2) University of Chinese Academy of Sciences, Beijing 100049, China;
3) Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
4) Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
5) School of Marine Science and Technology, Tianjin University, Tianjin 300072, China;
6) State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361005, China
The Hongyanhe Nuclear Power Plant is currently the only nuclear power plant operating in the northern seas of China. The plant is located along the west coast of the sou-thern central part of the Liaodong Peninsula, where the coast generally runs from northeast to southwest (Larson, 2014). Its surrounding seawater has high productivity and is influenced by seasonal circulation. During operation of the power plant, the discharge of warm seawater inevitably affects the environment, biological communities, and eco-logical characteristics in the nearby sea (Fang et al., 2000; Zhou et al., 2009; Liu et al., 2017). In recent years, the enrichment of gelatinous zooplankton, such as jellyfish, has occasionally blocked the water inlet of the power plant (Li and Wang 2009; Lin and Holbert, 2009). In the 1960s in Japan, jellyfish and other gelatinous zooplankton block-ed the water-intake system of power plants in coastal areas numerous times. Clogging incidents can cause emergency situations at nuclear power plants and may jeopardize the safe operation of the units, resulting in significant power loss and economic damage to affected cities (Purcell et al., 2007). Wu et al. (2017) have analyzed the oceanic matter that can block the nuclear power plant cold source sys-tems.
As widely distributed marine creatures, zooplankton is important for the energy flow and biogeochemical cycles of marine ecosystem (Biard et al., 2016). It plays a key role in the conversion of primary production to higher trophic levels in all pelagic ecosystems (Irigoien et al., 2004; Sun et al., 2010). Zooplankton is also one of the main biological groups affected by environmental factors and can be regarded as one important indicator of the marine ecosystem (Roemmich and Mcgowan, 1995; Ri-chardson and Schoeman, 2004). Thus, it is necessary to determine how the environment affects the zooplankton community.
Many studies have confirmed that seawater temperature, salinity, and chlorophyll a (Chl a) are important factors in marine ecosystem (Håkanson and Eklund, 2010; Loeb et al., 2010; Petrou et al., 2011; Feng et al., 2017). Most marine organisms can only live within a relatively narrow range of seawater temperatures (Gillooly, 2000; Petrou et al., 2011). Salinity could directly affect the osmotic balance of marine organisms (Håkanson and Eklund, 2010; Petrou et al., 2011). In the photosynthetic ecosystem, the Chl a concentration determines the primary productivity of the entire ecosystem and the amount of dissolved oxygen in the seawater (Håkanson and Eklund, 2010). In most cases, the various ecological factors are interrelated and have an integrative impact on marine organisms. Exploring the im-pact of environmental factors changes on zooplankton is important for studying the changes of ecological commu-nity and the interaction between nuclear power plant and marine organisms.
To date, many studies have focused on the ecosystem of the Bohai Sea. Lin et al. (2001) investigated the salin-ity and temperature of the Bohai Sea and analyzed their influence on the ecosystem. Tang et al. (2003) evaluated the ecosystem productivity of the Bohai Sea. Many stud-ies on Bohai Sea zooplankton have also been published. Microzooplankton in Bohai Sea and their grazing pres-sure were studied (Zhang and Wang, 2000). Zhang et al. (2002) investigated the Bohai Sea zooplankton commu-nity in spring and autumn. Wang et al. (2014) studied the zooplankton community structure of Bohai Bay in the spring and analyzed its relationship with environmental factors. Gao et al. (2014) studied the diversity of the zoo-plankton community in Bohai Bay and showed its varia-tions. But the zooplankton in the surrounding seawater of the Hongyanhe plant has rarely been the focus of research. Meanwhile, the effects of environmental factors and the Hongyanhe Nuclear Power Plant on the distribution and structure of zooplankton community have not been assess-ed. Here, we investigated the impact of seawater tempera-ture, salinity, Chl a concentration, and other environmen-tal factors on the structure and distribution of the zoo-plankton community in the sea area surrounding Hong-yanhe Nuclear Power Plant in summer. The aim of this study was to clarify the distribution mechanism of the zooplankton community in the seawater surrounding the Hongyanhe plant and to determine how it was affected by the power plant's drainage outlet.
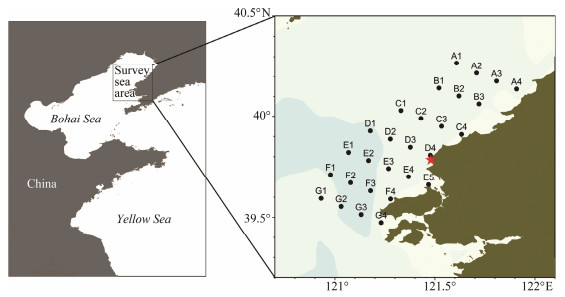
2 Materials and Methods 2.1 Field Sampling and Measurement of Environmental FactorsThree surveys were conducted in the seawater surround-ing the Hongyanhe Nuclear Power Plant during 13-20 July, 12-19 August, and 14-18 September of 2017. The effects of extreme seawater temperature resulting from warm drainage during these high-temperature months on zoo-plankton can be studied in the three months. Additionally, the jelly organisms bloom in the area during these months. Samples were collected from 24 stations along seven tran-sects (Fig. 1). D4 is the nearest station to the outlet of the nuclear power plant. Because of bad weather and unfore-seen circumstances during the surveys, three stations (F1, G1, G4) and four stations (F1, F3, G1, G3) were not in-vestigated in July and September, respectively. The tem-perature and salinity of seawater column were obtained at all stations using an RBR XR-420 CTD. To determine Chl a, 500 mL of surface seawater was collected using a water collector (5 L) and filtered through a 25-mm Whatman GF/ F filter, which was then stored in a freezer at -20℃. Con-sidering the shallow depth of the sampling area and the uniform mixing of the seawater column, the seawater for the detection of Chl a was sampled from the surface layer. After extracting Chl a with 90% aqueous acetone solution at ≤ 4℃ for 24 h, the Chl a concentration was measured using a Turner Designs Fluorometer (Parsons et al., 1984). Zooplankton larger than 500 μm were collected with a max-zooplankton net (mesh size: 500 μm; diameter: 50 cm) and others with a midi-zooplankton net (mesh size: 160 μm, dia-meter: 31.6 cm) (Sun et al., 2010; Chen et al., 2016). Nets were towed vertically from 2 m above the bottom (max bottom depth: 33 m) up to the surface at a rate of about 0.8 m s-1. Zooplankton samples were then immediately preserved in 5% neutral formalin seawater solution. We measured and calibrated the volume of seawater that fil-tered through a calibrated flowmeter (HYDROBIOS, Ger-many), which was equipped at the net mouth.
|
Fig. 1 The study area and location of the sampling stations in the surrounding seawater of the Hongyanhe Nuclear Power Plant during July, August, and September of 2017. The red star marks the outlet of the nuclear power plant. |
All specimens were identified and counted under a ste-reo zoom microscope (Nikon SMZ-745T, Japan) in the laboratory. Samples from the maxi-zooplankton net were used to count the species/groups larger than 500 μm (i.e., macrozooplankton and giant zooplankton). The others (i.e., mesozooplankton and microzooplankton) were counted using the samples from the midi-zooplankton net. The spe-cific sampling nets for each zooplankton species/group are shown in Table 2. To calculate zooplankton abundance, the count under the microscope was divided by the volu-me of the filtered seawater. The biological abundance data in this study are presented as ind m-3.
2.3 Data Analysis and Statistical MethodsThe zooplankton community diversity and structure were evaluated in terms of the Shannon-Wiener diversity index (H′) (Shannon and Weaver, 1949), Margalef index (Dm) (1957), and evenness coefficient (J′) (Pielou, 1969). The do-minance index (Y) was used to identify the dominant spe-cies each month (Odum, 1959). The formulas were as fol-lows:
| $ H\prime = - {\rm{Sum }}({P_i} \times \ln {P_i}), $ |
| $ D{\rm{m}} = \frac{{(S - 1)}}{{\ln N}}; J\prime = \frac{{H\prime }}{{\ln S}}, $ |
| $ Y = \frac{{ni}}{N} \times fi, $ |
| $ Pi = \frac{{ni}}{N}, $ |
where ni is species abundance; S is the number of all zoo-plankton species; N is the total abundance of all zooplank-ton species; and fi is the occurrence frequency of species in all stations. Y > 0.02 indicates that the species is dominant (Odum, 1959).
Correlations between the abundance of zooplankton and environmental factors (temperature, salinity, and Chl a con-centration) were statistically evaluated using the Pearson rank correlation with SPSS V16.0 software. A combina-tion of environmental factors that can best explain the zoo-plankton community changes is identified using the BIO-ENV procedure in PRIMER V6.0 software. To assess the similarity of the zooplankton community between stations, a cluster analysis was conducted with PRIMER V6.0. More-over, CANOCO for Windows V5.0 was also used to per-form principal component analysis and redundancy analy-sis (RDA), which we used to analyze the impact of dif-ferent environmental factors on the zooplankton commu-nity.
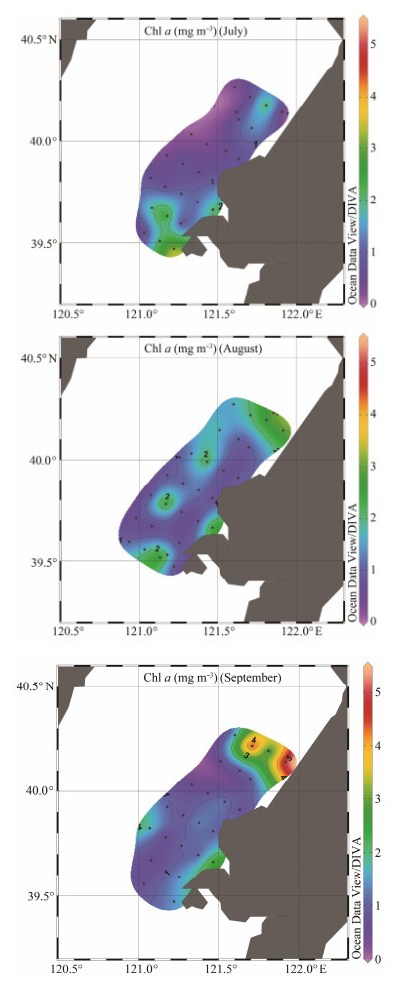
3 Results 3.1 Environmental Factors in the Seawater Surround-ing the Hongyanhe Nuclear Power PlantChanges in seawater temperature (ST), seawater sali-nity (SS), and Chl a concentration over three months are shown in Table 1. The average ST increased from 23.29 ± 1.59℃ in July to the highest in August (24.34 ± 1.50℃), and then decreased to 24.12 ± 0.44℃ in September. The minimum bottom seawater temperature (BST) (19.96℃) in August was lower than that in September (23.41℃). In contrast, SS decreased first and then increased. In Septem-ber, the average Chl a concentration reached its maximum value (1.46 ± 0.56 mg m-3), and the highest Chl a concen-tration was 5.12 mg m-3. The Chl a concentration was low in July, with an average of 1.04 ± 0.56 mg m-3 and a mini-mum of 0.13 mg m-3.
|
|
Table 1 The temperature, salinity, and Chl a concentration in the seawater surrounding the Hongyanhe Nuclear Power Plant in July, August, and September 2017 |
In July, the average ST in the investigation area was (23.29 ± 1.59)℃. The surface seawater temperature (SST) distribution in July was high in the southwest and low in the northeast. The SST at stations D4 and E5 were higher than those in the surrounding area, and this trend also applied to BST. The ST distribution on the bottom layer was the opposite of that of the surface layer, which was high in the southwest and low in the northeast. The dis-tribution pattern of surface seawater salinity (SSS) was un-clear. The bottom seawater salinity (BSS) was high in the west and low in the east. The three highest Chl a concen-trations were observed at stations A3 (2.16 mg m-3), E5 (2.02 mg m-3), and F2 (1.89 mg m-3). The average Chl a con-centration was (1.04 ± 0.56) mg m-3. The lowest Chl a con-centration was only 0.13 mg m-3 at station C1 (Fig. 2). The average ST of the whole seawater column increased to (24.34 ± 1.50)℃, and the average SST was (26.10 ± 1.10)℃ in August. There was no obvious trend in horizontal dis-tribution. The SST and BST of stations D4 and E5 were still higher than those of the other stations. The BST was high in the southwest and low in the northeast, which was also the case in July. The average Chl a concentration was (1.41 ± 0.77) mg m-3. The distribution of Chl a concentra-tions was similar to the average ST. Except for the two stations (E2 and E5) in transect A, the concentration of Chl a was lower than 2.30 mg m-3, and the lowest Chl a was recorded at station F2 (0.56 mg m-3). The average ST in September was high in the southwest and low in the northeast, which was consistent with the BST. We observed the highest SST (25.50℃) and BST (24.98℃) at station D4. The average Chl a concentration was (1.46 ± 0.56) mg m-3, and the Chl a concentration at all stations in transect A were higher than in other transects. At four stations the Chl a concentrations were below 0.6 mg m-3 and the low-est Chl a concentration was 0.53 mg m-3 at station E2. During the three months, the SST of station D4 was high-er than the average ST of the remaining stations by 0.48- 0.78℃, and the difference of BST was larger (0.79-2.33℃). The Chl a concentration was low at station D4, and it was lower than the average Chl a concentration by 0.09-0.73 mg m-3. There was only a slight difference in the salinity distribution.
|
Fig. 2 Horizontal distribution of Chl a concentrations (mg m-3) in the seawater surrounding the Hongyanhe Nuclear Power Plant during July, August, and September of 2017. |
From July to September 2017, we collected and identi-fied 49 species/groups of zooplankton from the seawater surrounding the Hongyanhe Nuclear Power Plant, includ-ing 16 species/groups of larvae and 33 species of adults (Table 2).
|
|
Table 2 Zooplankton species/groups in the seawater surrounding the Hongyanhe Nuclear Power Plant in July, August, and September of 2017 |
The average abundance of zooplankton first increased and then decreased from July to September. The average abundance across all stations in July was (4653.95 ± 2750.66) ind m-3, and the maximum was 10000 ind m-3 at station E3. The average abundance in August was (8116.70 ± 7204.30) ind m-3, and the maximum abundance was 32320 ind m-3 at station D3. In September, the average abundance was (3124.59 ± 3231.48) ind m-3. The highest abundance was observed at station A3 and it was 40987.5 ind m-3, of which Noctiluca scintillans contributed 39500 ind m-3.
The total numbers of zooplankton species/groups de-clined, with 38 species/groups in July, 29 in August, and 27 in September. In July, there were 12 species of cope-pods, three species of small jellyfish, and 14 groups of lar-vae. Copepod species richness was stable in July and Au-gust, and then declined to eight groups in September. Groups of larvae declined from 14 to 9 over the course of these months. In September, we observed five species of jellyfish, which was the maximum.
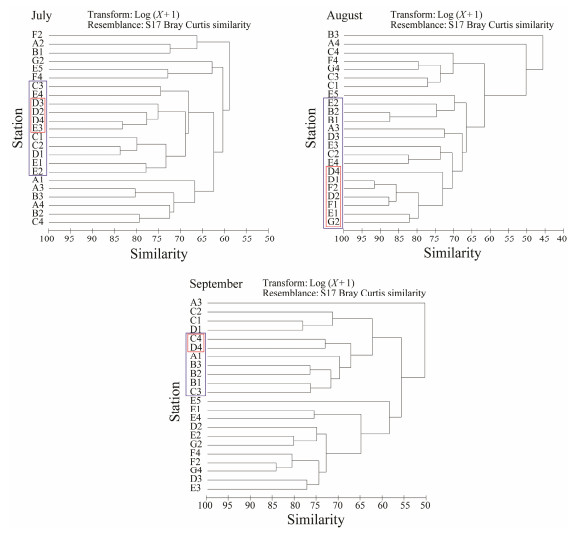
The cluster analysis of the zooplankton distribution is shown in Fig. 3. In July, the zooplankton community at station D4, which was closed to the outlet of the power plant, was similar to those of three adjacent stations D2, D3 and E3, with the similarity exceeding 70%. The simi-larity between station D4 and most of the stations in the C and E sections exceeded 65%. No obvious distribution characteristics were observed from cluster analysis results in August. The similarity between D4 and other stations in the same section (D1 and D2) and the southern section (F2, F1, E1, and G2) was higher than 70%. In September, only station C4 had over 70% similarity with D4. Stations C3 and A1 and stations in section B had more than 65% similarity with station D4.
|
Fig. 3 Cluster analysis of zooplankton community in different sample stations. The red block represents stations with more than 70% similarity with station D4. The blue block represents stations with more than 65% similarity with station D4. |
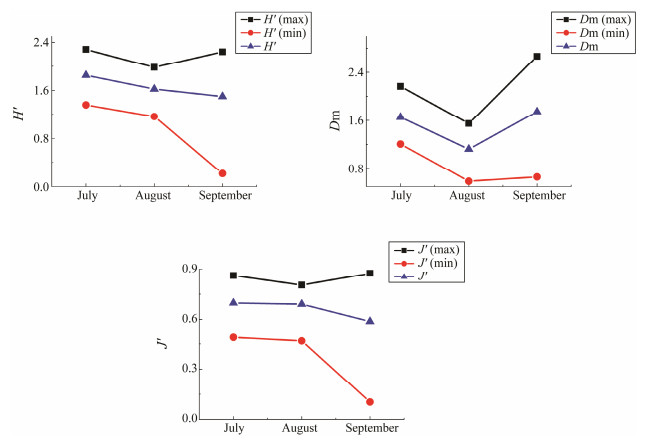
The trends of the Shannon-Wiener index (H'), Margalef index (Dm), and evenness coefficient (J′) are shown in Fig. 4. The Shannon-Wiener index showed a gentle downward trend, and the maximum value for all of the stations in August was 1.16-1.98 lower than in the other two months. The minimum value of H′ dropped significantly from Au-gust to September. The J′ value was fairly consistent with the H′ value, which also showed a downward trend over the three months. The Dm in the seawater surrounding the Hongyanhe Nuclear Power Plant was the lowest in Au-gust, ranging from 0.95 to 1.55. The maximum and aver-age values of Dm were significantly lower in August, and rose sharply in September. The minimum value rebound in September was slight.
|
Fig. 4 The diversity index of the seawater surrounding the Hongyanhe Nuclear Power Plant during July, August, and Sep-tember of 2017. |
In July 2017, Calanus sinicus, Paracalanus parvus, cope-podid, and Bivalvia larvae accounted for 49% of the total zooplankton abundance in seawater surrounding the Hong-yanhe Nuclear Power Plant. The maximum zooplankton abundances were 5017.60, 1779.31, 2320.00, and 4057.14 ind m-3, respectively. This abundance ratio increased to 70% in August, and the maximum abundances for each group reached 1010.00, 7120.00, 8640.00, and 6577.78 ind m-3, respectively. Acartia bifilosa became the domi-nant species, with a maximum abundance of 2466.67 ind m-3. In September, N. scintillans alone accounted for 72% of the total zooplankton abundance. Calanus sinicus, P. par-vus, copepodid, and Bivalvia larvae accounted for 56% of the zooplankton abundance when N. scintillans was not in-cluded. Their maximum abundances were 106.67, 250.00, 283.33, and 1590.00 ind m-3, respectively. The abundance of Chaetognatha larvae was also high, with a maximum of 415.79 ind m-3.
The dominance index (Y) of C. sinicus showed a down-ward trend with values of 0.495, 0.328, and 0.048 in July, August, and September, respectively. Y of P. parvus was 0.039, 0.274, and 0.022 in July, August, and September, respectively, with a maximum in August. The copepodid also reached their maximum value in August, with 0.161, 0.265, and 0.037 for each of the respective three months. The index of Bivalvia larvae decreased from 0.259 to 0.042 during the summer (Table 3). In September, N. scintillans comprised a great percentage (72%) of the total zooplank-ton abundance, leading to the low dominance indexes of the four groups.
|
|
Table 3 Dominance index of four dominant species/groups in the Hongyanhe Nuclear Power Plant in July, August, and September of 2017 |
The distribution of dominant copepod species C. sini-cus and P. parvus is shown in Fig. 5. The distribution of C. sinicus was uneven in July. It reached 5017.60 ind m-3 at the southernmost station (G4). Without considering the G4 station, the average abundance of the remaining stations was (106.17 ± 50.23) ind m-3. The distribution of C. sinicus was more even in August than in July, with an average abundance of (183.13 ± 43.34) ind m-3. At transects C and D, the abundance of C. sinicus was relatively high, with an average of (467.79 ± 139.66) ind m-3 and (284.03 ± 80.21) ind m-3, and the maximum abundance was 1010.00 ind m-3 at station C2. In September, the total abundance decreased rapidly, with an average of (16.62 ± 11.87) ind m-3. The high-est abundance was 73.33 ind m-3, which was found at sta-tion E1. This station was located the farthest from the shore. The overall distribution of the zooplankton community was balanced and was trending away from the shore.
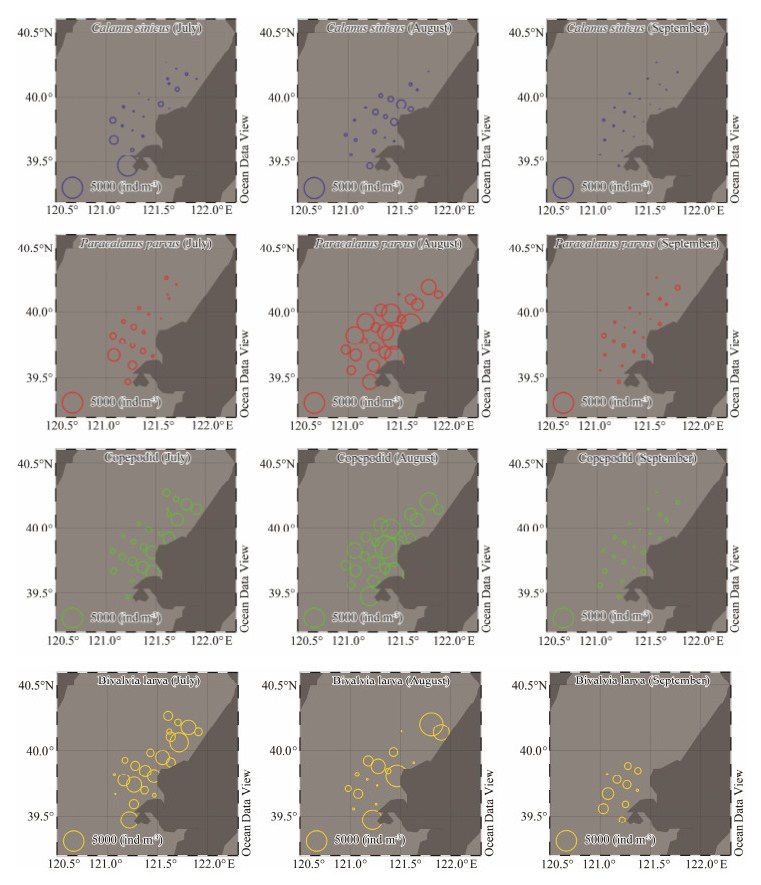
|
Fig. 5 Horizontal distribution of the dominant species/groups in the seawater surrounding the Hongyanhe Nuclear Power Plant during July, August, and September of 2017. |
In July, the average abundance of P. parvus was 231.60 ± 183.55 ind m-3. It was generally distributed in the south-west. The highest abundance was 1779.31 ind m-3 and was observed at station F2. The abundance of P. parvus in August increased sharply, with an average abundance of 2226.40 ± 1549.35 ind m-3 and a maximum abundance of 7120.00 ind m-3 (station D3). In September, the average and maximum abundances of P. parvus were 69.80 ± 82.73 ind m-3 and 250.00 ind m-3, respectively (station A3). The over-all abundance at transect E was high in September, with an average abundance of (118.30 ± 39.25) ind m-3.
3.4 Relationship Between Environmental Factors and the Zooplankton CommunityThe indicators of community changes were different for each of the three months. We used the BIO-ENV proce-dure to identify the combination of Chl a, BST, and aver-age SS as the most reliable indicators (ρ = 0.427) in July (Table 4). Chl a concentrations appeared in all the eight combinations with the highest ρ value. In August, the cor-relation was weaker than in the other two months (best ρ = 0.204), and the most reliable combination of factors in-cluded Chl a concentration and average ST. Chl a was also found to be among the most reliable five combinations. Chl a, average ST, and average SS were the most reliable indicators (ρ= 0.238) of community changes in Septem-ber.
|
|
Table 4 Results from BIO-ENV analysis, correlation of environmental factors, and zooplankton community in the seawater surrounding the Hongyanhe Nuclear Power Plant in July, August, and September of 2017 |
The results of the RDA are shown in Fig. 6. The aver-age ST, BST, and average SS were the three environmen-tal variables with the greatest influence in July. The inf-luence of BST and average ST was basically the same, showing a significant negative correlation with the abun-dance of C. sinicus and P. parvus. Conversely, the BST was positively correlated with the Gastropoda larvae, A. bifilo-sa, copepodid, Macrura larvae, bivalve larvae, Oikopleura dioica, and nauplius. P. parvus abundance was signifi-cantly affected by SS, and we identified a significant po-sitive correlation between its abundance and average SS. Corycaeus affinis and C. sinicus abundances were also po-sitively correlated with average SS, but C. sinicus was ne-gatively correlated with average ST. O. dioica abundance was significantly negatively correlated with salinity.
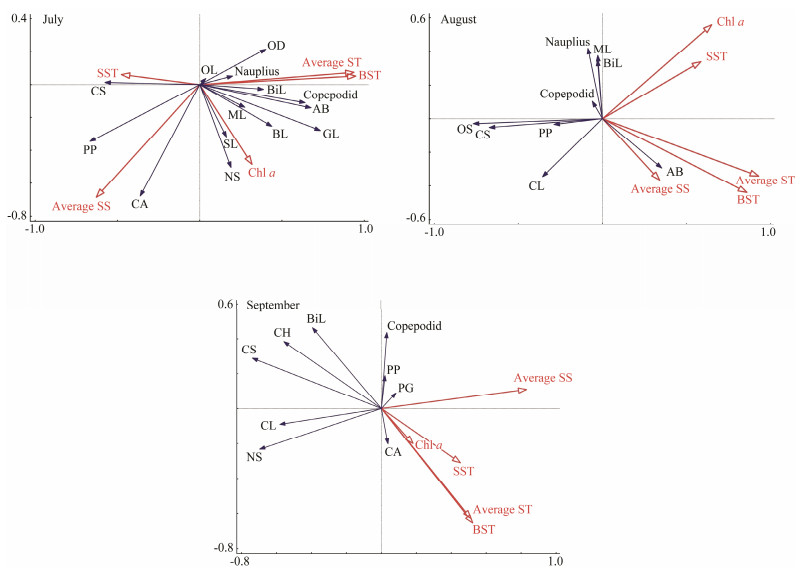
|
Fig. 6 RDA of zooplankton abundance and environmental factors in the seawater surrounding the Hongyanhe Nuclear Power Plant during July, August, and September of 2017. CS, Calanus sinicus; ML, Macrura larvae; BL, Brachyura larva; PP, Paracalanus parvus; AB, Acartia bifilosa; CA, Corycaeus affinis; OD, Oikopleura dioica; CL, Chaetognatha larvae; BiL, Bivalvia larvae; OL, Ophiopluteus larvae; NS, Noctiluca scintillans; GL, Gastropoda larvae; OS, Oithona similis. |
In August, average ST still had the greatest impact on zooplankton among all the environmental factors examined. Gastropoda larvae were no longer the dominant group. Zoo-plankton community composition was affected by changes in ST. The abundances of copepodid, bivalve larvae, and Macrura larvae were negatively correlated with BST in August, yet positively correlated with BST in July. The distribution of nauplii was not affected by other environ-mental factors. Only A. bifilosa was still positively corre-lated with ST and salinity. Both C. sinicus and P. parvus were negatively correlated with SST and Chl a. Similarly, the abundance of new dominant species of Chaetognatha larva and Oithona similis were significantly negatively af-fected by SST and Chl a in August (Fig. 6).
In September, SS, BST, and average ST were the most significant variables (Fig. 6). A. bifilosa, Macrura larvae, and O. similis were no longer the dominant species/group. Ins-tead, C. affinis and N. scintillans were the dominant spe-cies. Phialidium hemisphaericum and Pleurobrachia glo-bosa were the newly collected jellyfish species in Sep-tember. The abundances of C. sinicus, bivalve larvae, P. hemisphaericum, and copepodid showed a significantly negative correlation with average ST. Only C. affinis was positively correlated with ST. We did not observe a sig-nificant positive relationship between the dominant spe-cies/group with SS, but the abundance of bivalve larvae, P. hemisphaericum, C. sinicus, Chaetognatha larva, and N. scintillans were negatively correlated with SS.
The two species of small jellyfish P. globosa and P. he-misphaericum that appeared in September were consid-ered to be important influencing factors. The RDA show-ed that P. globosa and P. hemisphaericum had a significant effect on the zooplankton community. The abundances of C. sinicus, bivalve larvae, and copepodid were significantly positively correlated with the total abundance of jellyfish. Conversely, N. scintillans, O. similis, P. parvus, and C. af-finis were unaffected by the jellyfish.
4 Discussion 4.1 Effects of the Liaoning Hongyanhe Nuclear Power Plant on EnvironmentIn general, the cooling systems of nuclear power plants cause an increase in the surrounding ST (Hung et al., 2006; Ma et al., 2014; Cardoso-Mohedano et al., 2015; Lee et al., 2018), which was consistent with the findings of this study. On the basis of remote-sensing data from NASA, the SST change at station D4 was evident from 2010 to 2017 (Ta-ble 5). The average SST in the summer started to increase in 2013, after the nuclear power plant units were succes-sively put into operation. In June 2016, Wang et al. (2018) found that in the seawater surrounding the Hongyanhe Nuclear Power Plant, the ST at the outlet was about 4℃ higher than that in the area 200 m away. The SST values at station D4, which is located near the location of the hot-water exchange port of the power plant, were 1.55℃, 2.26℃, and 1.22℃ higher in July, August, and September, respectively, than the temperatures at the neighboring sta-tion D3. The ST at station D4 was 0.48-2.33℃ higher than the average ST, which was lower than that reported by other researchers (Bamber and Seaby, 2004; Cardoso-Mo-hedano et al., 2015). Cardoso-Mohedano et al. (2015) found that the mean thermal impact was distributed along the shoreline within approximately 100 m of the outlet of power plant. Hot-water exchange was supposed to mostly and directly affect the ST of station D4. The BST in July, how-ever, showed a decreasing trend from the southwest to the northeast. We speculated that the bottom cold-water flow caused the outflow of surface hot-water flow. The BST distribution in September was different from those in July and August, and it was almost the same as the average ST. In September, the ST at station D4 was the highest among all stations. It may be that the seawater convection ex-change for each seawater layer was weakened in Septem-ber.
|
|
Table 5 Average SST in summer months at station D4 based on NASA remote-sensing data |
The Chl a concentration ranged from 0.13 to 5.12 mg m-3, which is consistent with previous studies in Liao-dong Bay and the Bohai Sea (Song et al., 2010; Liu et al., 2014). The average Chl a concentration first increased and then decreased from July to September, which was consistent with the change of ST. In August, the highest Chl a concentration and salinity were found in transect A. It is likely that the ST did not increase to a point that sur-passed the thermal tolerance of the phytoplankton during these three months. The distributions of Chl a concentra-tion and ST in July and September were not consistent. The reasons might be that the phytoplankton populations at different stations were different, and that the population changed with different degrees of temperature adaptation.
The average SS values for the three months were simi-lar, which is consistent with previous studies in Liaodong Bay (Song et al., 2010), and slightly higher than the re-sults from the central Bohai Sea (Liu et al., 2014). Liao-dong Bay is far from the Yellow River estuary, which makes the higher salinity reasonable. The value of SS at station D4 did not differ from those of other stations. Thus SS was not significantly affected by the outlet of the nu-clear power plant.
4.2 Dominant Species/Groups Affected by Environmental FactorsEnvironmental factors greatly affect the zooplankton com-munity (Plourde et al., 2002; Poornima et al., 2005; Tachi-bana et al., 2013; Dessier et al., 2018). The zooplankton community may also be a reliable indicator for evaluating the state of aquatic ecosystems (Mayer-Pinto et al., 2012; Vandysh, 2012). Following a 30-year study from 1959 to 1992 in the Bohai Sea, Tang et al. (2003) suggested that a progressive depletion in zooplankton may be a response to the warming trend. In this study, we also found that the proportion of small-sized copepods increased over a re-latively short period, and this was closely related to tem-perature. Our results are consistent with predictions of the effects of environmental warming on ectotherms (Daufres-ne et al., 2009).
C. sinicus and P. parvus were the dominant species of the zooplankton community in the seawater surrounding the Hongyanhe Nuclear Power Plant in July, August, and September of 2017. Recent studies found that, in addition to Bivalvia larvae, the other three groups were common dominant groups in Bohai Bay (Yang et al., 2018). We re-corded a total of 49 zooplankton species/groups in the seawater surrounding the Hongyanhe Nuclear Power Plant from July to September in 2017. There were 43 species/ groups found in Liaodong Bay from July to September in 2005 (Song et al., 2010) and 48 species/groups collected from the coastal seawater of Tianjin of the Bohai Sea over three months (May, July, and August) in three years (Gao et al., 2014). In July, the highest abundances of C. sinicus were found mainly at the three stations (E1, F2, and G4) with the lowest average ST (about 22℃). This is likely related to the adaptability of copepod species to low ST (Huntley and Lopez, 1992; Karas, 1992; Pu et al., 2004b). High abundances of C. sinicus were found at transects B and C in August. The distribution of C. sinicus was af-fected by the interactive effects of several environmental factors. A negative correlation existed among them, indi-cating that the high ST and Chl a concentration brought survival stress to C. sinicus in August. Since C. sinicus cannot live in the surface seawater of Yellow Sea in sum-mer (Pu et al., 2004a), the ST was probably near the up-per temperature limit of C. sinicus in the Bohai Sea. In September, C. sinicus was significantly negatively corre-lated with all the environmental factors, and its abun-dance was low in the nearshore and high offshore. With regard to environmental factors, ST and salinity tended to be high in the nearshore and low offshore. Additionally, the jellyfish bloom may have also had a negative impact on C. sinicus abundance (Kawahara, 2006; Uye, 2011, 2014; Shi et al., 2015). According to our field observations, a large number of giant jellyfish Nemopilema nomurai and Aurelia aurita appeared in the seawater surrounding the Hongyanhe Nuclear Power Plant in the summer, and the correlation coefficient also showed that the distribution of large jellyfish was negatively correlated with C. sinicus in the summer (R = -0.22 and -0.31, respectively; P < 0.05) (unpublished data).
In July, P. parvus was affected by environmental factors in a similar way to C. sinicus. The P. parvus abundance is high in the southwest and low in the northeast seawater, which was negatively correlated with the average ST. In August and September, there was no significant correla-tion between the abundance of P. parvus and ST, salinity, or Chl a concentration. RDA results showed that P. par-vus was less affected by environmental factors. Addition-ally, the distribution of P. parvus in the summer was also affected by A. aurita, with a correlation coefficient of -0.41.
The copepodid were distributed close to the nearshore in July, and we did not detect obvious distribution regu-larity in August. According to the RDA, copepodid were less affected by environmental factors in August. In Sep-tember, there was a low abundance of copepodid in the south and a high abundance in the north seawater, whereas the average ST was also low in the southwest and high in the northeast seawater. The RDA showed that copepodid were negatively correlated with SST over the three months, suggesting that copepodid are relatively sensitive to ST and prefer a lower ST.
It is relatively difficult to directly judge whether the dis-tribution of bivalve larvae was affected by environmental factors. Different larvae groups had different adaptability to environmental factors. Bivalve larvae uniformly dis-tributed in July may be two different species, which are difficult to identify with the traditional morphological ta-xonomy method. From the RDA, the correlation of bi-valve larvae with ST changed from positive to negative in the three months. Considering that the planktonic larvae of benthic organisms had certain movement limitations, their response to the impact of environmental factors may have been delayed.
4.3 Impact of Power Plants on ZooplanktonThe similarities between the zooplankton communities at the stations close to the nuclear power plant were rela-tively high, especially in July and September. In August, zooplankton diversity was relatively low, and the abun-dance of the dominant species/groups including P. parvus, copepodid, and bivalve larvae was significantly higher than those in the other two months. Meanwhile, the three dominant species/groups were less affected than others by environmental factors in August (Fig. 6), which may have resulted in the unobvious cluster of the zooplankton com-munity. ST plays an important role in the impact of power plants on the zooplankton community (Dias and Bonecker, 2008; Wang et al., 2012; Ma et al., 2014; Lee et al., 2018). In the seawater surrounding the Hongyanhe Nuclear Power Plant, the zooplankton community was negatively corre-lated with ST in the summer of 2017. When ST peaked in August, zooplankton diversity was the lowest. Song et al. (2010) and Li et al. (2014) found that zooplankton diver-sity was positively correlated with ST in Liaodong Bay and Daya Bay. The highest BST (up to 28.16 in August) could exceed the optimal survival range of some zoo-plankton such as C. sinicus (Karas, 1992; Bi et al., 2001; Pu et al., 2004b), which could result in a negative correla-tion between zooplankton diversity and ST. Furthermore, increased ST resulting from nuclear power plants was found to raise the respiratory and metabolism rates and carbon dioxide emissions of zooplankton, affecting their survival (Shiah et al., 2005). A study on pelagic copepods in the Yangtze River Estuary, which has higher temperature than Hongyanhe sea area, also found a negative correlation between temperature and zooplankton diversity (Li and Qian, 2009). Song et al. (2010) found that in Liaodong Bay, the total abundance was positively correlated with Chl a concentration in August when the temperature was the highest. The number of zooplankton species decreased, but species diversity increased in September. Total zoo-plankton abundance was positively correlated with Chl a concentration in September, which was higher than the concentrations in July and August. This could have been caused by the outbreak of small hydromedusae in Sep-tember, which compete with zooplankton for food (Li and Qian, 2009).
In this study, the primary influence of the nuclear power plant on environmental factors was increasing the ST. High temperature was not conducive to the survival and repro-duction rates of copepods, such as C. sinicus (Karas, 1992; Zhang et al., 2002; Li and Qian, 2009). The negative im-pact of the Hongyanhe plant on the nearby zooplankton community owing to its influence on ST is consistent with previous studies. Many studies have found that high water temperatures caused by power plants may lead to a de-crease in the abundance of the zooplankton (Karas, 1992; Perissinotto and Wooldridge, 2010; Ma et al., 2014), and that higher ST in low-temperature months were positively correlated with the abundances of some copepods (Bi et al., 2001; Pu et al., 2004b; Lee et al., 2018). Wang et al. (2009) found that the Daya Bay Power Plant had a greater impact on the phytoplankton community than on the zoo-plankton community. Ye et al. (2018) suggested that the nuclear power plant changed the zooplankton community through its effect on phytoplankton in Daya Bay. Several environmental factors, including ST, dissolved oxygen, and nitrogen, could play important roles in determining zoo-plankton biomass in Daya Bay (Wang et al., 2012). The precise mechanism how nuclear power plants affect the zooplankton community requires additional research.
5 ConclusionsThe zooplankton community in the seawater surround-ing the Hongyanhe Nuclear Power Plant was affected by ST, SS, and Chl a concentration, and the degree of influ-ence varied over the three summer months. The influence of temperature was relatively strong while that of salinity was relatively weak. The zooplankton abundance first in-creased and then decreased from July to September in 2017, and the biodiversity of the zooplankton communi-ty declined from July to September. The abundances of three dominant copepod species were significantly nega-tively affected by ST. In addition to the dominant cope-pod, the bivalve larvae had a different distribution mode in relation to the changes of ST. The Hongyanhe Nuclear Power Plant can affect the zooplankton community struc-ture as it can increase the ST near the outlet of the power plant.
AcknowledgementsThis work was supported by the National Key R & D Program of China (Nos. 2017YFC1404401, 2017YFC140 4402), the Science & Technology Basic Resources Inves-tigation Program of China (No. 2017FY100803), and the National Natural Science Foundation of China (No. 4130 6155). We thank Dr. Natalie Kim for improving this ma-nuscript.
Bamber, R. N. and Seaby, R. M. H., 2004. The effects of power station entrainment passage on three species of marine plank-tonic crustacean, Acartia tonsa (Copepoda), Crangon crangon (Decapoda) and Homarus gammarus (Decapoda). Marine En-vironmental Research, 57: 281-294. DOI:10.1016/j.marenvres.2003.08.002 (  0) 0) |
Bi, H. S., Sun, S., Gao, S. W. and Zhang, G. T., 2001. The eco-logical characteristics of zooplankton community in the Bo-hai Sea Ⅱ. The distribution of copepoda abundance and sea-sonal dynamics.. Acta Ecologica Sinica, 21(2): 177-185. (  0) 0) |
Biard, T., Stemmann, L., Picheral, M., Mayot, N., Vandromme, P., Hauss, H., Gorsky, G., Guidi, L., Kiko, R. and Not, F., 2016. In situ imaging reveals the biomass of giant protists in the global ocean. Nature, 532: 504-507. DOI:10.1038/nature17652 (  0) 0) |
Cardoso-Mohedano, J. G., Bernardello, R., Sanchez-Cabeza, J. A., Ruiz-Fernández, A. C., Alonso-Rodriguez, R. and Cruzado, A., 2015. Thermal impact from a thermoelectric power plant on a tropical coastal lagoon. Water, Air, & Soil Pollution, 226: 1-11. DOI:10.1007/s11270-014-2202-8 (  0) 0) |
Chen, H. J., Yu, H. and Liu, G. X., 2016. Comparison of cope-pod collection efficiencies by three commonly used plankton nets: A case study in Bohai Sea, China. Journal of Ocean University of China, 15(6): 1007-1013. DOI:10.1007/s11802-016-3122-6 (  0) 0) |
Daufresne, M., Lengfellner, K., Sommer, U. and Carpenter, S. R., 2009. Global warming benefits the small in aquatic eco-systems. Proceedings of the National Academy of Sciences, 106: 12788-12793. DOI:10.1073/pnas.0902080106 (  0) 0) |
Dessier, A., Bustamante, P., Chouvelon, T., Huret, M., Pagano, M., Marquis, E., Rousseaux, F., Pignon-Mussaud, C., Mornet, F. and Bréret, M., 2018. The spring mesozooplankton varia-bility and its relationship with hydrobiological structure over year-to-year changes (2003-2013) in the southern Bay of Bis-cay (Northeast Atlantic). Progress in Oceanography, 166: 76-87. DOI:10.1016/j.pocean.2018.04.011 (  0) 0) |
Dias, C. D. and Bonecker, S. L. C., 2008. Long-term study of zoo-plankton in the estuarine system of Ribeira Bay, near a power plant (Rio de Janeiro, Brazil). Hydrobiologia, 614: 65-81. DOI:10.1007/s10750-008-9537-3 (  0) 0) |
Fang, Y., Fang, G. H. and Zhang, Q. H., 2000. Numerical simu-lation and dynamic study of the wintertime circulation of the Bohai Sea. Chinese Journal of Oceanology & Limnology, 18(1): 1-9. DOI:10.1007/BF02842535 (  0) 0) |
Feng, S., Lin, J. N., Sun, S., Zhang, F. and Li, C. L., 2018. Hy-posalinity and incremental micro-zooplankton supply in early-developed Nemopilema nomurai polyp survival, growth, and podocyst reproduction. Marine Ecology Progress Series, 591: 117-128. DOI:10.3354/meps12204 (  0) 0) |
Gao, W. S., Liu, X. B., Zhang, Q. F., Xu, Y. S., Ma, Y. Y., He, R. and Liu, Y., 2014. Species diversity of zooplankton in the coastal area of Bohai Bay. Marine Sciences, 38: 55-60. (  0) 0) |
Gillooly, F. J., 2000. Effect of body size and temperature on ge-neration time in zooplankton. Journal of Plankton Research, 22: 241-251. DOI:10.1093/plankt/22.2.241 (  0) 0) |
Håkanson, L. and Eklund, J. M., 2010. Relationships between chlorophyll, salinity, phosphorus, and nitrogen in lakes and ma-rine areas. Journal of Coastal Research, 26: 412-423. DOI:10.2112/08-1121.1 (  0) 0) |
Hung, T. C., Huang, C. C. and Shao, K. T., 2006. Ecological sur-vey of coastal water adjacent to nuclear power plants in Tai-wan. Chemistry and Ecology, 15: 129-142. DOI:10.1080/02757549808037625 (  0) 0) |
Huntley, M. E. and Lopez, M. D., 1992. Temperature-depend-ent production of marine copepods: A global synthesis. The American Naturalist, 140(2): 201-242. DOI:10.1086/285410 (  0) 0) |
Irigoien, X., Huisman, J. and Harris, R. P., 2004. Global biodi-versity patterns of marine phytoplankton and zooplankton. Nature, 429(6994): 863-867. DOI:10.1038/nature02593 (  0) 0) |
Karas, P., 1992. Zooplankton entrainment at Swedish nuclear power plants. Marine Pollution Bulletin, 24: 27-32. DOI:10.1016/0025-326X(92)90313-U (  0) 0) |
Kawahara, M., 2006. Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Marine Ecology Progress Series, 307: 161-173. DOI:10.3354/meps307161 (  0) 0) |
Larson, A., 2014. Hongyanhe Nuclear Power Plant, Liaoning Pro-vince, China. Power, 158(11): 24-25. (  0) 0) |
Lee, P. W., Tseng, L. C. and Hwang, J. S., 2018. Comparison of mesozooplankton mortality impacted by the cooling systems of two nuclear power plants at the northern Taiwan coast, sou-thern East China Sea. Marine Pollution Bulletin, 136: 114-124. DOI:10.1016/j.marpolbul.2018.09.003 (  0) 0) |
Li, J. Q. and Wang, L. H., 2009. Numerical simulation of tem-perature field in turbo-generators stator on cooling water blo-ckage. Proceedings of the CSEE, 29(12): 70-74 (in Chinese with English abstract). (  0) 0) |
Li, K. Z., Yin, J. Q., Tan, Y. H., Huang, L. M. and Song, X. Y., 2014. Short-term variation in zooplankton community from Daya Bay with outbreaks of Penilia avirostris. Oceanologia, 56: 583-602. DOI:10.5697/oc.56-3.583 (  0) 0) |
Li, X. Z. and Qian, G., 2009. Labidocera euchaeta: Its distribu-tion in Yangtze River Estuary and responses to global warming. Chinese Journal of Applied Ecology, 20: 1196 (in Chinese with English abstract). (  0) 0) |
Lin, C. L., Su, J. L., Xu, B. R. and Tang, Q. S., 2001. Longterm variations of temperature and salinity of the Bohai Sea and their influence on its ecosystem. Progress in Oceanography, 49(1-4): 7-19. DOI:10.1016/S0079-6611(01)00013-1 (  0) 0) |
Lin, K. and Holbert, K. E., 2009. Blockage diagnostics for nu-clear power plant pressure transmitter sensing lines. Nuclear Engineering & Design, 239(2): 365-372. DOI:10.1016/j.nucengdes.2008.10.012 (  0) 0) |
Liu, L. X., Wang, Y. J., Di, B. P. and Liu, D. Y., 2014. Spatial distribution of chlorophyll a and environmental factors in the Bohai Sea in spring of 2012. Marine Sciences, 38(12): 8-15 (in Chinese with English abstract). (  0) 0) |
Liu, Y. W., Wang, Z. Y., Cao, G. W., Cao, Y. and Huo, Y., 2017. Study on corrosion behavior of zinc exposed in coastal-indus-trial atmospheric environment. Materials Chemistry and Phy-sics, 198: 243-249. DOI:10.1016/j.matchemphys.2017.05.043 (  0) 0) |
Loeb, V., Hofmann, E. E., Klinck, J. M. and Holm-Hansen, O., 2010. Hydrographic control of the marine ecosystem in the South Shetland-Elephant Island and Bransfield Strait region. Deep-Sea Research Part Ⅱ-Topical Studies in Oceanography, 57: 519-542. DOI:10.1016/j.dsr2.2009.10.004 (  0) 0) |
Ma, Y. E., Ke, Z. X., Huang, L. M. and Tan, Y. H., 2014. Iden-tification of human-induced perturbations in Daya Bay, China: Evidence from plankton size structure. Continental Shelf Re-search, 72: 10-20. DOI:10.1016/j.csr.2013.10.012 (  0) 0) |
Margalef, R., 1957. La teoria de la informacion en ecologia. Me-morias de la Real Academia de Ciencias y Artes de Barcelona, 32: 373-499. (  0) 0) |
Mayer-Pinto, M., Ignacio, B. L., Szechy, M. T. M., Viana, M. S., Curbelo-Fernandez, M. P., Lavrado, H. P., Junqueira, A. O. R., Vilanova, E. and Silva, S. H. G., 2012. How much is too little to detect impacts? A case study of a nuclear power plant. PLoS One, 7: e47871. DOI:10.1371/journal.pone.0047871 (  0) 0) |
Odum, E. P., 1959. Fundamentals of Ecology. W. B. Saunders Co, Philadelphia, 384pp.
(  0) 0) |
Parsons, T. R., Lebrasseur, R. J. and Fulton, J. D., 1967. Some observations on the dependence of zooplankton grazing on the cell size and concentration of phytoplankton blooms. Oceano-graphical Society of Japan, 23: 10-17. DOI:10.5928/kaiyou1942.23.10 (  0) 0) |
Perissinotto, R. and Wooldridge, T. H., 2010. Short-term ther-mal effects of a power-generating plant on zooplankton in the Swartkops Estuary, South Africa. Marine Ecology, 10: 205-219. DOI:10.1111/j.1439-0485.1989.tb00473.x (  0) 0) |
Petrou, K., Doblin, M. A. and Ralph, P. J., 2011. Heterogeneity in the photoprotective capacity of three Antarctic diatoms du-ring short-term changes in salinity and temperature. Marine Biology, 158: 1029-1041. DOI:10.1007/s00227-011-1628-4 (  0) 0) |
Pielou, E. C., 1966. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13: 131-144. DOI:10.1016/0022-5193(66)90013-0 (  0) 0) |
Plourde, S., Dodson, J. J., Runge, J. A. and Therriault, J. C., 2002. Spatial and temporal variations in copepod community struc-ture in the lower St. Lawrence Estuary, Canada.. Marine Eco-logy Progress Series, 230: 211-224. DOI:10.3354/meps230211 (  0) 0) |
Poornima, E. H., Rajadurai, M., Rao, T. S., Anupkumar, B., Ra-jamohan, R., Narasimhan, S. V., Rao, V. N. R. and Venugo-palan, V. P., 2005. Impact of thermal discharge from a tropical coastal power plant on phytoplankton. Journal of Thermal Biology, 30: 307-316. DOI:10.1016/j.jtherbio.2005.01.004 (  0) 0) |
Pu, X. M., Sun, S., Yang, B., Ji, P., Zhang, Y. and Zhang, F., 2004. The combined effects of temperature and food supply on Calanus sinicus in the southern Yellow Sea in summer. Jour-nal of Plankton Research, 26: 1049-1057. DOI:10.1093/plankt/fbh097 (  0) 0) |
Purcell, J. E., Uye, S. I. and Lo, W. T., 2007. Anthropogenic cause of jellyfish blooms and their direct consequences for humans: A review. Marine Ecology Progress Series, 350: 153-174. DOI:10.3354/meps07093 (  0) 0) |
Richardson, A. J. and Schoeman, D. S., 2004. Climate impact on plankton ecosystems in the Northeast Atlantic. Science, 305(5690): 1609-1612. DOI:10.1126/science.1100958 (  0) 0) |
Roemmich, D. and Mcgowan, J., 1995. Climatic warming and the decline of zooplankton in the California Current. Science, 267(5202): 1324-1326. DOI:10.1126/science.267.5202.1324 (  0) 0) |
Shannon, C. and Weaver, W., 1949. The Mathematical Theory of Communication. University of Illinois Press, Urbana, IL, 94pp.
(  0) 0) |
Shi, Y. Q., Sun, S., Zhang, G. T., Wang, S. W. and Li, C. L., 2015. Distribution pattern of zooplankton functional groups in the Yellow Sea in June: A possible cause for geographical sepa-ration of giant jellyfish species. Hydrobiologia, 754: 43-58. DOI:10.1007/s10750-014-2070-7 (  0) 0) |
Shiah, F. K., Tu, Y. Y., Tsai, H. S., Kao, S. J. and Jan, S., 2005. A case study of system and planktonic responses in a subtro-pical warm plume receiving thermal effluents from a power plant. Terrestrial Atmospheric and Oceanic Sciences, 16: 513-528. DOI:10.3319/TAO.2005.16.2.513(O) (  0) 0) |
Song, L., Zhou, Z. C., Wang, N. B., Ma, Z. Q., Xue, K., Tian, J., Yang, S., Wang, Z. H. and Wu, J. H., 2010. Zooplankton di-versity of Liaodong Bay and relationship with oceanic envi-ronmental factors. Marine Sciences, 34: 35-39 (in Chinese with English abstract). (  0) 0) |
Sun, S., Huo, Y. Z. and Yang, B., 2010. Zooplankton functional groups on the continental shelf of the Yellow Sea. Deep-Sea Research Part Ⅱ-Topical Studies in Oceanography, 57(11-12): 1006-1016. DOI:10.1016/j.dsr2.2010.02.002 (  0) 0) |
Tachibana, A., Itoh, H. and Yoshida, Y., 2013. Seasonal and an-nual change in community structure of meso-sized copepods in Tokyo Bay, Japan. Journal of Oceanography, 69: 545-556. DOI:10.1007/s10872-013-0191-7 (  0) 0) |
Tang, Q. S., Jin, X. S., Wang, J., Zhuang, Z. M., Cui, Y. and Meng, T. X., 2003. Decadal-scale variations of ecosystem produc-tivity and control mechanisms in the Bohai Sea. Fisheries Oceanography, 12(4-5): 223-233. DOI:10.1046/j.1365-2419.2003.00251.x (  0) 0) |
Uye, S. I., 2011. Human forcing of the copepod-fish-jellyfish tri-angular trophic relationship. Hydrobiologia, 666: 71-83. DOI:10.1007/s10750-010-0208-9 (  0) 0) |
Uye, S. I., 2014. The giant jellyfish Nemopilema nomurai in East Asian marginal seas. In: Jellyfish Blooms. Pitt, K. A., and Lucas, C. H., eds., Springer, Dordrecht, 185-205, https://doi.org/10.1007/978-94-007-7015-7_8.
(  0) 0) |
Vandysh, O. I., 2012. Specific features of zooplankton commu-nity in industrially polluted areas of Subarctic Lake Imandra (Monche, Belaya, and Molochnaya Bays). Russian Journal of Ecology, 43: 390-397. DOI:10.1134/S1067413612050153 (  0) 0) |
Wang, Y., Fang, E. J., Guo, B., Gao, Y. and Hou, C. Q., 2014. Zoo-plankton community structure and its relationship with envi-ronmental factors in spring of Bohai Bay in Tianjin sea area. Marine Fisheries, 36: 300-305 (in Chinese with English abstract). (  0) 0) |
Wang, X., Wang, X. X., Su, X., Meng, Q. H., Zou, D. J., Yin, X. D., Wang, L., Wen, S. Y. and Zhao, J. H., 2018. Thermal dis-charge monitoring of nuclear power plant with aerial remote sensing technology using a UAV platform: Take Hongyanhe Nuclear Power Plant, Liaoning Province, as example. Remote Sensing for Land and Resources, 30(4): 182-186 (in Chinese with English abstract). (  0) 0) |
Wang, Y. S., Lou, Z. P., Sun, C. C., Wang, H. L., Mitchell, B. G., Wu, M. L. and Deng, C., 2012. Identification of water quality and zooplankton characteristics in Daya Bay, China, from 2001 to 2004. Environmental Earth Sciences, 66: 655-671. DOI:10.1007/s12665-011-1274-7 (  0) 0) |
Wang, Z. H., Zhao, J. G., Zhang, Y. J. and Yu, C., 2009. Phyto-plankton community structure and environmental parameters in aquaculture areas of Daya Bay, South China Sea. Journal of Environmental Sciences, 21: 1268-1275. DOI:10.1016/S1001-0742(08)62414-6 (  0) 0) |
Wu, Y. N., Wang, Y. Q., Hou, Q. M., Jiao, F. and Sun, G. C., 2017. Experience feedbacks on events of nuclear power plants cold source systems blocked by oceanic foreign matter. Nuclear Safety, 16(1): 26-32 (in Chinese with English abstract). (  0) 0) |
Yang, L., Liu, J., Zhang, J., Wang, X. L., Xu, Y., Li, X. and He, L., 2018. Zooplankton community variation and its relation-ship with environmental variables in Bohai Bay. Journal of Marine Sciences, 36(1): 93-101. (  0) 0) |
Ye, Y. Y., Chen, K. B., Zhou, Q. Q., Xiang, P., Huo, Y. L. and Lin, M., 2018. Impacts of thermal discharge on phytoplankton in Daya Bay. Journal of Coastal Research, 83: 135-147. DOI:10.2112/SI83-022.1 (  0) 0) |
Zhang, W. C. and Wang, R., 2000. Microzooplankton and their grazing pressure on phytoplankton in Bohai Sea. Oceanologia et Limnologia Sinica, 31: 252-258 (in Chinese with English abstract). (  0) 0) |
Zhang, W. C., Wang, K., Gao, S. W. and Wang, R., 2002. Zoo-plankton in the Bohai Sea in spring and autumn. Oceanologia et Limnologia Sinica, 33: 630-639. (  0) 0) |
Zhou, F., Huang, D. J. and Su, J. L., 2009. Numerical simulation of the dual-core structure of the Bohai Sea cold bottom water in summer. Chinese Science Bulletin, 54(23): 4520-4528. DOI:10.1007/s11434-009-0019-4 (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


