Mollusca, Class Cephalopoda. Science Press, Beijing, 201pp.
(  0) 0) |
Fukusumi, S., Yoshida, H., Fujii, R., Maruyama, M., Komatsu, H., Habata, Y., et al., 2003. A new peptidic ligand and its receptor regulating adrenal function in rats. Journal of Biological Chemistry, 278: 46387-46395. DOI:10.1016/s0378-1119(01)00651-5 (  0) 0) |
Georgsson, J., BergströM, F., Nordqvist, A., Watson, M. J., Blundell, C. D., Johansson, M. J., et al., 2014. GPR103 antagonists demonstrating anorexigenic activity in vivo: Design and development of pyrrolo [2,3-c] pyridines that mimic the C-terminal Arg-Phe motif of QRFP26. Journal of Medicinal Chemistry, 57: 5935-5948. DOI:10.1021/jm401951t (  0) 0) |
Jiang, X., Fu, F. Y., Li, Z., and Feng, X. D., 2007. Study on the oogenesis and ovarial development of Sepiella maindroni. Journal of Fisheries of China, 5: 607-617. DOI:10.3321/j.issn:1000-0615.2007.05.007 (  0) 0) |
Jiang, Y., Luo, L., Gustafson, E. L., Yadav, D., Laverty, M., Murgolo, N., et al., 2003. Identification and characterization of a novel RF-amide peptide ligand for orphan G-protein-coupled receptor SP9155. Journal of Biological Chemistry, 278: 27652-27657. DOI:10.1074/jbc.M302945200 (  0) 0) |
Kampe, J., Wiedmer, P., Pfluger, P., Castaneda, T., Burget, L., Mondala, H., et al., 2006. Effect of central administration of QRFP (26) peptide on energy balance and characterization of a second QRFP receptor in rat. Brain Research, 1119: 133-149. DOI:10.1016/j.brainres.2006.08.055 (  0) 0) |
Larhammar, D., Xu, B., and Bergqvist, C. A., 2014. Unexpected multiplicity of QRFP receptors in early vertebrate evolution. Frontiers in Neuroscience, 8: 337. DOI:10.3389/fnins.2014.00337 (  0) 0) |
Lectez, B., Jeandel, L., El-Yamani, F., Arthaud, S., Alexandre, D., Mardargent, A., et al., 2009. The orexigenic activity of the hypothalamic neuropeptide 26RFa is mediated by the neuropeptide Y and proopiomelanocortin neurons of the arcuate nucleus. Endocrinology, 150: 2342-2350. DOI:10.1210/en.2008-1432 (  0) 0) |
Lee, D. K., Nguyen, T., Lynch, K. R., Cheng, R., Vanti, W. B., Arkhitko, O., et al., 2001. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene, 275: 83-91. DOI:10.1016/s0378-1119(01)00651-5 (  0) 0) |
Leprince, J., Bagnol, D., Bureau, R., Fukusumi, S., Granata, R., Hinuma, S., et al., 2017. The Arg-Phe‐amide peptide 26RFa/glutamine RF‐amide peptide and its receptor: IUPHAR Review 24. British Journal of Pharmacology, 174: 3573-3607. DOI:10.1111/bph.13907 (  0) 0) |
Li, Y., Cao, Z., Li, H., Liu, H., Lü, Z., and Chi, C., 2018. Identification, characterization, and expression analysis of a FMR-Famide-like peptide gene in the common chinese cuttlefish (Sepiella japonica). Molecules, 23: 742. DOI:10.3390/molecules23040742 (  0) 0) |
Liu, Y., Zhang, Y., Li, S., Huang, W., Liu, X., Lu, D., et al., 2009. Molecular cloning and functional characterization of the first non-mammalian 26RFa/QRFP orthologue in goldfish, Carassius auratus. Molecular and Cellular Endocrinology, 303: 82-90. DOI:10.1016/j.mce.2009.01.009 (  0) 0) |
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25: 402-408. DOI:10.1006/meth.2001.1262 (  0) 0) |
Lü, Z., Liu, W., Liu, L., Shi, H., Ping, H., Wang, T., et al., 2016. De novo assembly and comparison of the ovarian transcriptomes of the common Chinese cuttlefish (Sepiella japonica) with different gonadal development. Genomics Data, 7: 155-158. DOI:10.1016/j.gdata.2015.12.011 (  0) 0) |
Luo, J., Jiang, X., Liu, M., Tang, F., and Peng, R., 2014. Oogenesis and ovarian development in Sepia lycidas. Acta Hydrobiologica Sinica, 38: 1107-1116. DOI:10.7541/2014.162 (  0) 0) |
Mirabeau, O., and Joly, J. S., 2013. Molecular evolution of peptidergic signaling systems in bilaterians. Proceedings of the National Academy of Sciences, 110: E2028-E2037. DOI:10.1073/pnas.1219956110 (  0) 0) |
Mitra, V., and Metcalf, J., 2012. Metabolic functions of the liver. Anaesth. Intensive Care Medicine, 13: 54-55. DOI:10.1016/j.mpaic.2011.11.006 (  0) 0) |
Montague, T. G., Rieth, I. J., Gjerswold-Selleck, S., Garcia-Rosales, D., Aneja, S., Elkis, D., et al., 2023. A brain atlas for the camouflaging dwarf cuttlefish, Sepia bandensis. Current Biology, 33: 2794-2801. DOI:10.1016/j.cub.2023.06.007 (  0) 0) |
Patel, S. R., Murphy, K. G., Thompson, E. L., Patterson, M., Curtis, A. E., Ghatei, M. A., et al., 2008. Pyroglutamylated Rfamide peptide 43 stimulates the hypothalamic-pituitary-gonadal axis via gonadotropin-releasing hormone in rats. Endocrinology, 149: 4747-4754. DOI:10.1210/en.2007-1562 (  0) 0) |
Polese, G., Bertapelle, C., and Di Cosmo, A., 2015. Role of olfaction in Octopus vulgaris reproduction. General and Comparative Endocrinology, 210: 55-62. DOI:10.1016/j.ygcen.2014.10.006 (  0) 0) |
Prevost, G., Jeandel, L., Arabo, A., Coëffier, M., El Ouahli, M., Picot, M., et al., 2015. Hypothalamic neuropeptide 26RFa acts as an incretin to regulate glucose homeostasis. Diabetes, 64: 2805-2816. DOI:10.2337/db14-1864 (  0) 0) |
Qiu, J. Y., Zheng, L. B., and Chi, C. F., 2022. Identification, characterization, and expression of a PRQFVamide-related peptide in cephalopod Sepiella japonica. Frontiers in Marine Science, 9: 805209. DOI:10.3389/fmars.2022.805209 (  0) 0) |
Ramanjaneya, M., Karteris, E., Chen, J., Rucinski, M., Ziolkowska, A., Ahmed, N., et al., 2013. QRFP induces aldosterone production via PKC and T-type calcium channel-mediated pathways in human adrenocortical cells: Evidence for a novel role of GPR103. American Journal of Physiology-Endocrinology and Metabolism, 305: E1049-E1058. DOI:10.1152/ajpendo.00191.2013 (  0) 0) |
Shigeno, S., and Yamamoto, M., 2002. Organization of the nervous system in the pygmy cuttlefish, Idiosepius paradoxus Ortmann (Idiosepiidae, Cephalopoda). Journal of Morphology, 254: 65-80. DOI:10.1002/jmor.10020 (  0) 0) |
Song, C. P., Sun, L. L., Zheng, L. B., and Chi, C. F., 2021. Gonadotropin-releasing hormone-like gene in the cephalopod, Sepia pharaonis: Characterization, expression analysis, and localization in the brain. Invertebrate Reproduction & Development, 65: 226-234. DOI:10.1080/07924259.2021.1935335 (  0) 0) |
Takayasu, S., Sakurai, T., Iwasaki, S., Teranishi, H., Yamanaka, A., Williams, S. C., et al., 2006. A neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proceedings of the National Academy of Sciences, 103: 7438-7443. DOI:10.1073/pnas.0602371103 (  0) 0) |
Tobari, Y., Iijima, N., Tsunekawa, K., Osugi, T., Haraguchi, S., Ubuka, T., et al., 2011. Identification, localisation and functional implication of 26RFa orthologue peptide in the brain of zebra finch (Taeniopygia guttata). Journal of Neuroendocrinology, 23: 791-803. DOI:10.1111/j.1365-2826.2011.02179.x (  0) 0) |
Ukena, K., Osugi, T., Leprince, J., Vaudry, H., and Tsutsui, K., 2014. Molecular evolution and function of 26RFa/QRFP and its cognate receptor. Journal of Molecular Endocrinology, 52: T119-131. DOI:10.1530/JME-13-0207 (  0) 0) |
Ukena, K., Tachibana, T., Iwakoshi-Ukena, E., Saito, Y., Minakata, H., Kawaguchi, R., et al., 2010. Identification, localization, and function of a novel avian hypothalamic neuropeptide, 26RFa, and its cognate receptor, G protein-coupled receptor-103. Endocrinology, 151: 2255-2264. DOI:10.1210/en.2009-1478 (  0) 0) |
Wang, W., Jiang, C., Xu, Y., Ma, Q., Yang, J., Shi, Y., et al., 2020. Functional characterization of neuropeptide 26RFa receptors GPR103A and GPR103B in zebrafish, Danio rerio. Cell Signaling, 73: 109677. DOI:10.1016/j.cellsig.2020.109677 (  0) 0) |
Wu, D., Wang, C., Shao, Y., and Jiang, X., 2010. Effects of starvation on fatty acid composition of liver and ovary in Sepiella maindroni. South China Fisheries Science, 6: 46-52. DOI:10.3969/j.issn.1673-2227.2010.02.008 (  0) 0) |
Wu, J., Cao, Z., Zheng, L., and Chi, C., 2021. Identification, characterization, and expression analysis of a GnRH-like gene at mature stage in the cephalopod Sepiella japonica (Sepiidae). Oceanologia et Limnologia Sinica, 52: 1530-1539. DOI:10.11693/hyhz20210400083 (  0) 0) |
Xu, B., Bergqvist, C. A., Sundström, G., Lundell, I., Vaudry, H., Leprince, J., et al., 2015. Characterization of peptide QRFP (26RFa) and its receptor from amphioxus, Branchiostoma floridae. General and Comparative Endocrinology, 210: 107-113. DOI:10.1016/j.ygcen.2014.10.010 (  0) 0) |
Yamamoto, T., Miyazaki, R., Yamada, T., and Shinozaki, T., 2011. Anti-allodynic effects of intrathecally and intracerebroventricularly administered 26RFa, an intrinsic agonist for GRP103, in the rat partial sciatic nerve ligation model. Peptides, 32: 1262-1269. DOI:10.1016/j.peptides.2011.03.008 (  0) 0) |
Yamamoto, T., Wada, T., and Miyazaki, R., 2008. Analgesic effects of intrathecally administered 26RFa, an intrinsic agonist for GPR103, on formalin test and carrageenan test in rats. Neuroscience, 157: 214-222. DOI:10.1016/j.neuroscience.2008.08.061 (  0) 0) |
Yan, Y. J., Wang, T. M., Liu, W., Wu, C. W., Zhu, A. Y., Chi, C. F., et al., 2016. Identification and expression profile of the gonadotropin‐releasing hormone receptor in common Chinese cuttlefish, Sepiella japonica. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 325: 453-466. DOI:10.1002/jez.2030 (  0) 0) |
Yang, D., Zhou, Q., Labroska, V., Qin, S., Darbalaei, S., Wu, Y., et al., 2021. G protein-coupled receptors: Structure-and function-based drug discovery. Signal Transduction and Targeted Therapy, 6: 7. DOI:10.1038/s41392-020-00435-w (  0) 0) |
Yun, S., Furlong, M., Sim, M., Cho, M., Park, S., Cho, E. B., et al., 2015. Prevertebrate local gene duplication facilitated expansion of the neuropeptide GPCR superfamily. Molecular Biology and Evolution, 32: 2803-2817. DOI:10.1093/molbev/msv179 (  0) 0) |
Zhang, D., Gao, F., Jakovlić, I., Zou, H., Zhang, J., Li, W. X., et al., 2020. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20: 348-355. DOI:10.1111/1755-0998.13096 (  0) 0) |
Zhou, X., Fang, P. X., Cao, H. M., Xie, J. J., Li, S., and Chi, C. F., 2023. Molecular characterization and expression of twenty interleukin-17 transcripts in the common Chinese cuttlefish (Sepiella japonica) in response to Vibrio harveyi infection. Fish & Shellfish Immunology, 140: 108903. DOI:10.1016/j.fsi.2023.108903 (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


