2) Shandong Key Laboratory of Corrosion Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266100, China
Silver oxide nanomaterials (Ag2O NMs) have been extensively studied because of their remarkable physical and chemical properties that give rise to unique electrocatalytic and photocatalytic performances (Haq et al., 2018; Azevedo et al., 2019; Rashmi et al., 2020). As a result, they have been applied in many fields, such as in sensors (Rahman et al., 2013; Bhanjana et al., 2019; Jamal et al., 2020), environmental remediation (Rahman et al., 2018; Ullah et al., 2020), wastewater treatment (Sun et al., 2019; Yaseen et al., 2021), solar cells (Jo et al., 2018), supercapacitors (Kariper et al., 2020; Oje et al., 2021), and anti-cancer agents (Iqbal et al., 2019). The antibacterial effect of silver is concentration-dependent, and a low silver concentration cannot manifest a sufficient inhibition of bacterial growth. Thus, for the successful application of silver-based antibacterial agents, a silver source needs to be more active and exhibit a controlled release of silver ion to avoid any toxic effects (Wang et al., 2011).
The ability of Ag2O NMs to easily integrate with many substrates to form structurally and functionally diverse nanocomposites has encouraged a significant amount of research (Akbarzadeh et al., 2020; Eid et al., 2020; Hou et al., 2020; Sinha et al., 2020; Liu et al., 2021). Because of this, nanocomposites have attracted considerable attention as promising materials for a diversity of applications in various fields (Ahmad et al., 2020). Among the variety of substrates that can be employed in the development of Ag2O-based nanocomposites, natural polymers and their derivatives have attracted myriad attention due to their low-costs, low toxicities, and modifiability. Carboxymethyl starch (CMS), one of the most important commercially derived starch derivatives, is synthesized commercially by the etherification of the free hydroxyl groups of starch with sodium monochloroacetate. In contrast to native starch, CMS is soluble in cold water; this physical property, among others, makes CMS an attractive derivative of starch that has led it to be widely studied in many fields (Qi et al., 2020; Quadrado et al., 2020). For example, the preparation of nanocomposite hydrogels comprising CMS/polyvinyl alcohol/silver nanoparticles (Ag NPs) have been reported for use as biomedical materials. In addition, the antibacterial activities of synthesized NCs against Escherichia coli and Staphylococcus aureus were evaluated using the agar diffusion method (Ounkaew et al., 2020).
Although Ag2O NMs was reported as effective antibacterial agents (Dharmaraj et al., 2021; Silva et al., 2021), to the best of our knowledge, the fabrication of Ag2O NPs loaded onto CMS and the evaluation of their antibacterial activities have not been well-reported. Therefore, this work entails on the synthesis, as well as structural and bactericidal characterization, of CMS-Ag2O NCs, which were prepared by modifying the surface of CMS with loading Ag2O NPs. The resulting CMS-Ag2O NCs were characterized by the X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). In addition, the antibacterial activities of CMS-Ag2O NC were evaluated against E. coli, S. aureus, and marine bacteria (Pseudoalteromonas tetraodonis, Micrococcus luteus, and Shewanella putrefaciens).
2 Materials and Methods 2.1 MaterialsThe chemical reagents used in this study included starch derived from corn (Food grade, Qingdao Lingniao Food Co., Ltd.), chloroacetic acid (C2H3ClO2, Shanghai Sinopharm Chemical Reagent Co., Ltd.), sodium hydroxide (NaOH, Shanghai Sinopharm Chemical Reagent Co., Ltd.), silver nitrate (AgNO3, Shanghai Sinopharm Chemical Reagent Co., Ltd.), 2216E liquid medium and 2216E agar (Qingdao Hope Biotechnology Co., Ltd.), nutrient broth and nutrient agar (Beijing Land Bridge Technology Co., Ltd.), and deionized water (Conductivity: 2.10–2.20 μS cm−1, Qingdao distilled water plant). The bacterial strains E. coli, S. aureus, P. tetraodonis, M. luteus, and S. putrefaciens were supplied by College of Marine Life Science, Ocean University of China.
2.2 Methods 2.2.1 Synthesis of CMSCMS was prepared according to the reference reported by Sun (2001). Starch (40 g) was mixed with 12 mL of 45 wt.% NaOH aqueous solution and 200 mL of ethanol. The resulting suspension was stirred continuously at 35℃ for 60 min. Chloroacetic acid was added to the suspension, and the reaction temperature was controlled between 40 and 45℃. The pH was adjusted to 8–10 by the slow addition of a 45 wt% NaOH solution, and the reaction was allowed to continue stirring for 60 min. The reaction temperature was increased to 60℃, after which the reaction was allowed to continue stirring for another 60 min. Finally, the CMS gained (denoted S0) was isolated by filtration, washed with an 80% (v/v) solution of ethanol in water, and dried at 60℃ overnight.
2.2.2 Synthesis of CMS-Ag2O NCsFirst, 1.0 g of CMS was added to different concentrations (1.5, 2.6, 5.2, and 13 mmol L−1) of aqueous AgNO3. Ion-exchange reaction was performed at 50℃ for 3 h to enable the association of Ag+ ion with CMS. The resulting CMS-Ag suspension was filtered, and the filter cake was washed with deionized water. To oxidize the bonded Ag+, 2 mol L−1 solution of NaOH was added to the suspension until the pH was approximately 10, after which the suspension was stirred at room temperature for 1 h. The CMS-Ag2O NCs were then collected by filtration, washed with deionized water, and dried at 60℃ overnight. The final prepared CMS-Ag2O NCs were accordingly labeled as S1, S2, S3, and S4 according to the order of Ag+ concentration from low to high.
2.2.3 CharacterizationThe structural features of CMS and CMS-Ag2O NCs were evaluated by X-ray diffraction (XRD, Bruker D8-Advance, Germany) using λ = 1.5418 Å, Cu Kα radiation in the wide-angle range of 2θ (5˚–70˚) at a scan speed of 6˚ min−1. The surface morphologies of CMS and CMS-Ag2O NCs were investigated by scanning electron microscopy (SEM, Hitachi S-4800, Japan). Elemental analysis was performed by X-ray photoelectron spectroscopy (XPS, ESCALAB 250XI, USA).
2.2.4 Antibacterial activities testNutrient broth (19.0 g) for cultivating E. coli, and S. aureus (or 37.4 g of 2216E liquid medium for cultivating marine bacteria) was dissolved in 1 L of deionized water. The liquid culture medium was obtained after the solution was sterilized in an autoclave at 121℃ for 15 min and cooled to room temperature. The bacterial strain was activated in an incubator at 37℃ for 24 h, after which the activated bacterial strain and 15 mL of the above liquid culture medium was transferred into a sterile test tube. The bacterial culture solution was obtained after the tube was sealed and incubated in a shaking incubator at 37℃ for 24 h.
Nutrient agar (33.0 g) for cultivating E. coli, and S. aureus (or 52.4 g of 216E agar for cultivating marine bacteria) was dissolved in 1 L of deionized water. The agar solution was sterilized at 121℃ for 15 min and cooled to approximately 45℃. About 30 mL of the sterilized agar solution was added to a sterile Petri dish and cooled until solidification to obtain agar plates.
15 mL of the liquid culture medium, and 200 μL of the bacterial culture solution were mixed together in a sterile test tube. The tube was sealed and incubated in a shaking incubator at 37℃ for 24 h, then the 'blank group' was obtained. According to the same experimental method, the 'experimental group' was obtained by adding sterile CMS-Ag2O NCs.
The solutions corresponding to the blank and experimental groups were diluted with sterilized 0.9 wt.% NaCl solution and inoculated onto different agar plates, respectively. The solutions were evenly coated on the agar, and the plates were incubated in an incubator at 37℃ until the numbers of live bacteria colonies are visible to the naked eye. The antibacterial efficiency (A) was evaluated according to the following equation:
| $ A = \left({\frac{{{N_0} - N}}{{{N_0}}}} \right) \times 100\%, $ | (1) |
where N0 and N are the numbers of live bacteria colonies corresponding to the blank group and experimental group.
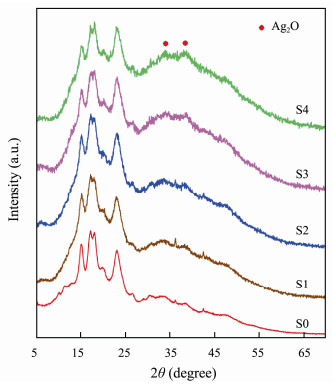
3 Results and Discussion 3.1 CharacterizationThe influences of the silver ion concentrations (1.5, 2.6, 5.2, and 13 mmol L−1) on the structure of CMS-Ag2O NCs were studied by XRD. Fig.1 displays the XRD spectra of the synthetic CMS and CMS-Ag2O NCs. As shown in the XRD spectrum of S0, the diffraction peaks were consistent with the standard spectrum of CMS. In the XRD spectra of S1 and S2, new diffraction peaks were observed, but these peaks were relatively weak and not obvious, which indicated that the Ag2O NPs were small in size and quantity. As the Ag+ ion concentration increased, noticeable diffraction peaks appeared at 33.88˚ and 38.08˚ (S3 and S4), which corresponded to the characteristic peaks of Ag2O (Khalid et al., 2020; Akram et al., 2021). The intensities of these peaks also gradually increased as the Ag+ ion concentration increased.
|
Fig. 1 XRD patterns of CMS (S0) and CMS-Ag2O NCs (S1–S4). |
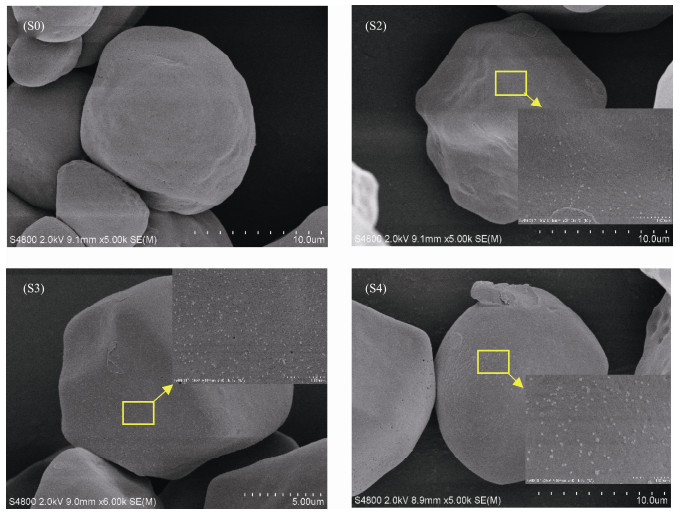
Fig.2 shows the SEM images of CMS and CMS-Ag2O NCs, which were used to determine the surface morphologies of the samples. As shown in the SEM image of S0, CMS particles featured an irregular granule structure. The SEM images of samples S2–S4 indicated that only minor changes on the surfaces of CMS-Ag2O NCs were observed compared to CMS. The Ag2O NPs that formed on the surface of CMS-Ag2O NCs exhibited a granular shape, and their sizes increased gradually as the concentration of Ag+ ion increased. As shown in the image of S4, the granular-shaped Ag2O NPs were the largest and most obvious when the Ag+ ion concentration was 13 mmol L−1 compared to a lower concentration. Overall, the diameter of the Ag2O NPs was in the range of 50–100 nm. These SEM results corroborated the formation of Ag2O NPs and were consistent with the XRD patterns in Fig.1 (S2–S4).
|
Fig. 2 SEM images of CMS (S0) and CMS-Ag2O NCs (S2–S4). |
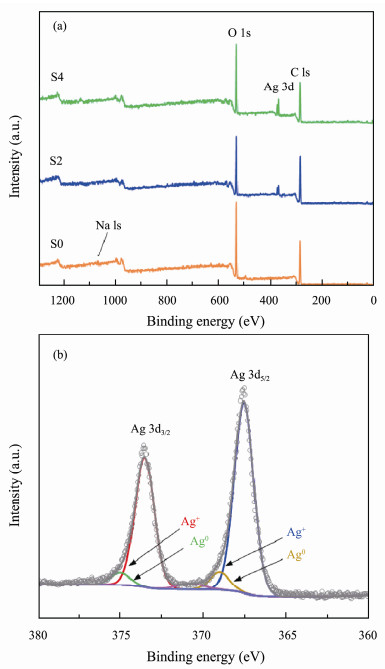
The elemental compositions of the prepared CMS and CMS-Ag2O NCs were determined by XPS, and the results are shown in Figs.3a, b. As shown in Fig.3a, the survey spectrum clearly indicated that CMS (S0) was mainly composed of C and O, with a small amount of Na, while CMS-Ag2O NCs (S2 and S4) mainly comprised Ag, C, and O. As the concentration of Ag+ increased, the XPS peaks corresponding to silver appeared, and their intensity increased. The high-resolution XPS spectrum of Ag 3d is shown in Fig.3b. The Ag 3d5/2 and Ag 3d3/2 peaks were each fitted into two peaks corresponding to Ag+ and Ag0. Ag+ was the main valence state, accounting for the vast majority. The main peaks centered at 367.6 eV and 373.6 eV were attributed to Ag+ (Hájková et al., 2014; Hu et al., 2015). The presence of Ag+ was also corroborated by the XPS results.
|
Fig. 3 XPS patterns of S0, S2, and S4. (a), survey; (b), Ag 3d. |
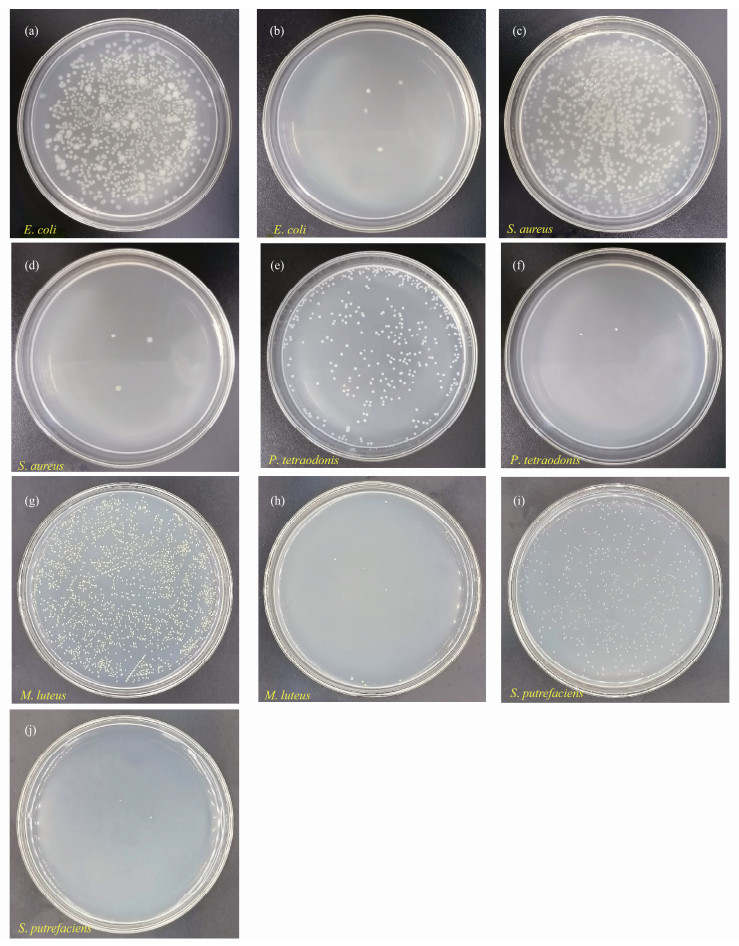
The antibacterial activities of CMS-Ag2O NC (S4) were evaluated against E. coli, S. aureus, and marine bacteria (P. tetraodonis, M. luteus, and S. Putrefaciens). The amounts of CMS and S4 were 7 mg used for assessing the antibacterial activity against marine bacteria, respectively. Whereas 15 mg of CMS and S4 were used respectively against E. coli and S. aureus. The results of the antibacterial activity tests are shown in Table 1 and Fig.4. After several hours of incubation, the numbers of live bacterial colonies in the experimental groups significantly decreased compared to those in the blank groups in Table 1.
|
|
Table 1 Antibacterial activities of CMS-Ag2O NC (S4) |
|
Fig. 4 Antibacterial activity tests: (a, c, e, g, i) blank group, (b, d, f, h, j) experimental group. |
As shown in Figs.4a, b, the numbers of live bacterial colonies were 1150 in the blank group versus 5 in the experimental group, which amounted to an antibacterial efficiency of 99.6% against E. coli. From Figs.4c, d, the numbers of live S. aureus colonies were 915 in the blank group, and 3 in the experimental group. Therefore, the antibacterial efficiency against S. aureus was calculated to be 99.7%. As shown in Figs.4e, f, the numbers of live bacteria colonies remained 361 in the blank group, while only 2 in the experimental group. The resulting antibacterial efficiency of CMS-Ag2O NC against P. tetraodonis was calculated to be 99.4%. In Figs.4g, h, the numbers of live M. luteus colonies counted were 2000 in the blank group compared to only 11 in the experimental group, which equated to an antibacterial efficiency of 99.5%. From Figs.4i, j, the numbers of live bacteria colonies were 462 and 2 in the blank group and experimental group, respectively. The antibacterial efficiency reached 99.6% against S. putrefaciens.
The reproduction rate of E. coli and S. aureus are much faster than marine bacteria. Therefore, a greater amount of CMS-Ag2O NC was required to improve the antibacterial efficiency against E. coli and S. aureus. The antibacterial efficiencies of CMS-Ag2O NC against the bacterial species tested in this study were all greater than 99%. Therefore, these results indicated that CMS-Ag2O NC was highly effective as a bactericidal agent against multiple bacterial species.
The results presented herein indicated that CMS was a suitable substrate for hosting silver oxide, and CMS-Ag2O NC exhibited a controlled release of silver ion. It has been suggested that AgNPs inhibit bacterial growth by adhering to the surface of the bacterial cell membrane, resulting in damage to surface proteins and ultimately destruction to the cell membrane and obstructing the respiratory system of microbe (Wu et al., 2018; Sable et al., 2020). Another proposed mechanism is that AgNPs can be phagocytized, potentially causing cytolysis by interacting with phosphorus and sulfur containing compounds, such as DNA (Marslin et al., 2015; Sable et al., 2020). A third proposed mechanism involves AgNPs inducing cellular toxicity and oxidative stress through the formation of reactive oxygen species (ROS) and free radicals (Neves et al., 2021).
4 ConclusionsCMS-Ag2O NCs were synthesized by modifying CMS with Ag2O. The XRD and XPS results corroborated the formation and loading of Ag2O NPs on the surface of CMS. The SEM images demonstrated that, when the concentration of Ag+ was 13 mmol L−1, the Ag2O NPs presented a clear and large granular shape, and the diameters of the Ag2O NPs were between 50 and 100 nm. In addition, CMS-Ag2O NC demonstrated remarkable antibacterial properties against E. coli, S. aureus, P. tetraodonis, M. luteus, and S. putrefaciens. The antibacterial efficiencies of CMS-Ag2O NC against E. coli, S. aureus, P. tetraodonis, M. luteus, and S. putrefaciens reached 99.6%, 99.7%, 99.4%, 99.5%, and 99.6%, respectively. These results indicated that CMS-Ag2O NC was highly effective as a bactericidal agent against multiple bacterial species. CMS-Ag2O NC exhibited a controlled release of silver ion CMS-Ag2O NC has the potential to be further applied to antifouling coating.
AcknowledgementsThe project was supported by the National Key Research and Development Project (No. 2019YFC0312103) and the Open Fund of Shandong Key Laboratory of Corrosion Science (No. KLCS201905).
Ahmad, I., Islam, M., Habis, N. A., and Parvez, S., 2020. Hotpressed graphene nanoplatelets or/and zirconia reinforced hybrid alumina nanocomposites with improved toughness and mechanical characteristics. Journal of Materials Science & Technology, 40: 135-145. (  0) 0) |
Akbarzadeh, E., Rasteh, M., and Gholami, M. R., 2020. Visible light photocatalytic performance of Ag2O/ZnCr-LDH nanocomposite. Chemical Physics Letters, 751: 137558. DOI:10.1016/j.cplett.2020.137558 (  0) 0) |
Akram, N., Guo, J., Guo, Y., Kou, Y. L., Suleman, H., and Wang, J., 2021. Enhanced synergistic catalysis of novel Ag2O/CuO nanosheets under visible light illumination for the photodecomposition of three dyes. Journal of Environmental Chemical Engineering, 9(2): 104824. DOI:10.1016/j.jece.2020.104824 (  0) 0) |
Azevedo, V. H. R., Silva, J. L., and Stradiotto, N. R., 2019. Silver oxide nanoparticles in reduced graphene oxide modified electrode for amino acids electrocatalytic oxidation. Journal of Electroanalytical Chemistry, 845: 57-65. DOI:10.1016/j.jelechem.2019.05.037 (  0) 0) |
Bhanjana, G., Chaudhary, G. R., Dilbaghi, N., Chauhan, M., Kim, K. H., and Kumar, S., 2019. Novel electrochemical sensor for mononitrotoluenes using silver oxide quantum dots. Electrochimica Acta, 293: 283-289. DOI:10.1016/j.electacta.2018.10.042 (  0) 0) |
Dharmaraj, D., Krishnamoorthy, M., Rajendran, K., Karuppiah, K., Annamalai, J., Durairaj, K. R., et al., 2021. Antibacterial and cytotoxicity activities of biosynthesized silver oxide (Ag2O) nanoparticles using Bacillus paramycoides. Journal of Drug Delivery Science and Technology, 61: 102111. DOI:10.1016/j.jddst.2020.102111 (  0) 0) |
Eid, M., Arnaouty, M. B. E., Salah, M., Soliman, E. S., and Hegazy, E. S. A., 2020. Radiation synthesis and characterization of poly (aniline/glycidyl methacrylate)-Ag2O nanocomposites. Inorganic Chemistry Communications, 114: 107844. DOI:10.1016/j.inoche.2020.107844 (  0) 0) |
Hájková, P., Matoušek, J., and Antoš, P., 2014. Aging of the photocatalytic TiO2 thin films modified by Ag and Pt. Applied Catalysis B: Environmental, 160-161: 51-56. DOI:10.1016/j.apcatb.2014.04.045 (  0) 0) |
Haq, S., Rehman, W., Waseem, M., Meynen, V., Awan, S. U., Saeed, S., et al., 2018. Fabrication of pure and moxifloxacin functionalized silver oxide nanoparticles for photocatalytic and antimicrobial activity. Journal of Photochemistry and Photobiology B: Biology, 186: 116-124. DOI:10.1016/j.jphotobiol.2018.07.011 (  0) 0) |
Hou, J. W., Zhou, J. Y., Liu, Y. F., Yang, Y., Zheng, S. X., and Wang, Q. Y., 2020. Constructing Ag2O nanoparticle modified TiO2 nanotube arrays for enhanced photocatalytic performances. Journal of Alloys and Compounds, 849: 156493. DOI:10.1016/j.jallcom.2020.156493 (  0) 0) |
Hu, X. H., Zhu, Q., Wang, X. L., Kawazoe, N., and Yang, Y. N., 2015. Nonmetal-metal-semiconductor-promoted P/Ag/Ag2O/ Ag3PO4/TiO2 photocatalyst with superior photocatalytic activity and stability. Journal of Materials Chemistry A, 3(34): 17858-17865. DOI:10.1039/C5TA05153C (  0) 0) |
Iqbal, S., Fakhar-e-Alam, M., Akbar, F., Shafiq, M., Atif, M., Amin, N., et al., 2019. Application of silver oxide nanoparticles for the treatment of cancer. Journal of Molecular Structure, 118: 203-209. (  0) 0) |
Jamal, R. K., Mutlak, F. A. H., Ibrahim, F. T., and Nayef, U. M., 2020. Synthesis of Ag2O films by pulsed laser deposited on porous silicon as gas sensor application. Optik, 218: 164971. DOI:10.1016/j.ijleo.2020.164971 (  0) 0) |
Jo, H., Yang, J. H., Lee, J. H., Lim, J. W., Lee, J., Shin, M., et al., 2018. Transparent bifacial a-Si: H solar cells employing silver oxide embedded transparent rear electrodes for improved transparency. Solar Energy, 170: 940-946. DOI:10.1016/j.solener.2018.05.096 (  0) 0) |
Kariper, İ. A., and Tezel, F. M., 2020. Synthesis and characterization of magnesium oxide/silver oxide electrode for supercapacitors by simple Sol-Gel process. Journal of Energy Storage, 32: 101958. DOI:10.1016/j.est.2020.101958 (  0) 0) |
Khalid, N. R., Bilal, M., Tahir, M. B., Shakil, M., Iqbal, T., Rafique, M., et al., 2020. Interfacial coupling effect of Ag2O nanorods over MoS2 microflowers for improved photocatalytic activity. Ceramics International, 46(5): 6856-6859. DOI:10.1016/j.ceramint.2019.11.179 (  0) 0) |
Liu, C., Xu, J. J., Du, X. Y., Li, Q. H., Fu, Y. H., and Chen, M. D., 2021. Synthesis of Ag2O-KNbO3 heterojunction photocatalysts with enhanced visible-light-responsive photocatalytic performance for sulfamethoxazole degradation. Optical Materials, 112: 110742. DOI:10.1016/j.optmat.2020.110742 (  0) 0) |
Marslin, G., Selvakesavan, R. K., Franklin, G., Sarmento, B., and Dias, A. C. P., 2015. Antimicrobial activity of cream incorporated with silver nanoparticles biosynthesized from Withania somnifera. International Journal of Nanomedicine, 10: 5955-5963. (  0) 0) |
Neves, A. C. O., Viana, A. D., Menezes, F. G., Neto, A. O. W., Melo, M. C. N., and Gasparotto, L. H. S., 2021. Biospectroscopy and chemometrics as an analytical tool for comparing the antibacterial mechanism of silver nanoparticles with popular antibiotics against Escherichia coli. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 253: 119558. DOI:10.1016/j.saa.2021.119558 (  0) 0) |
Oje, A. I., Ogwu, A. A., and Oje, A. M., 2021. Effect of temperature on the electrochemical performance of silver oxide thin films supercapacitor. Journal of Electroanalytical Chemistry, 882: 115015. DOI:10.1016/j.jelechem.2021.115015 (  0) 0) |
Ounkaew, A., Kasemsiri, P., and Jetsrisuparb, K., 2020. Synthesis of nanocomposite hydrogel based carboxymethyl starch/ polyvinyl alcohol/nanosilver for biomedical materials. Carbohydrate Polymers, 248: 116767. DOI:10.1016/j.carbpol.2020.116767 (  0) 0) |
Qi, T. M., Lü, S. Y., Zhang, S. F., Bai, X., Chen, J., Huang, M. J., et al., 2020. Zein coated porous carboxymethyl starch fertilizer for iron promoting and phosphate sustainable release. Journal of Cleaner Production, 258: 120778. DOI:10.1016/j.jclepro.2020.120778 (  0) 0) |
Quadrado, R. F. N., and Fajardo, A. R., 2020. Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arabian Journal of Chemistry, 13(1): 2183-2194. DOI:10.1016/j.arabjc.2018.04.004 (  0) 0) |
Rahman, M. M., Khan, S. B., Asiri, A. M., and Al-Sehemi, A. G., 2013. Chemical sensor development based on polycrystalline gold electrode embedded low-dimensional Ag2O nanoparticles. Electrochimica Acta, 112: 422-430. DOI:10.1016/j.electacta.2013.08.164 (  0) 0) |
Rahman, M. M., Alam, M. M., Hussain, M. M., Asiri, A. M., and Zayed, M. E. M., 2018. Hydrothermally prepared Ag2O/ CuO nanomaterial for an efficient chemical sensor development for environmental remediation. Environmental Nanotechnology, Monitoring & Management, 10: 1-9. (  0) 0) |
Rashmi, B. N., Harlapur, S. F., Avinash, B., Ravikumar, C. R., Nagaswarupa, H. P., Kumar, M. R. A., et al., 2020. Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic and biological studies. Inorganic Chemistry Communications, 111: 107580. DOI:10.1016/j.inoche.2019.107580 (  0) 0) |
Sable, S. V., Kawade, S., Ranade, S., and Joshi, S., 2020. Bioreduction mechanism of silver nanoparticles. Materials Science and Engineering: C, 107: 110299. DOI:10.1016/j.msec.2019.110299 (  0) 0) |
Silva, L. C. A., Neto, F. G., Pimentel, S. S. C., Palácios, R. S., Satob, F., Retamiro, K. M., et al., 2021. The role of Ag2O on antibacterial and bioactive properties of borate glasses. Journal of Non-Crystalline Solids, 55: 120611. (  0) 0) |
Sinha, S., Devi, N. A., Nongthombam, S., Bhujel, R., Rai, S., Sarkar, G., et al., 2020. Investigation of optical, electrical and electrochemical properties of polyaniline/rGO/Ag2O nanocomposite. Diamond and Related Materials, 107: 107885. DOI:10.1016/j.diamond.2020.107885 (  0) 0) |
Sun, F. J., Zeng, Q. H., Tian, W., Zhu, Y. M., and Jiang, W., 2019. Magnetic MFe2O4-Ag2O (M = Zn, Co, & Ni) composite photocatalysts and their application for dye wastewater treatment. Journal of Environmental Chemical Engineering, 7(2): 103011. DOI:10.1016/j.jece.2019.103011 (  0) 0) |
Sun, Y. M., Liu, H. L., Chen, G. H., and Song, J. M., 2001. Preparation and properties of starch oil gelling agent on seawater surface. Marine Sciences, 25(8): 37-41. (  0) 0) |
Ullah, S., Fayeza, D., Khan, A. A., Jan, A., Aain, S. Q., Neto, E. P. F., et al., 2020. Enhanced photoactivity of BiVO4/Ag/Ag2O Z-scheme photocatalyst for efficient environmental remediation under natural sunlight and low-cost LED illumination. Colloid Surface A, 600: 124946. DOI:10.1016/j.colsurfa.2020.124946 (  0) 0) |
Wang, J. C., Wang, Z. P., Guo, S., Zhang, J. Y., Song, Y., Dong, X. M., et al., 2011. Antibacterial and anti-adhesive zeolite coatings on titanium alloy surface. Microporous and Mesoporous Materials, 146(1-3): 216-222. DOI:10.1016/j.micromeso.2011.04.005 (  0) 0) |
Wu, Y. P., Yang, Y., Zhang, Z. J., Wang, Z. H., Zhao, Y. B., and Sun, L., 2018. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Advanced Powder Technology, 29(2): 407-415. DOI:10.1016/j.apt.2017.11.028 (  0) 0) |
Yaseen, M., Farooq, M. U., Ahmad, W., and Subhan, F., 2021. Fabrication of rGO-CuO and/or Ag2O nanoparticles incorporated polyvinyl acetate based mixed matrix membranes for the removal of Cr6+ from anti-corrosive paint industrial wastewater. Journal of Environmental Chemical Engineering, 9(2): 105151. DOI:10.1016/j.jece.2021.105151 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


