2) Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China;
3) Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy of Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China;
4) Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK
The ciliated protists are a large group of microbial eukaryotes that play vital roles in cycling of materials and transfer of energy in aquatic ecosystems (Caron et al., 2012; Gao et al., 2017; Worden et al., 2015; Xu et al., 2017). The class Spirotrichea is an extremely diverse group, which typically has a paroral membrane and a well-developed adoral zone of polykinetids that spirals out over the anterior end, and sometimes completely encloses it. The class is divided into seven subclasses, including the subclasses Hypotrichia and Oligotrichia (Lynn, 2008).
Oligotrich ciliates are important components of the marine microzooplankton and episodically dominate marine planktonic ciliate communities (Pierce and Turner, 1992). They are characterized by their conspicuous adoral zone of membranelles arranged in two sections and somatic ciliature comprising a girdle and a ventral kinety (Lynn, 2008). In recent years, many known species have been redescribed based on their infraciliature revealed by protargol-staining techniques (Agatha, 2004, 2011; Krainer, 1991; Liu et al., 2011a, 2011b, 2015, 2017; Lynn and Gilron, 1993; Petz et al., 1995; Song and Bradbury, 1998; Song et al., 2015a, 2015b, 2018a, 2018b; Wang et al., 2018; Xu et al., 2009; Yan et al., 2017; Zhang et al., 2017; Zhao et al., 2017;).
The subclass Hypotrichia Stein, 1859, comprising about 800 described species, is a highly diverse group with respect to morphological and ontogenetic patterns (Berger, 1999, 2006, 2008, 2011; Chen et al., 2013, 2017a; Dong et al., 2016; Foissner, 2016; Huang et al., 2018; Kumar et al., 2015, 2017; Luo et al., 2017; Pan et al., 2016; Shao et al., 2014). Recent investigations of new habitats and the increasing applications of molecular techniques have shown that the diversity of hypotrich ciliates is underestimated (Chen et al., 2011, 2015, 2017b; Liu et al., 2017; Lu et al., 2017; Paiva et al., 2016; Park et al., 2017; Wang et al., 2016, 2017).
During a faunistic study of spirotrich ciliates in China, two oligotrich and two hypotrich species were isolated, giving the opportunity to reinvestigate their morphology.
2 Materials and Methods 2.1 Collection, Isolation and Morphological StudiesBoth Spirostrombidium apourceolare Liu et al., 2013 and Parallelostrombidium obesum Liu et al., 2015 were isolated from samples collected from mangrove wetlands near Zhanjiang, China (21°22'N, 110°25'E) on 3 November 2012 and 26 November 2012, respectively (Figs. 1D, E). Two populations of Protogastrostyla pulchra (Pereyaslawzewa, 1886) Gong et al., 2007 were collected on 30 August 2012, from Taipingjiao beach in Qingdao, China (36°03'N, 120°20'E), and on 5 November 2013, from the mangrove wetlands near Zhanjiang (21°37'N, 110°43'E), respectively (Figs. 1A, C). Samples from Taipingjiao beach were collected from the surface of rocky sediments using a sponge. Samples from mangrove wetlands comprised a mixture of water and organic debris. Uncinata bradburyae (Gong et al., 2001) Luo et al., 2015 was collected on 26 May 2015, from ZhongYuan maritime square in Qingdao (36°03'N, 120°18'E) (Fig. 1B). In this case, samples were collected using artificial substrates, i.e., glass slides that are fixed to a frame and immersed to a depth of 1.5 m for about 10 days to allow the colonization of ciliates.
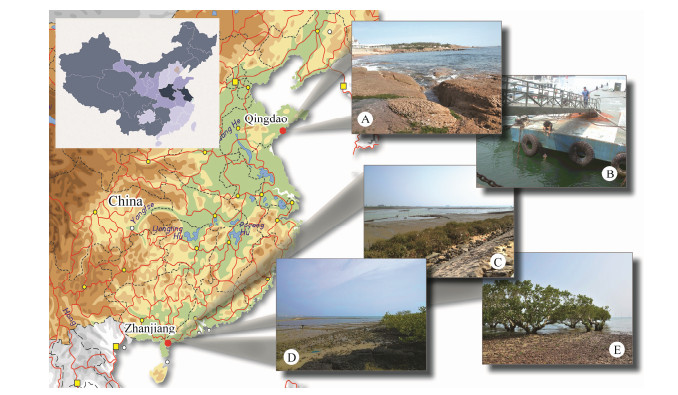
|
Fig. 1 Map of South-East China and photographs of sampling sites (A-E). A, B, Coastal waters off Qingdao. C-E, Mangrove wetlands in Zhanjiang. |
The samples were transferred to Petri dishes and the ciliates were immediately isolated under a dissecting microscope for further studies. No pure cultures were established. The morphology of live cells was studied using bright-field and differential interference contrast microscopy (Nikon eclipse E600). Protargol staining (Wilbert, 1975) was used to reveal the infraciliature and nuclear apparatus. The protargol reagent was made according to Pan et al. (2013). Drawings of live cells were based on photomicrographs and those of silver-stained cells were made using a camera lucida. Counts and measurements were performed at a magnification of 1000×. Terminology is according to Agatha and Riedel-Lorjé (2006) and Berger(2006, 2008), and the taxonomic system is mainly according to Lynn (2008) and Gao et al. (2016).
3 Results 3.1 Subclass: Oligotrichia Butschli, 1887Order: Strombidiida Petz & Foissner, 1992
Family: Strombidiidae Fauré-Fremiet, 1970
Genus Parallelostrombidium Agatha, 2004
Parallelostrombidium obesum Liu et al., 2015
3.1.1 Locality and habitatCoastal waters off Zhanjiang (21°22xN, 110°25xE), Guang-dong Province, China: salinity 26.0, temperature 28.0℃ and pH 6.6.
3.1.2 Deposition of slidesTwo voucher slides with protargol-stained specimens of the Zhanjiang population were deposited in the Laboratory of Protozoology, Ocean University of China with registration numbers SW2012110301-1, SW2012110301-2.
3.1.3 Description of Zhanjiang population (Fig. 2; Table 1)
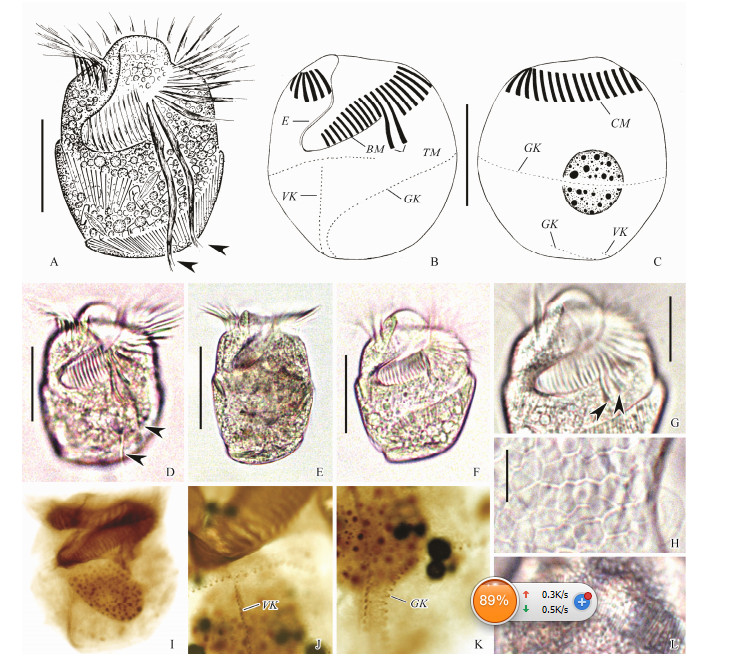
|
Fig. 2 Living Parallelostrombidium obesum Liu et al., 2015 from life (A, D-H, L) and samples after protargol staining (B, C, I-K). (A, D-F) Ventral views of different individuals, arrowheads mark thigmotactic membranelles. (B, C) Ventral and dorsal views of one specimen showing oral and somatic ciliature and macronucleus. (G) Anterior ventral view, arrowheads mark bases of thigmotactic membranelles. (H) Hemitheca. (L) Extrusomes. (I) Ventral view, showing oral and somatic ciliature. (J, K) Detail of somatic kineties. BM, buccal membranelles; CM, collar membranelles; E, endoral membrane; GK, girdle kinety; TM, thigmotactic membranelles; VK, ventral kinety. Scale bars = 35 μm (A-F); 20 μm (G); 5 μm (H). |
|
|
Table 1 Morphometric data of Parallelostrombidium obesum Liu et al., 2015 (first row) and Spirostrombidium apourceolare Liu et al., 2013 (second row) |
Body (60-95) × (50-70) μm in vivo, (64-102) × (59-90) μm in protargol-stained specimens. Body doliform, anterior and posterior ends transversely truncated, widest at mid-body, with conspicuous apical protrusion about 10 μm high (Figs. 2A, D-F). Dorso-ventrally flattened, ratio of width: thickness about (2-3):1. Hemitheca covers posterior dorsal and left ventral portion posterior to girdle kinety, composed of polygonal platelets about 3 μm across in vivo (Fig. 2H).
Cytoplasm colourless, containing numerous droplets, dark particles and food vacuoles about 3 μm across containing algae. Extrusomes acicular, about (10-12) × 0.5 μm in size, oriented slightly obliquely to cell surface arranged in rows above girdle kinety (Fig. 2L). One macronucleus, centrally located, ellipsoidal, (37-54) × (28-36) μm in size after protargol staining (Fig. 2C). Cytopyge, contractile vacuole and micronucleus not recognized. Locomotion fast, sometimes stationary temporarily attached to bottom of Petri dish via its thigmotactic membranelles.
Buccal cavity about 40% of cell length. Adoral zone of membranelles composed of 25-32 collar, 12-17 buccal and two thigmotactic membranelles (Fig. 2G). Each collar membranelle consists of three rows of basal bodies with cilia up to 25 μm long and bases of membranelles about 10-15 μm wide. Each buccal membranelle with cilia up to 12 μm long, bases of membranelles decrease in width from 15 μm at distal end to 7 μm at proximal end of zone portion. Thigmotactic membranelles located between collar and buccal portions, each consists of three rows of basal bodies, with cilia up to 45 μm long and bases of membranelles about 25-30 μm wide (Figs. 2A, D). Endoral membrane located on inner wall of buccal lip, composed of a single row of densely packed basal bodies.
Somatic ciliature composed of a girdle kinety and a ventral kinety (Figs. 2B, C, I). Girdle kinety dextrally spiralled with one and a half whorls, starting ventrally about 2/5 of way down cell length and about 12-17 dikinetids left of ventral kinety, extending horizontally across both right and dorsal sides, extends obliquely across ventral side, then curves posteriorly, crosses posterior margin, extends obliquely and terminates on dorsal side. Girdle kinety consists of 124-162 dikinetids (Fig. 2K). Each dikinetid bears a 2 μm long cilium on left basal body, right basal body barren. Ventral kinety starts about two dikinetids below girdle kinety, extends posteriorly, crosses posterior margin, terminates on dorsal side, posterior 10-18 dikinetids of ventral kinety parallel to girdle kinety. Ventral kinety composed of 30-39 dikinetids, each dikinetid bearing a 2 μm long cilium on anterior basal body, posterior basal body barren (Fig. 2J).
3.2 Genus Spirostrombidium Jankowski, 1978Spirostrombidium apourceolare Liu et al., 2013 (Fig. 3; Table 1)
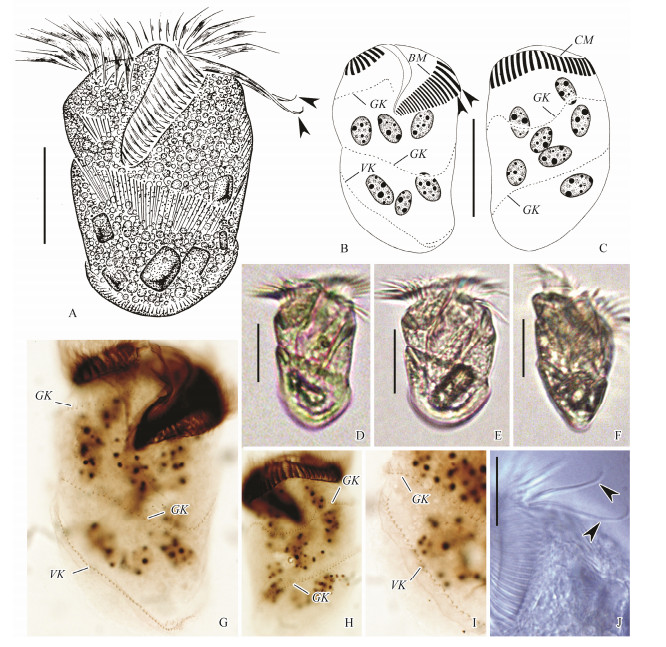
|
Fig. 3 Living Spirostrombidium apourceolare Liu et al., 2013 (A-C) and samples after protargol staining (G-I). (A, D-F) Ventral and ventral-lateral views of different individuals, arrowheads mark thigmotactic membranelles. (B, C) Ventral and dorsal views of one specimen showing oral and somatic ciliature and macronucleus, arrowheads mark thigmotactic membranelles. (G, H) Ventral and dorsal views, showing oral and somatic ciliature. (I) Posterior right ventral view, showing detail of somatic kineties. (J) Anterior ventral view, arrowheads mark thigmotactic membranelles. BM, buccal membranelles; CM, collar membranelles; E, endoral membrane; GK, girdle kinety; VK, ventral kinety. Scale bars=30 μm (A-F); 20 μm (J). |
A mangrove wetland near Zhanjiang (21°22xN, 110°25xE), Guangdong Province, China: salinity 27.0, temperature 28.0℃ and pH 6.8.
3.2.2 Deposition of slidesTwo voucher slides with protargol-stained specimens of Zhanjiang population were deposited in the Laboratory of Protozoology, Ocean University of China with registration numbers SW2012112603-1, SW2012112603-2.
3.2.3 Description of Zhanjiang populationBody (65-80) × (40-45) μm in vivo, (79-95) × (46-56) μm in protargol-stained specimens. Body elongate ellipsoidal, asymmetrical with apical protrusion inconspicuous and posterior end broadly rounded (Fig. 3A, D, E). Dorsal ventrally flattened, ratio of width: thickness about 3:1 (Fig. 3F). Hemitheca not recognized.
Cytoplasm colourless, containing numerous lipid droplets and food vacuoles with algae, posterior portion of cell containing dark particles; cell transparent at lower magnifications. Extrusomes rod-shaped, about 10 μm in length, oriented slightly obliquely to cell surface, usually arranged in three to eight rows along girdle kinety and one row spaced along ventral kinety. About 11-15 ovoid macronuclear nodules scattered in cytoplasm, each about (6-13) × (5-8) μm in size after protargol staining (Fig. 3G). Cytopyge, contractile vacuole and micronucleus not recognized. Locomotion by swimming while rotating about main cell axis, sometimes crawling while attached to substratum via thigmotactic membranelles.
Buccal cavity about 30%-50% of cell length in vivo. Adoral zone of membranelles composed of 26-29 collar, 17-23 buccal and two thigmotactic membranelles, and collar and buccal portions continuous (Fig. 3B). Cilia of collar membranelles about 20 μm long in vivo, bases of membranelles about 6-8 μm wide. Cilia of buccal membranelles about 10 μm long in vivo, bases of membranelles about 15-17 μm wide. Cilia of thigmotactic membranelles about 30 μm long in vivo, bases of membranelles about 18 μm wide and difficult to distinguish from buccal membranelles in protargol preparations (Fig. 3J). Endoral membrane located on inner wall of buccal lip, composed of a single row of densely packed basal bodies.
Somatic ciliature composed of a girdle kinety and a ventral kinety (Figs. 3B, C). Girdle kinety dextrally spiralled with two whorls, starting ventrally right of buccal membranelles with a small curve, spirals around body with dorsal and left lateral undulations on first whorl, terminates on posterior ventral side. Girdle kinety consists of 126-168 dikinetids (Figs. 3G, H). Each dikinetid bears a 2 μm long cilium on left basal body, right basal body barren. Ventral kinety composed of 31-48 dikinetids, each bearing a 2 μm long cilium on anterior basal body, posterior basal body barren (Fig. 3I), extends longitudinally along right margin of cell to posterior end, posterior portion inversely parallel to girdle kinety and comprises three to seven dikinetids (Fig. 3B, G).
3.3 Subclass: Hypotrichia Stein, 1859Order: Stichotrichida Fauré-Fremiet, 1961
Family: Amphisiellidae Jankowski, 1979
Genus Protogastrostyla Gong et al., 2007
Protogastrostyla pulchra (Pereyaslawzewa, 1886) Gong et al., 2007
3.3.1 Locality and habitatPopulation Ⅰ: coastal waters off Qingdao (36°03xN, 120°20xE), Shandong Province, China, salinity 29.0, temperature 26.0℃; population Ⅱ: coastal waters off Zhan-jiang (21°37xN, 110°43xE), Guangdong Province, China, salinity 17.0, temperature 20.0℃.
3.3.2 Deposition of slidesVoucher slides with protargol-stained specimens of Qingdao and Zhanjiang populations were deposited in the Laboratory of Protozoology, Ocean University of China with registration numbers LXT2012083001/1-3 and LXT 2013110506/1-4, respectively.
3.3.3 Description of Qingdao and Zhanjiang populations (Fig. 4; Table 2)
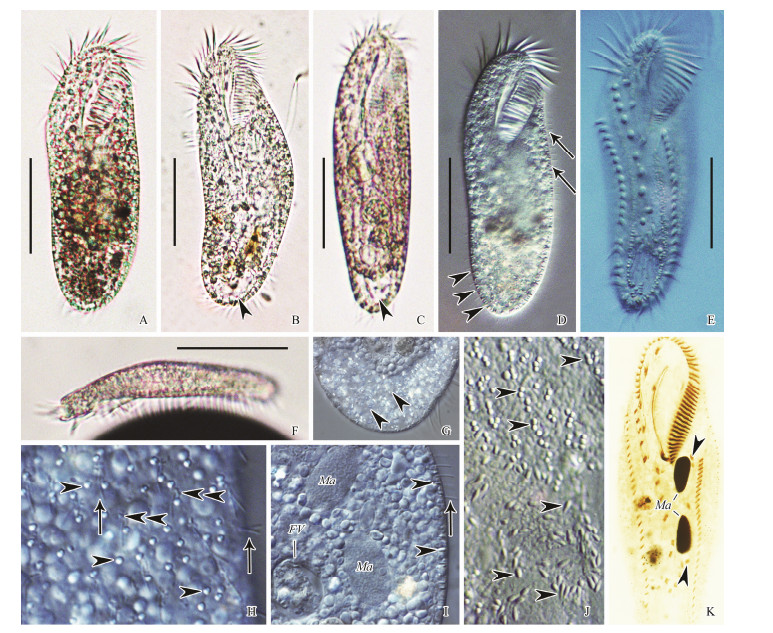
|
Fig. 4 Living Protogastrostyla pulchra (Pereyaslawzewa, 1886) Gong et al., 2007 (A-C, F, bright field; D, E, G-J, differential interference contrast) and samples after protargol staining (K). (A-E) Ventral views of different individuals, showing body shape, cortical granules, and cirral pattern. Arrowheads in (B, C) indicate the large food vacuole containing numerous bacteria; arrowheads in (D) show the lateral view of cortical granules; arrows in (D) show dorsal bristles. (E) Lateral view of a representative specimen. (G) Detail of the large food vacuole containing numerous bacteria. (H) Two kinds of cortical granules on dorsal side. Arrowheads indicate the larger ones, while double arrowheads show the smaller ones around the dorsal bristles (arrows). (I) Portion of ventral view, showing macronuclear nodules, food vacuole, dorsal bristles (arrow) and cortical granules (arrowheads). (J) Cortical granules (arrowheads) on ventral side. (K) Ventral view of a representative specimen, showing ciliature and nuclear apparatus. Arrowheads indicate micronuclei. Scale bars = 50 μm. |
|
|
Table 2 Morphometric data of Protogastrostyla pulchra (Pereyaslawzewa, 1886) Gong et al., 2007 from Qingdao (first row) and Zhanjiang (second row) |
Remarks: The Qingdao (Figs. 4A, B, D, G-I) and Zhan-jiang (Figs. 4C, E, F, J, K) populations correspond closely in living morphology and infraciliature both with each other and with the original description. The following description is based on the two Chinese populations. Cell size constant in Qingdao population: (120-150) × (45-50) μm in vivo, (95-120) × (42-49) (on average 106 × 48) μm after protargol staining; more variable in Zhanjiang population, (100-170) × (30-40) μm in vivo, (115-206) × (43-72) (on average 159 × 59) μm after protargol staining. Body elongate in shape, with both ends widely rounded. Body length to width ratio about (3-4):1, dorsoventrally flattened about 2:1 (Figs. 4A-F). Body flexible but non-contractile. Cytoplasm colourless, packed with irregular shaped (2-4 μm) granules (Fig. 4I). One large food vacuole characteristically present in caudal region, usually filled with numerous bacteria (Fig. 4G). Two types of cortical granules: type Ⅰ larger, about 2 × 0.7 μm in size, colorless, rod-like in lateral view, orthogonal to pellicle, grouped together in rows between ventral cirral rows and roughly in a single row along dorsal kineties, although some irregularly distributed on dorsal side (Figs. 4D, E, H-J); type Ⅱ smaller, less than 0.5 μm in size, clustered around dorsal bristles (Fig. 4H, double arrowheads). Contractile vacuole not observed. Constantly two ellipsoidal macronuclear nodules located in about mid-body, slightly left of cell midline (Fig. 4K). In Qingdao population, one to three (on average two) micronuclei, about 7 × 6 μm; in Zhanjiang population, three to seven (on average five) micronuclei, about 4 × 2 μm. Locomotion by moderately rapid crawling on substrate.
Infraciliature as shown in Fig. 4K. Adoral zone occupied about 35% and 37% of body length, composed of 33-42 (on average 38) and 42-51 (on average 47) membranelles in protargol-stained specimens in Qingdao and Zhanjiang populations, respectively. Cilia of membranelles approximately 10-15 μm long in vivo. Distal end of adoral zone extends down right-ventral side of cell to level of rightmost frontal cirrus. Paroral and endoral membranes in Oxytricha pattern, almost equal in length, slightly curved, optically intersect below mid-point.
On average 16 frontal-ventral cirri (invariably three frontal, one buccal and two pretransverse cirri; usually ten ventral cirri). Nine of the ten ventral cirri roughly arranged in a row. Five enlarged transverse cirri (cilia about 20 μm long in vivo) arranged in a J-shaped row, positioned in the posterior 16%-23% (on average 19%) of body in Qingdao population and 19%-29% (on average 23%) of body in Zhanjiang population, without protruding beyond posterior end of cell. Marginal cirral rows confluent posteriorly. Left marginal cirral row composed of 28-35 and 32-40 cirri in Qingdao population and Zhanjiang population, respectively. Right marginal row composed of 25-34 and 28-36 cirri in Qingdao population and Zhanjiang population, respectively. Constantly three caudal cirri, not recognizable in vivo. Dorsal cilia about 3-4 μm in length, arranged in 7-9 (on average 8) and 7-8 (on average 7) kineties in Qingdao and Zhanjiang populations, respectively.
3.4 Subclass: Hypotrichia Stein, 1859Order: Urostylida Jankowski, 1979
Family: Urostylidae Jankowski, 1979
Genus Uncinata Bullington, 1940
Uncinata bradburyae (Gong et al., 2001) Luo et al., 2015
3.4.1 Locality and habitatCoastal waters off Qingdao (36°03xN, 120°18xE), Shan-dong Province, China, salinity 30.0, temperature 19.0℃.
3.4.2 Deposition of slidesFour voucher slides with protargol-stained specimens of the Qingdao population were deposited in the Laboratory of Protozoology, Ocean University of China with registration numbers LXT2015052601/1-4.
3.4.3 Description of Qingdao population (Fig. 5; Table 3)
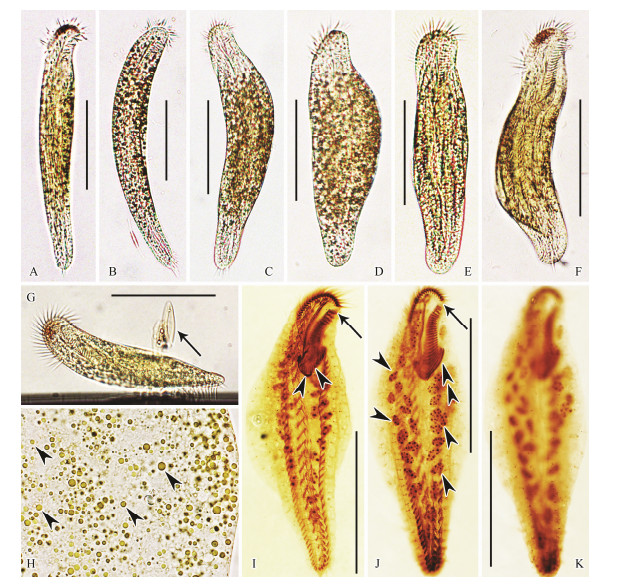
|
Fig. 5 Living Uncinata bradburyae (Gong et al., 2001) Luo et al., 2015 (A-H, bright field) and samples after protargol staining (I-K). (A-F) Ventral views of different individuals, showing body shape and flexibility (A, B, fully extended specimens; C-F, more or less contracted specimens). (G) Lateral view of a representative specimen, arrow indicates diatoms. (H) Yellow-brownish cortical granules (arrowheads). (I-K) Ventral (I, J) and dorsal (K) views of representative specimens, showing ciliature and nuclear apparatus. Arrows show the conspicuous gap of the adoral zone. Double arrowheads indicate the elongated adoral membranelles. Arrowheads in (I) indicate transversely oriented left marginal cirri. Arrowheads in (J) show the macronuclear nodules. Scale bars = 80 μm. |
|
|
Table 3 Morphometric data of Uncinata bradburyae (Gong et al., 2001) Luo et al., 2015 |
Cell size (160-250) × (30-55) μm in vivo, (105-180) × (46-71) μm in protargol-stained specimens. Body generally elongate-fusiform in shape, and anterior end more or less bent to left forming a snout-like projection, posterior region narrowed and terminally rounded (Figs. 5A-G).
Body flexible and contractile. Ratio of length to width about (6-8):1 when fully extended and about (4-5):1 when contracted; dorsoventrally flattened about 2:1. Pellicle soft and flexible. Cytoplasm colourless, usually packed with food vacuoles containing diatoms. Cortical granules yellow-brownish, spherical, approx. 0.5-2 μm across, distributed irregularly throughout cortex rendering cells brownish at low magnifications (Fig. 5H). Contractile vacuole not observed. On average, 39 (26 to 57) macronuclear nodules, spherical to ovoid in outline, each with variable-sized nucleoli, arranged in an elongateelliptical pattern around central region of cell (Fig. 5J). Locomotion typified by slowly crawling on substrate and among debris, occasionally jerking back and forth.
Cilia of membranelles approximately 10-15 μm long. All ventral cirri about 10 μm long except those of frontal and transverse cirri which are about 15 μm long.
Infraciliature as shown in Figs. 5I-K. Adoral zone occupies about 34% of body length in protargol-stained specimens. Buccal cavity narrow, right margin near cell midline. Adoral zone typically bipartite with conspicuous gap, distal and proximal parts comprising 18-27 (on average 22) and 15-26 (on average 23) membranelles, respectively. Bases of membranelles unequal in length, those in distal part distinctly shorter than those in proximal part, those of two or three proximalmost membranelles distinctly elongated (Fig. 5J). Distal end of adoral zone extends down right-ventral side of cell to about themed-region of oral area. Paroral and endoral membranes almost equally long, parallel to each other.
Constantly one buccal cirrus located near anterior end of undulating membranes. Five to nine enlarged frontal cirri and one parabuccal cirrus in frontal area. Two relatively fine frontoterminal cirri positioned close to distal end of adoral zone. Midventral complex extends almost to level of pretransverse ventral cirri, composed of 17-26 (on average 23) pairs of cirri arranged in a typical zigzag pattern. On average 18 (12 to 22) well developed transverse cirri arranged in a J-shaped row that extends anteriorly almost to mid-region of cell. Posterior ends of marginal rows are separated by transverse cirri. Left marginal row with 35-51 cirri, anteriormost 9 to 12 of which overlap elongated proximalmost adoral membranelles, transversely oriented and characteristically bent towards central region of body. Right marginal row with 39-56 cirri and commencing close to frontoterminal cirri. Dorsal cilia closely spaced, about 3-4 μm long, arranged in 8-11 (on average 9) kineties; caudal cirri absent.
4 Discussion 4.1 Parallelostrombidium obesum: Comparison with Original PopulationParallelostrombidium obesum was originally reported by Liu et al. (2015) based on a Shenzhen population. Our population matches well with this original one in all characters except the anterior end of girdle kinety is left to the ventral kinety with more dikinetids ((12-17) vs. (7-10)), and larger size of macronucleus after protargol staining ((37-54) × (28-36) vs. (24-39) × (18-28) μm). We consider these differences are population-dependent, and a larger size of macronucleus may be related to over bleaching during protargol staining process. We think our population is conspecific with Parallelostrombidium obesum.
4.2 Spirostrombidium apourceolare:Comparison with Original PopulationSpirostrombidium apourceolare was originally reported by Liu et al. (2013) based on a population from Daya Bay, Guangdong Province, China. Our population matches well with the original one in all characters except it possesses a larger cell size after protargol staining ((79-95) × (46-56) vs. (48-66) × (24-45) μm) and fewer macronuclear nodules ((11-15) vs. (15-27)). As the cell size and number of macronuclear nodules can be population-dependent, our population is conspecific with Spirostrombidium apourceolare.
4.3 Protogastrostyla pulchra: Comparison with Original PopulationProtogastrostyla pulchra was firstly described by Pereyaslawzewa (1886) with the name Stylonychia pulchra, while no formal diagnosis was provided and no type material was available. Since then on more populations have been reported but with no clear definition until Hu and Song (2000) supplied a new diagnosis based on a neotype population collected from the coast of Qingdao (Berger, 2008). Gong et al. (2007) established the genus Protogastrostyla with P. pulchra as the type species and reported three new populations. The body size of P. pulchra reported in previous studies is rather variable ((110-300) × (30-67) μm) (Borror, 1963; Burkovsky, 1970; Gong et al., 2007; Hu and Song, 2000; Kahl, 1932). Both the Qingdao ((120-150) × (45-50) μm) and Zhanjiang ((100-170) × (30-40) μm) populations are in the range of body size described previously. Except for the smaller cortical granules which are easily overlooked, and the slight differences in the number of adoral membranelles and cirri, which nevertheless overlap among populations, both the Qingdao and the Shenzhen populations correspond well with the previous descriptions in terms of body shape, infraciliature, and nuclear apparatus, especially the neotype population described by Hu and Song (2000). Thus, the species identification is definite.
4.4 Uncinata bradburyae: Comparison with Original PopulationUncinata bradburyae was firstly reported by Gong et al. (2001) with the name Holosticha bradburyae. This species was transferred to the genus Uncinata Bullington, 1940 by Luo et al. (2015) based on the following diagnostic generic features: 1) prominent beak-like projection in anterior region; 2) proximalmost adoral membranelles distinctly elongated; 3) numerous dorsal kineties; 4) unique developmental mode of the dorsal kineties with two groups of anlagen, and 5) a bipartite adoral zone. The main differences are that our population has a relatively smaller body size ((160-250) × (30-55) vs. (150-320) × (25-75) μm in vivo; (105-180) × (46-71) vs. (148-236) × (64-96) μm after protargol staining), fewer cirri on average (midventral pairs, (17-26) vs. (27-32); transverse cirri (12-22) vs. (20-26), left marginal cirri, (35-51) vs. (49-60); right marginal cirri (39-56) vs. (53-63)). Additionally, the cortical granulesin our samples are yellowbrownish, about 0.5-2 μm in size, and irregularly distributed, while they are colorless, circular and flattened with a central depression, about 2 μm in size, and arranged in about 10 lines on the dorsal side in the type population (Gong et al., 2001). Nevertheless, considering these differences are population-dependent, our population is conspecific with Uncinata bradburyae.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (Nos. 31801955, 31702009) and the China Postdoctoral Science Foundation (Nos. 2017M622276, BX20180348)
Agatha, S., 2004. Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications. Zoology, 107: 153-168. DOI:10.1016/j.zool.2004.02.003 (  0) 0) |
Agatha, S., 2011. Updated hypothesis on the evolution of oligotrichid ciliates (Ciliophora, Spirotricha, Oligotrichida) based on somatic ciliary patterns and ontogenetic data. European Journal of Protistology, 47: 51-56. DOI:10.1016/j.ejop.2010.09.001 (  0) 0) |
Agatha, S. and Riedel-Lorjé, J.C., 2006. Redescription of Tintinnopsis cylindrica Daday, 1887 (Ciliophora: Spirotricha) and unification of tintinnid terminology. Acta Protozoologica, 45: 137-151. (  0) 0) |
Berger, H., 1999. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monographiae Biologicae, 78: 1-1080. (  0) 0) |
Berger, H., 2006. Monograph of the Urostyloidea (Ciliophora, Hypotricha). Monographiae Biologicae, 85: 1-1304. (  0) 0) |
Berger, H., 2008. Monograph of the Amphisiellidae and Trachelostylidae (Ciliophora, Hypotrichida). Monographiae Biologicae, 88: 1-737. (  0) 0) |
Berger, H., 2011. Monograph of the Gonostomatidae and Kahliellidae (Ciliophora, Hypotrichida). Monographiae Biologicae, 90: 1-741. (  0) 0) |
Borror, A. C., 1963. Morphology and ecology of the benthic ciliated protozoa of Alligator Harbor, Florida. Archiv für Protistenkunde, 106: 465-534. (  0) 0) |
Burkovsky, I. V., 1970. The ciliates of the mesopsammon of the Kandolaksha Gulf (White Sea) II. Acta Protozoologica, 8: 47-64. (  0) 0) |
Caron, D. A., Countway, P. D., Jones, A. C., Kim, D. Y. and Schnetzer, A., 2012. Marine protistan diversity. Annual Review of Marine Science, 4: 467-493. DOI:10.1146/annurev-marine-120709-142802 (  0) 0) |
Chen, L. Y., Liu, W. W., Liu, A., Al-Rasheid, K. A. S. and Shao, C., 2013. Morphology and molecular phylogeny of a new marine hypotrichous ciliate, Hypotrichidium paraconicum n. sp. (Ciliophora, Hypotrichia). Journal of Eukaryotic Microbiology, 60: 588-600. DOI:10.1111/jeu.12064 (  0) 0) |
Chen, L. Y., Zhao, X. L., Shao, C., Miao, M. and Clamp, J., 2017a. Morphology and phylogeny of two new ciliates, Sterkiella sinica sp. nov. and Rubrioxytricha tsinlingensis sp. nov. (Protozoa, Ciliophora, Hypotrichia) from north-west China. Systematics and Biodiversity, 15: 131-142. DOI:10.1080/14772000.2016.1219426 (  0) 0) |
Chen, X. M., Dong, J., Lin, X. F. and Al-Rasheid, K. A. S., 2011. Morphology and phylogeny of a new urostylid ciliate, Monocoronella carnea n. gen., n. sp. (Ciliophora, Hypotricha) from Daya Bay, southern China. Journal of Eukaryotic Microbiology, 58: 497-503. DOI:10.1111/j.1550-7408.2011.00581.x (  0) 0) |
Chen, X. M., Gao, F., Al-Farraj, S. A., Al-Rasheid, K. A. S., Xu, K. D. and Song, W. B., 2015. Morphology and morphogenesis of a novel mangrove ciliate, Sterkiella subtropica sp. nov. (Protozoa, Ciliophora, Hypotrichia), with phylogenetic analyses based on small-subunit rDNA sequence data. International Journal of Systematic and Evolutionary Microbiology, 65: 2292-2303. DOI:10.1099/ijs.0.000253 (  0) 0) |
Chen, X. M., Lu, X. T., Luo, X. T., Jiang, J. M., Shao, C., Al-Rasheid, K. A. S., Warren, A. and Song, W. B., 2017b. The diverse morphogenetic patterns in spirotrichs and philasterids: Researches based on five-year-projects supported by IRCN-BC and NSFC. European Journal of Protistology, 61: 439-452. DOI:10.1016/j.ejop.2017.05.003 (  0) 0) |
Dong, J. Y., Lu, X. T., Shao, C., Huang, J. A. and Al-Rasheid, K. A. S., 2016. Morphology, morphogenesis and molecular phylogeny of a novel saline soil ciliate, Lamtostyla salina n. sp. (Ciliophora, Stichotrichida). European Journal of Protistology, 56: 219-231. DOI:10.1016/j.ejop.2016.09.005 (  0) 0) |
Foissner, W., 2016. Terrestrial and semiterrestrial ciliates (Protozoa, Ciliophora) from Venezuela and Galápagos. Denisia, 35: 1-912. (  0) 0) |
Gao, F., Huang, J., Zhao, Y., Li, L. F., Liu, W. W., Miao, M., Zhang, Q. Q., Li, J. M., Yi, Z. Z., El-Serehy, H. A., Warren, A. and Song, W. B., 2017. Systematic studies on ciliates (Alveolata, Ciliophora) in China: Progress and achievements based on molecular information. European Journal of Protistology, 61: 409-423. DOI:10.1016/j.ejop.2017.04.009 (  0) 0) |
Gao, F., Warren, A., Zhang, Q. Q., Gong, J., Miao, M., Sun, P., Xu, D. P., Huang, J., Yi, Z. Z. and Song, W. B., 2016. The all-data-based evolutionary hypothesis of ciliated protists with a revised classification of the phylum Ciliophora (Eukaryota, Alveolata). Scientific Reports, 6: 24874. DOI:10.1038/srep24874 (  0) 0) |
Gong, J., Kim, S. J., Kim, S. Y., Min, G. S., Roberts, D. M., Warren, A. and Choi, J. K., 2007. Taxonomic redescriptions of two ciliates, Protogastrostyla pulchra n. g., n. comb. and Hemigastrostyla enigmatica (Ciliophora: Spirotrichea, Stichotrichia), with phylogenetic analyses based on 18S and 28S rRNA gene sequences. Journal of Eukaryotic Microbiology, 54: 468-478. (  0) 0) |
Gong, J., Song, W. B., Hu, X. Z., Ma, H. G. and Zhu, M. Z., 2001. Morphology and infraciliature of Holosticha bradburyae n. sp. (Ciliophora, Hypotrichida) from the Yellow Sea, China. Hydrobiologia, 464: 63-69. DOI:10.1023/A:1013901621439 (  0) 0) |
Hu, X. Z. and Song, W. B., 2000. Morphology and morphogenesis of a marine ciliate, Gastrostyla pulchra (Perejaslawzewa, 1885) Kahl, 1932 (Ciliophora, Hypotrichida). European Journal of Protistology, 36: 201-210. DOI:10.1016/S0932-4739(00)80038-3 (  0) 0) |
Huang, J. B., Zhang, T. T., Zhang, Q. Q., Li, Y., Warren, A., Pan, H. B. and Yan, Y., 2018. Further insights into the highly derived haptorids (Ciliophora, Litostomatea): Phylogeny based on multigene data. Zoologica Scripta, 47: 231-242. DOI:10.1111/zsc.12269 (  0) 0) |
Kahl, A., 1932. Urtiere oder Protozoa Ⅰ: Wimpertiere oder Ciliata (Infusoria). Tierwelt Deutschlands, 25: 399-650. (  0) 0) |
Krainer, K. H., 1991. Contributions to the morphology, infraciliature and ecology of the planktonic ciliates Strombidium pelagicum n. sp., Pelagostrombidium mirabile (Penard, 1916) n. g., n. comb., Pelagostrombidium fallax (Zacharias, 1896) n. g., n. comb. (Ciliophora, Oligotrichida). European Journal of Protistology, 27: 60-70. DOI:10.1016/S0932-4739(11)80428-1 (  0) 0) |
Kumar, S., Bharti, D., Shazib, S. U. A. and Shin, M. K., 2017. Discovery of a new hypotrich ciliate from petroleum contaminated soil. PLoS One, 12: e0178657. DOI:10.1371/journal.pone.0178657 (  0) 0) |
Kumar, S., Kamra, K., Bharti, D., Terza, A. L., Sehgal, N., Warren, A. and Sapra, G. R., 2015. Morphology, morphogenesis, and molecular phylogeny of Sterkiella tetracirrata n. sp. (Ciliophora, Oxytrichidae), from the Silent Valley National Park, India. European Journal of Protistology, 51: 86-97. DOI:10.1016/j.ejop.2014.12.002 (  0) 0) |
Liu, W., Yi, Z., Li, J., Warren, A., Al-Farraj, S. A. and Lin, X., 2013. Taxonomy, morphology and phylogeny of three new oligotrich ciliates (Protozoa, Ciliophora, Oligotrichia) from southern China. International Journal of Systematic and Evolutionary Microbiology, 63: 4805-4817. DOI:10.1099/ijs.0.052878-0 (  0) 0) |
Liu, W., Yi, Z., Lin, X. and Al-Rasheid, K. A. S., 2011a. Morphologic and molecular data suggest that Lynnella semiglobulosa n. g., n. sp. represents a new family within the subclass Choreotrichia (Ciliophora, Spirotrichea). Journal of Eukaryotic Microbiology, 58: 43-49. DOI:10.1111/j.1550-7408.2010.00519.x (  0) 0) |
Liu, W., Yi, Z., Warren, A., Al-Rasheid, K. A. S., Al-Farraj, S. A., Lin, X. and Song, W. B., 2011b. Taxonomy, morphology and molecular systematics of a new oligotrich ciliate, Williophrya maedai gen. nov., sp. nov., with redescriptions of Strombidium basimorphum and Pseudotontonia simplicidens (Protozoa, Ciliophora, Oligotrichia). Systematics and Biodiversity, 9: 247-258. DOI:10.1080/14772000.2011.605812 (  0) 0) |
Liu, W. W., Jiang, J. M., Xu, Y., Pan, X. M., Qu, Z. S., Luo, X. T., El-Serehy, H. A., Warren, A., Ma, H. G. and Pan, H. B., 2017. Diversity of free-living marine ciliates (Alveolata, Ciliophora): Faunal studies in coastal waters of China during the years 2011-2016. European Journal of Protistology, 61: 424-438. DOI:10.1016/j.ejop.2017.04.007 (  0) 0) |
Liu, W. W., Yi, Z. Z., Lin, X. F., Li, J. Q., Al-Farraj, S. A., Al-Rasheid, K. A. S. and Song, W. B., 2015. Morphology and molecular phylogeny of three new oligotrich ciliates (Protozoa, Ciliophora) from the South China Sea. Zoological Journal of the Linnean Society, 174: 653-665. DOI:10.1111/zoj.12257 (  0) 0) |
Lu, X. T., Huang, J. A., Shao, C., Al-Farraj, S. A. and Gao, S., 2017. Morphology and morphogenesis of a novel saline soil hypotrichous ciliate, Gonostomum sinicum nov. spec. (Ciliophora, Hypotrichia, Gonostomatidae), including a report on the small subunit rDNA sequence. Journal of Eukaryotic Microbiology, 64: 632-646. DOI:10.1111/jeu.12398 (  0) 0) |
Luo, X. T., Gao, F., Al-Rasheid, K. A. S., Warren, A., Hu, X. Z. and Song, W. B., 2015. Redefinition of the hypotrichous ciliate Uncinata, with descriptions of the morphology and phylogeny of three urostylids (Protista, Ciliophora). Systematics and Biodiversity, 13: 455-471. DOI:10.1080/14772000.2015.1046967 (  0) 0) |
Luo, X. T., Gao, F., Yi, Z. Z., Pan, Y., Al-Farraj, S. A. and Warren, A., 2017. Taxonomy and molecular phylogeny of two new brackish hypotrichous ciliates, with the establishment of a new genus (Protozoa, Ciliophora). Zoological Journal of the Linnean Society, 179: 475-491. (  0) 0) |
Lynn, D. H., 2008. The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature. 3rd edition. Springer, Dordrecht, 605pp.
(  0) 0) |
Lynn, D. H. and Gilron, G. L., 1993. Strombidiid ciliates from coastal waters near Kingston Harbor, Jamaica (Ciliophora, Oligotrichia, Strombidiidae). Journal of the Marine Biological Association of the United Kingdom, 73: 47-65. DOI:10.1017/S0025315400032641 (  0) 0) |
Paiva, T. S., Shao, C., Fernandes, N. M., Borges, B. N. and Silva-Neto, I. D., 2016. Description and phylogeny of Urostyla grandis wiackowskii subsp. nov. (Ciliophora, Hypotricha) from an estuarine mangrove in Brazil. Journal of Eukaryotic Microbiology, 63: 247-261. DOI:10.1111/jeu.12273 (  0) 0) |
Pan, X. M., Bourland, W. A. and Song, W. B., 2013. Protargol synthesis: An in-house protocol. Journal of Eukaryotic Microbiology, 60: 609-614. DOI:10.1111/jeu.12067 (  0) 0) |
Pan, X. M., Fan, Y. B., Gao, F., Qiu, Z. J., Al-Farraj, S. A., Warren, A. and Shao, C., 2016. Morphology and systematics of two freshwater urostylid ciliates, with description of a new species (Protista, Ciliophora, Hypotrichia). European Journal of Protistology, 52: 73-84. DOI:10.1016/j.ejop.2015.11.003 (  0) 0) |
Park, K. M., Jung, J. H., Min, G. S. and Kim, S., 2017. Pseudonotohymena antarctica n. g., n. sp. (Ciliophora, Hypotricha), a new species from Antarctic soil. Journal of Eukaryotic Microbiology, 64: 447-456. DOI:10.1111/jeu.12381 (  0) 0) |
Pereyaslawzewa, S., 1886. Protozoaires de la mer Noire. Zap novoross Obshch Estest, 10: 79-114. (  0) 0) |
Petz, W., Song, W. B. and Wilbert, N., 1995. Taxonomy and ecology of the ciliate fauna (Protozoa, Ciliophora) in the endopagial and pelagial of the Weddell Sea, Antarctica. Stapfia, 40: 1-223. (  0) 0) |
Pierce, R. W. and Turner, J. T., 1992. Ecology of planktonic ciliates in marine food webs. Reviews in Aquatic Sciences, 6: 139-181. (  0) 0) |
Shao, C., Li, L. Q., Zhang, Q. Q., Song, W. B. and Berger, H., 2014. Molecular phylogeny and ontogeny of a new ciliate genus, Paracladotricha salina n. g., n. sp. (Ciliophora, Hypotrichia). Journal of Eukaryotic Microbiology, 61: 371-380. DOI:10.1111/jeu.12117 (  0) 0) |
Song, W., Li, J. M., Liu, W. W., Al-Rasheid, K. A. S., Hu, X. Z. and Lin, X. F., 2015a. Taxonomy and molecular phylogeny of four Strombidium species, including description of S. pseudostylifer sp. nov. (Ciliophora, Oligotrichia). Systematics and Biodiversity, 13: 76-92. DOI:10.1080/14772000.2014.970674 (  0) 0) |
Song, W., Li, J. M., Hu, X. Z., Liu, W. W., Al-Rasheid, K. A. S., and Miao, M., 2018a. Morphology and phylogeny of three oligotrichous ciliates (s.l.), including description of a new species Parastrombidinopsis zhuhaiensis sp. n. (Ciliophora, Choreotrichia). Acta Protozoologica (in press).
(  0) 0) |
Song, W., Wang, L., Li, L., Al-Farraj, S. A., Aleidan, A., Smith, S. and Hu, X., 2018b. Morphological characterizations of four species of Parallelostrombidium (Ciliophora, Oligotrichia), with a note on the phylogeny of the genus. Journal of Eukaryotic Microbiology, 65: 679-693. DOI:10.1111/jeu.12513 (  0) 0) |
Song, W., Zhao, X. L., Liu, W. W., Hu, X. Z., Al-Farraj, S. A., Al-Rasheid, K. A. S., Song, W. B. and Warren, A., 2015b. Biodiversity of oligotrich ciliates in the South China Sea: Description of three new Strombidium species (Protozoa, Ciliophora, Oligotrichia) with phylogenetic analyses. Systematics and Biodiversity, 13: 928-943. (  0) 0) |
Song, W. B. and Bradbury, P. C., 1998. Studies on some new and rare reported marine planktonic ciliates (Ciliophora: Oligotrichida) from coastal waters in North China. Journal of the Marine Biological Association of the United Kingdom, 78: 767-794. DOI:10.1017/S0025315400044775 (  0) 0) |
Wang, J. Y., Lyu, Z., Warren, A., Wang, F. and Shao, C., 2016. Morphology, ontogeny and molecular phylogeny of a novel saline soil ciliate, Urosomoida paragiliformis n. sp. (Ciliophora, Hypotrichia). European Journal of Protistology, 56: 79-89. DOI:10.1016/j.ejop.2016.07.005 (  0) 0) |
Wang, J. Y., Ma, J. Y., Qi, S. Y. and Shao, C., 2017. Morphology, morphogenesis and molecular phylogeny of a new soil ciliate Paragonostomoides xianicum n. sp. (Ciliophora, Hypotrichia, Gonostomatidae). European Journal of Protistology, 61: 233-243. DOI:10.1016/j.ejop.2017.07.001 (  0) 0) |
Wang, R., Song, W., Yi, Z. Z., Lin, X. F., AL-Rasheid, K. A. S. and Hu, X. Z., 2018. Morphology and molecular phylogeny of two new species of Spirostrombidium (Ciliophora, Oligotrichia), with a key to species in the genus. Systematics and Biodiversity, 16: 743-756. DOI:10.1080/14772000.2018.1484393 (  0) 0) |
Wilbert, N., 1975. Eine verbesserte Technik der Protargolim-prägnation für Ciliaten. Mikrokosmos, 64: 171-179. (  0) 0) |
Worden, A. Z., Follows, M. J., Giovannoni, S. J., Wilken, S., Zimmerman, A. E. and Keeling, P. J., 2015. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science, 347: 1257594. DOI:10.1126/science.1257594 (  0) 0) |
Xu, D. P., Warren, A., and Song, W. B., 2009. Oligotrichs. In: Free-Living Ciliates in the Bohai and Yellow Seas, China. Song, W. B., et al., eds., Science Press, Beijing, 307-351.
(  0) 0) |
Xu, D. P., Jiao, N. Z., Ren, R. and Warren, A., 2017. Distribution and diversity of microbial eukaryotes in bathypelagic waters of the South China Sea. Journal of Eukaryotic Microbiology, 64: 370-382. DOI:10.1111/jeu.12372 (  0) 0) |
Yan, Y., Rogers, A. J., Gao, F. and Katz, L. A., 2017. Unusual features of non-dividing somatic macronuclei in the ciliate class Karyorelictea. European Journal of Protistology, 61: 399-408. DOI:10.1016/j.ejop.2017.05.002 (  0) 0) |
Zhang, Q. Q., Agatha, S., Zhang, W. C., Dong, J., Yu, Y., Jiao, N. Z. and Gong, J., 2017. Three rDNA loci-based phylogenies of tintinnid ciliates (Ciliophora, Spirotrichea, Choreotrichida). Journal of Eukaryotic Microbiology, 64: 226-241. DOI:10.1111/jeu.12354 (  0) 0) |
Zhao, X. L., Wang, Y. Y., Wang, Y. R., Liu, Y. F. and Gao, S., 2017. Histone methyltransferase TXR1 is required for both H3 and H3.3 lysine 27 methylation in the well-known ciliated protist Tetrahymena thermophila. Science China Life Sciences, 60: 264-270. DOI:10.1007/s11427-016-0183-1 (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


