2) Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3) Qingdao Conson Oceantec Valley Development Co., Ltd., Qingdao 266003, China
Neurogenesis, which mainly involves cell proliferation, migration, differentiation and survival, is an important pro-gress that the neural stem cells (NSCs) differentiate into functional neurons under conductive conditions. Similar to other stem cells, NSCs also display self-renewal and dif-ferentiation abilities, as well as participate in the formation of the nervous system during development. In mammals, neural cells are mainly generated during the pre-natal phase of development, and neurogenesis is restricted to two re-gions of the adult brain (Taupin, 2006). However, neurogenesis considerably differs among various vertebrate classes. For example, teleosts continuously produce a tremendous number of new neurons in many regions of the adult brain. Moreover, the cell proliferation rate in teleosts is one to two orders of magnitude higher than that in mammals (Hinsch and Zupanc, 2006). Therefore, studying the NSCs in teleosts is important to understand the development and evolution from teleosts to mammals. In addition, the study of NSCs in fish is still in its infancy (Grandel et al., 2006; Zupanc, 2008) when compared with that in mammals (Cai et al., 2002; Charrier et al., 2006; Taupin, 2006; Duan et al., 2008).
Cell lines are an important system for many studies be-cause of their several advantages, including similar micro-environment to that in vivo and abundant resources. Some scientists have attempted to establish cultures of brain cells from several fish species. For example, embryonic brain cell lines in the silky shark Carcharhinus falciformis were established in 1992, and they have been successfully main-tained through 45 passages (Poyer and Hartmann, 1992). In addition, intrinsic stem cells from the telencephalon, corpus cerebelli, and valvula cerebelli of the brown ghost knifefish Apteronotus leptorhynchus were isolated, culti-vated and then examined (Hinsch and Zupanc, 2006). Some primary cultures, such as those of tilapia brain from different ages, were also established to study the function of neural systems (Tsai et al., 2001). However, few studies established cell lines derived from fish brains. These cell lines include the neural progenitor cell line TB2 from tilapia (Wen et al., 2008a); GBC1 and GBC4 from the orange-spotted grouper Epinephelus coioides (Wen et al., 2008b); BB from the Asian seabass Lates calcarifer (Wu and Chi, 2006); EAGB from the Hong Kong grouper Epinephelus akaara (Huang et al., 2011); RGB from the Longfin grouper Epinephelus quoyanus (Ku et al., 2009); and SBB-W1 from European seabass Dicentrarchus labrax (Servili et al., 2009). These previously established cell lines indicate that neurogenesis continues at a high rate through-out the adult life in different regions of the fish central nervous system (Grandel et al., 2006; Hinsch and Zupanc, 2006). However, NSC lines have been reported in only a few fish species, including A. Leptorhynchus (Hinsch and Zupanc, 2006), tilapia (Wen et al., 2008a), sea bass (Ser-vili et al., 2009) and some groupers. Permanent cell lines provided opportunities to study neurogenesis in vitro, while the experiences can also be applied in fish brain cells (Wen et al., 2010; Hasoon et al., 2011; Huang et al., 2011).
The Japanese flounder Paralichthys olivaceus is an im-portant economic marine fish in China. The permanent cell lines from this fish have been broadly applied in toxicology and immunology studies (Tong et al., 1997). However, few cell lines have been established from fish neural tissues. Therefore, neural stem/progenitor cell lines from the brain of P. olivaceus need be established to offer a valuable in vitro system for the study of fish nervous regulation and endocrinology-related functions.
2 Materials and Methods 2.1 Animals and Tissue CollectionP. olivaceus individuals were collected from a commer-cial hatchery located in Haiyang City, China. The fish were kept in indoor facilities under natural light with constant temperature and salinity. After acclimatization, the fish were randomly selected for cell culture. Nine apparently healthy adult fish were used in accordance with the guidelines and regulations for animal experiments of the Ocean University of China and the local government of Yantai City.
2.2 Primary CultureThe whole brain (including the telencephalon, dience-phalon, midbrain, cerebellum, and medulla oblongata) of three individuals and the hypothalamus of six individuals were separated and mixed for the primary cell culture. The two types of tissues were finely minced with scissors in D-Hank's solution (containing 500 IU mL-1 penicillin and 500 µg mL-1 streptomycin) and then washed at least three times to remove any blood clots. The small brain fragments were washed several times with phosphate-buffered saline (PBS) supplemented with antibiotics (200 IU mL-1 penicillin and 200 µg mL-1 streptomycin), transferred into Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, Gibco) supplemented with antibiotics (100 IU mL-1 penicillin and 100 µg mL-1 streptomycin), and then further minced into small pieces (about 1 mm3).
For the hypothalamus part, TrypLETM Express (Gibco) was added into the cell suspension at a ratio of 1:1 for 5 min. Then the cell suspension was filtered through 40 μm bore diameter and then transferred into a 25 cm2 tissue culture flask (Nunc) containing 5 mL of DMEM/F12 medium supplemented with 20% fetal bovine serum (FBS, Gibco). This culture was named PoBh. The whole brain frag-ments were divided into two groups. The first group was transferred into a 25 cm2 tissue culture flask (Nunc) and se-parated in FBS without TrypLETM Express. Then DMEM/ F12 medium (5 mL) supplemented with 20% FBS was add-ed after these smaller fragments attached to the flask. After 3 days, the adherent cells were named PoB1. The upper non-adherent cells of the first group were transferred to a new flask and named PoBf. The second group was treated with TrypLETM Express as previously described and the resultant was named PoB2. All cultures were incubated at 24℃. Every 4 – 5 days, half of the growth medium was re-placed with fresh medium. Monolayers of primary cultured cells were obtained within a month.
2.3 Sub-Culture or PropagationWhen the confluent monolayers reached 95%, the cells were examined under an inverted microscope to ensure that their morphologies were normal without microbial con-tamination. After removing the culture medium, the cells were washed twice with PBS buffer to eliminate the FBS and dead cells. Then 1 mL of TrypLETM Express was added to each flask to dislodge cells from the surface. After the cells were dislodged, FBS was added to stop the reaction. The cells were sub-cultured at a split ratio of 1:2 to 1:3 in fresh DMEM/F12 medium supplemented with 10 mmol L-1 HEPES (pH 7.5), 10 mmol L-1 non-essential amino acid, antibiotics (100 IU mL-1 penicillin and 100 µg mL-1 strep-tomycin; Gibco), FBS, insulin-like growth factor 1 (IGF-1, Peprotech) and basic fibroblast growth factor (bFGF, Pe-protech). In addition, the PoB2 cell line also contained fi-broblast growth factor 9 (FGF9, Peprotech). The concentration of FBS was maintained at 20% before the 15th passage, at 15% before the 60th passage, and at 10% the-reafter. The concentrations of IGF-1, bFGF and FGF-9 were maintained at 10 ng mL-1 before the 50th passage, at 5 ng mL-1 before the 100th passage, and at 2 ng mL-1 the-reafter.
2.4 Effect of FBS on Cell GrowthThe effects of FBS concentration on the proliferation of the four brain cell lines were examined. In brief, 2.8×105 PoB1 cells, 2.8×105 PoB2 cells, 6.8×104 PoBf cells and 1×105 PoBh cells were inoculated in a six-well plate and then incubated at 24℃ for growth test. The concentration of FBS was set to 5%, 10%, 15% and 20%. Cell density was measured microscopically using a haemocytometer. Every experiment was repeated three times.
2.5 Cryopreservation and RecoveryFor cryopreservation, 24-hour-old brain cells at different passages were used. The cells were harvested by centrifugation and then suspended in 10% dimethyl sulfoxide, 15% FBS and 75% complete DMEM/F12 medium to a density of 1×106 cells mL-1. The cell suspensions were then transferred into cryovials. Cryopreservation was perform-ed by gradual freezing at 4℃ for 30 min, -20℃ for 2 h and -80℃ overnight, followed by prolonged storage in liquid.
For recovery, the cryovials from the liquid nitrogen were placed in running water at 37℃ and then finely shook until the cells were thawed. The cells in the frozen medium were immediately transferred into a fresh medium with 20% FBS and then cultured at 24℃. Meanwhile, the recovered cells were stained with trypan blue and counted with a haemo-cytometerto analyze the survival rate.
2.6 Chromosome AnalysisAfter inoculation, the cells were cultured for 5 h at nor-mal condition till the cells reached exponential stage. The cells were treated with 0.005% colcemid (Solarbio, China) for 2 h. The monolayer was trypsinized and pelleted as des-cribed above. The cells were suspended in 0.8 mL of hypotonic medium (0.075 mol L-1 KCl) for 40 min and prefixed with 0.4 mL of pre-cooling Carnoy's Fluid (3:1, me-thanol to glacial acetic acid) at room temperature for 25 min. After centrifugation at 3000 g for 5 min, the cells were su-spended thrice in 1 mL of pre-cooled Carnoy's fluid at -20℃ for 25 min. Finally, the cells were resuspended in appro-ximately 0.2 mL of fixative, and a drop of the cell suspension was immediately added to one side of a clean slide. The cells were dispersed and air-dried. Chromosomes were stained with Giemsa (Solarbio) for 10 min, washed with running water, air-dried, mounted and then photographed. Chromosome counts were performed for at least 150 me-taphase plates.
2.7 Molecular Identification of Brain Cell CulturesThe expression of specific genes was examined to cha-racterize NSCs and their sub-lineages. The following mar-ker genes were used: nestin, sex determining region Y-box 1 (sox1) and sex determining region Y-box 2 (sox2) for NSCs; glial fibrillary acidic protein (GFAP) and aldehyde dehydrogenase 1 family, member L1 (aldh1l1) for astrocytes; myelin-associated glycoprotein (MAG) for oligodendrocytes; glutamate decarboxylase 1 (GAD1) for GABAergic/ glutaminergic neurons; and solute carrier family 6 (neuro-transmitter transporter), member 4 (slc6a4) for serotonegic neurons. The primers (Table 1) used for gene expression detection were designed on the basis of the sequence data from NCBI.
|
|
Table 1 Nucleotide sequences of the primers |
Cells cultured in a flask for 24 h were harvested and washed with PBS. After centrifugation, the cells were add-ed with 1 mL of Trizol (Invitrogen) for RNA extraction. First-strand of cDNA was prepared as previously des-cribed (Song et al., 2015). PCR amplification for β-actin was performed using the following program: initial denaturation for 5 min at 94℃; 35 cycles of 30 s at 94℃, 30 s at 58℃ and 30 s at 72℃; and a final extension of 7 min at 72℃. PCR amplifications for other genes were performed using the following program: initial denaturation for 5 min at 94℃; 35 cycles of 30 s at 94℃, 30 s at 60℃, and 30 s at 72℃; and a final extension of 7 min at 72℃. An aliquot of PCR product was electrophoresed on 3% agarose gels, vi-sualized and then photographed for verification.
2.8 Transfection AnalysisThe pEGFP-C1 plasmid, which was conserved in the la-boratory, was used to evaluate the transfection ability of the four brain cell lines. The plasmid contains a cytome-galo virus promoter, an SV40 polyadenylation signal and a neomycin-resistant gene. The cells stored in DMEM/F12 medium with 10% FBS were seeded into a 24-well plate at a density of 1 × 105 cells mL-1 and a growth volume of 1 mL. After 10 h, the cells were treated with a complex of the pEGFP-C1 plasmid (0.5 μg) and the liposome transfection reagent LipofectamineⓇ 3000 (Invitrogen) or TurboFect (Thermo) in accordance with the manufacturer's instructions. After the cells were incubated at 24℃, green fluorescence signals were observed every 12 h under a Nikon fluorescence microscope. The transfection efficiency was evaluated by calculating the ratio of cells expressing green fluorescence signals to all cells employed for transfection.
2.9 Statistical AnalysisThe cell amount was analyzed with independent sample tests. Data were expressed as mean ± SD. Statistical significance was considered when P < 0.05. All statistical ana-lyses were computed using SPSS 18.0.
3 Results 3.1 Isolation and Cultivation of Brain CellsPrimary cultures of brains from P. olivaceus were estab-lished through explant culturing and trypsinization method. When the explant fragments of whole brains attached onto the bottom of the flask, some cells emerged from the edges of tissue explants 2 d after tissue explanting, whereas other cells did not emerge from the edges of tissue explants even 8 d after tissue explanting. On the basis of their different rates of attachment and migration, the PoB1 and PoBf cell cultures were separated and established. Cells suspensions of PoBh and PoB2 in DMEM/F12 with 20% FBS using trypsinization attached to the flask 1 d after inoculation. These cells grew rapidly and stably in DMEM/F12 medium. The two culture methods were found to be ideal for developing primary cultures and responded well to sub-culturing.
Primary cells from the brains showed various morpho-logies after explant culturing or trypsinization methods. PoB1 and PoBf cells adhered well and achieved conflu-ence within half a month after explant culturing. PoB1 cells merged from the edges of seeded tissue explants 2 d after tissue explanting. The cells in primary culture were main-ly large, round with distinct borders, or irregularly shaped (Fig. 1A). By contrast, PoBf cells adhered on the flask after 4 d and were small, polygonal and in patches (Fig. 1B). PoBh cells in primary cultures were larger than the other cell cultures and were mainly in irregular shape. Some cells exhibited many obvious pseudopodia which were similar to synapses (Fig. 1C). The brains cells cultured using tryp-siniztion showed a similar morphology to those cultured using explanting (Fig. 1D).
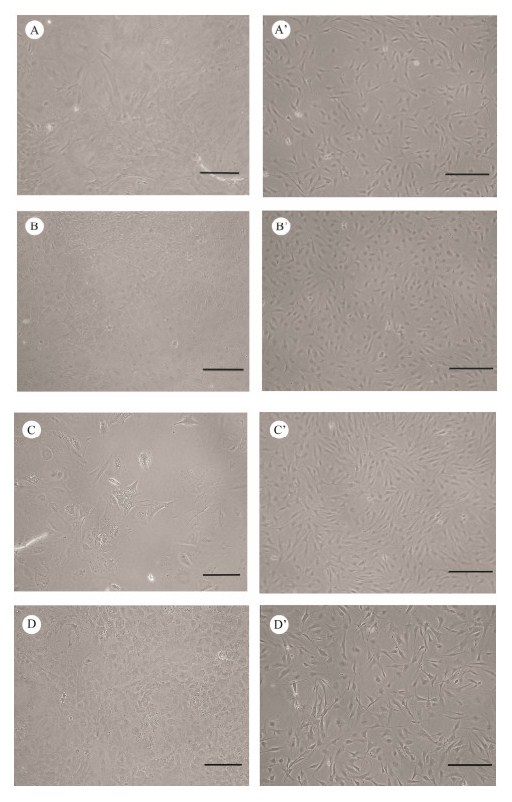
|
Fig. 1 Photomicrographs of cell lines established from brains of Japanese flounder. Primary cell culture of PoB1 (A), PoBf (B), PoB2 (C), and PoBh (D), and confluent monolayer of PoB1 (passage 157, A'), PoBf (passage 136, B'), PoB2 (passage 100, C'), and PoBh (passage 130, D') are shown. Bar = 200 μm. |
Brain cells grew slowly and formed a monolayer 5 – 6 d before passage 30 and then grew rapidly and formed a mo-nolayer within 3 – 4 d. Confluent cells were continuously sub-cultured at the ratio of 1:2 – 1:4. The sub-cultured cells could adhere in 2 h. After several sub-cultures, fibroblast-like cells were mainly observed. The concentrations of FBS and growth factors gradually reduced to 10% and 2 ng mL-1, respectively. These brain cell lines gave rise to long-term cultures which lasted over a year and have been sub-cul-tured for more than 150 times so far. The four cell lines were found composed of fibrocytes and epithelium in mor-phologies and showed similar sizes (Figs. 1A', 1B', 1C', and 1D').
Upon reaching confluence, the cells formed neurosphe-roidal bodies or cell clumps (Fig. 2). The cell clumps ad-hered again and grew contiguously after inoculation in a new flask with small suspension form.
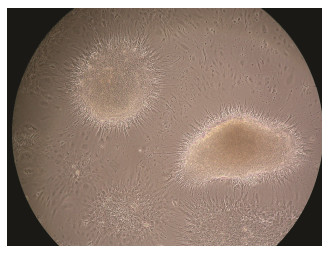
|
Fig. 2 The neurospheres of PoBh cells at passage 73 for 27 d. The neurospheres cultured attach to the surface, and the differentiating cells migrate away from the core of the neu-rosphere. Magnification = 100×. |
The effects of various FBS concentrations on the growth of PoB1, PoBf, PoB2 and PoBh cells at passages 138, 123, 86 and 81 were investigated. PoB1 cells exhibited no different growth characteristics at FBS concentrations ranging from 5% to 20% (Fig. 3A). PoBf and PoB2 cells showed poor growth at 5% and 10% FBS, and relatively good growth at 15% and 20% FBS (Figs. 3B and 3C). PoBh cells exhi-bited relatively poor growth at 5% FBS (Fig. 3D). No significant growth difference was observed at various FBS concentrations (Fig. 3). The growth curves involving various FBS concentrations indicate that FBS had minimal influence on the growth of all four cell lines after several passages. However, high FBS concentration in the medium facilitated better the adherence and spreading of cells com-pared with low FBS concentration (data not shown).
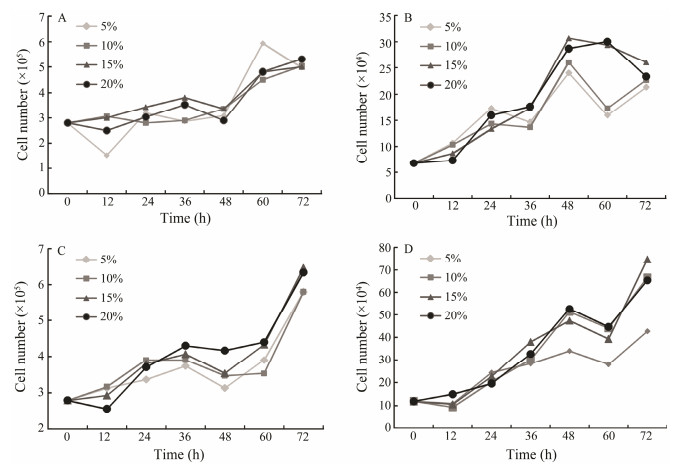
|
Fig. 3 Growth curves of brain cells in DMEM/F12 supplemented with different concentrations of fetal bovine serum. The cells were propagated at 24℃ and the cell number was determined every 12 h. The effect of FBS on growth of PoB1 cells at passage 138 (A), of PoBf cells at passage 123 (B), of PoB2 cells at passage 86 (C) and of PoBh cells at passages 81 (D) are shown. |
The brain cells were successfully cryopreserved in li-quid nitrogen and were recovered from different storage periods. The average viabilities of the cryopreserved PoB1, PoBf, PoB2 and PoBh cells were estimated to be appromately 94.4%, 95.57%, 90.52%, and 92.31% respectively. After recovering from storage, the cells grew confluency within 1 week and showed the same morphology. No significant changes in the morphology of recovered cells were observed after prolonged storage. However, the concentration of cells in frozen medium was vital for recovery. The suitable concentration for all cell lines was 106 cells mL-1.
3.4 Chromosomal AnalysisThe chromosomes numbers and karyotype are presented in Fig. 4. PoB1 at passage 142 showed that the chromo-somes number mainly ranged from 60 to 69 (Fig. 4A), while most of the cells had 64 or 66 chromosomes. In terms of karyotype (Fig. 4A'), both end-centromere chromosomes and central-centromere chromosomes were observed. PoBf at passage 139 also showed that the number of chromosomes varied from 60 to 69 (Fig. 4B), while most of the cells had 62, 64 or 66 chromosomes. Acrocentric, metacentric, subtelocentric and spot chromosomes were obser-ved (Fig. 4B'). PoB2 at passage 90 showed that the number of chromosomes ranged mainly from 60 to 79 (Fig. 4C), with a mode of 72 that was higher than the other three cell lines. PoBh at passage 121 showed that the chromosome number mainly ranged from 60 to 69 (Fig. 4D) with a distinct peak of 64.
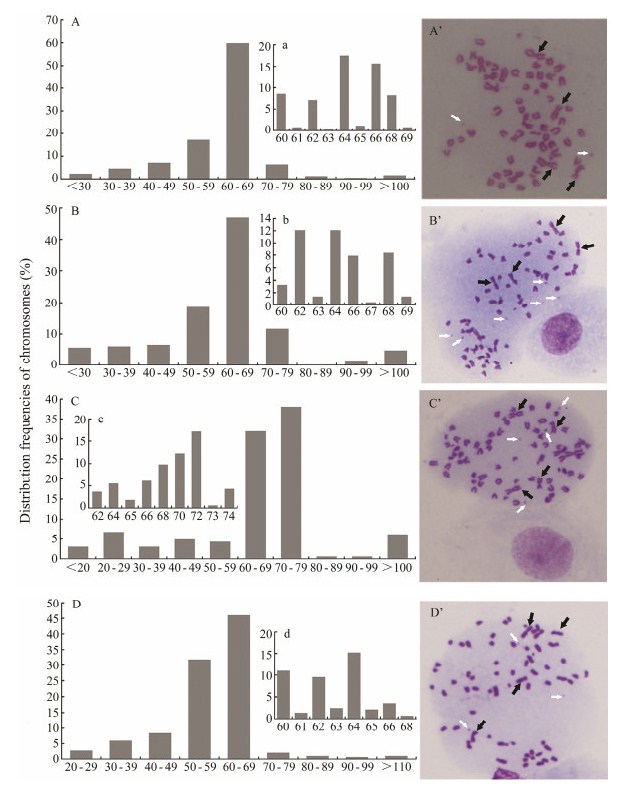
|
Fig. 4 The distribution frequencies of chromosomes and karyotypes of four cell lines. A, The distribution frequencies of chromosomes of PoB1 cells at passage 142; B, PoBf cells at passage 139; C, PoB2 cells at passage 90; D, PoBh cells at passage 121 (D). A', Karyotypes of PoB1 cells at passage 142; B', PoBf cells at passage 139; C', PoB2 cells at passage 90; D', PoBh cells at passage 121. White arrows showed the spot chromosomes and black arrows showed the metacentric chromosome. Cellular chromosomes arrested in metaphase were shown as magnification =1000×. The x axis stands for chromosome number, and the y axis stands for the percentage of cells. The figures A, B and D stand for the percentage of cells whose chromosome numbers are between 60 and 69. The figure C stands for the percentage of cells whose chromosome numbers are between 60 and 79. |
Karyotype analysis revealed that few cells had a diploid chromosome number of 2n = 48 (Yu et al., 1995; Wang et al., 2009) as the modal chromosome numbers in P. olivaceus. A high proportion of the cells displayed aneuploidy and heteroploidy (Figs. 4A', 4B', 4C' and 4D'). These pheno-mena were pronounced in some immortal cell lines (Tong et al., 1997; Wang et al., 2008).
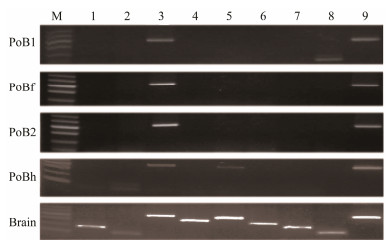
3.5 Molecular Identification of Brain Cell CulturesTo verify whether the four brain cell lines express specific neural genes, we performed reverse transcription (RT) PCR using the total RNA extracted from the cells. Electrophoretic analyses of the amplification of these genes from the cell lines and P. olivaceus brain tissue were con-ducted. The PCR products obtained from the cells' cDNA displayed the expected sizes that were the same with those obtained using cDNA from brain tissue, which was used as a positive control (Fig. 5). The expected 443, 357 and 450 bp PCR products of nestin, p53 and β-actin were observed in all samples, respectively (Fig. 5). In addition, an obvious band at approximately 421 bp for slc6a4 was observed in PoB1 cells (Fig. 5). Weak bands at about 182 bp for sox1 and 107 bp for sox2, whereas obvious bands at 421 bp for aldh1l1, were observed in PoBh cells (Fig. 5). However, no PCR product was obtained from the cDNA template prepared from PoBf and PoB2 cells (Fig. 5).
|
Fig. 5 Expressions of neural genes in brain cell lines PoB1, PoBf, PoB2, and PoBh, and brain tissue (positive control). M, 100 bp ladder DNA marker; lane 1, sox1 (182 bp); lane 2, sox2 (107 bp); lane 3, nestin (443 bp); lane 4, mag (352 bp); lane 5, aldh1l1 (421 bp); lane 6, gfap (304 bp); lane 7, gad1 (244 bp); lane 8, slc6a4 (166 bp); lane 9, actin (450 bp). |
The sequences of sox1 and sox2 were 100% identical to the mRNA of the P. olivaceus transcription factors SOX-1a (Sox1a, Genbank accession no. KR108250.1) and SOX-2 (Sox2, Genbank accession no. KF709692.1), respectively. The sequences of nestin, aldh1l1 and slc6a4 amplicons were 80%, 90% and 90% identical to the Stegastes partitus nes-tin mRNA sequence (Genbank accession no. XM_00829 7933.1), the Larimichthys crocea cytosolic 10-formyltetra-hydrofolate dehydrogenase-like mRNA sequences (Genbank accession no. XM_010752381.1) and the L. crocea slc6a4 mRNA sequences (Genbank accession no. XM_010739 044.1), respectively. The sequences of nestin, aldh1L1 and slc6a4 were 100% identical to those sequences in the ge-nome of P. olivaceus (data unpublished). These data indicate that the four cell lines are derived from P. olivaceus but have different expression patterns.
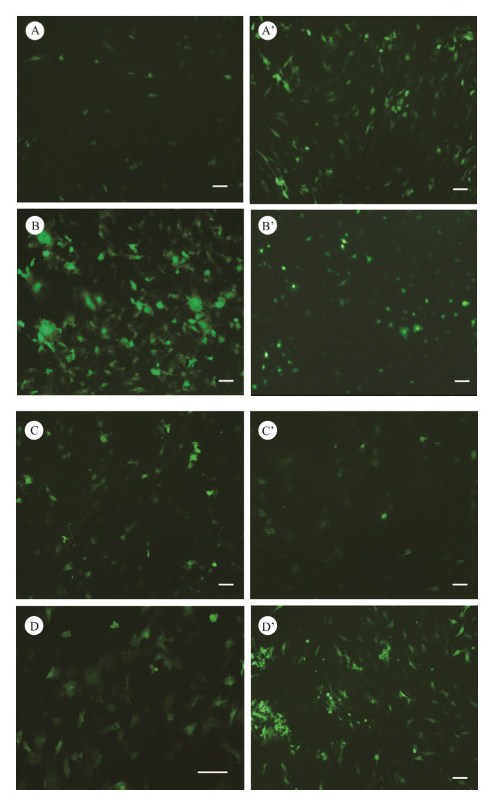
3.6 Transfection StudyThe four brain cell lines were successfully transfected with pEGFP-C1 by using LipofectamineⓇ 3000 (Figs. 6A – 6D) or TurboFect (Figs. 6A' – 6D'). The expression of GFP in these cell lines could be weakly detected under a fluorescent microscope as early as 12 h after transfection. Clear green fluorescent signals were detected at 24 h after trans-fection (Fig. 6). These results suggest that the four brain cell lines of P. Olivaceus are suitable for transfection with Li-pofectamineⓇ 3000 or TurboFect.
|
Fig. 6 Green fluorescent protein expression in Japanese flounder brain cells transfected with pEGFP-C1 using transfection reagents LipofectamineⓇ 3000 (A – D) or TurboFect (A' – D'). A and A', PoB1; B and B', PoBf; C and C', PoB2; D and D', PoBh. Green signals indicate the expression of EGFP. Bar = 100 µm. |
However, the transfection efficiency of one special cell line with different transfection reagents differed. The trans-fection efficiency of the PoB1 cell with TurboFect was ap-proximately 40% (Fig. 6A'), whereas that of the PoB1 cells with LipofectamineⓇ 3000 was only approximately 10% (Fig. 6A). By contrast, the PoBf cells showed a significant-ly higher transfection efficiency with LipofectamineⓇ 3000 (70%, Fig. 6B) than with TurboFect (approximately 15%, Fig. 6B'). The clear green fluorescent signals in the PoBf cells were stronger when they were transfected with LipofectamineⓇ 3000 than with TurboFect. The PoB2 cells transfected with LipofectamineⓇ 3000 and TurboFect show-ed no significant difference in transfection efficiency (i.e., approximately 20% – 25%, Figs. 6C and 6C'). The results of transfection in PoBh cells were similar to those in PoB1 cells; that is, a high efficiency of approximately 40% was caused by TurboFect transfection (Fig. 6D'), whereas low efficiency of approximately 25% was caused by LipofectamineⓇ 3000 transfection (Fig. 6D).
4 DiscussionIn the present study, four cell lines were established from the brain of P. olivaceus. These cell lines designated as PoB1, PoB2, PoBf and PoBh on the basis of their origin, isolation and culture methods of themselves showed distinct morphological and genetic characteristics. The four types of brain cell lines have been sub-cultured for more than 176, 140, 184 and 165 times since their establishment in January of 2014 and are still in active growth.
Brain tissues of P. olivaceus were either digested with enzyme trypsin or were cultured directly to initiate the pri-mary culture of cells. The establishment of the four brain cell lines proved that the two methods were helpful. All four cell lines adapted well for growth in DMEM/F12 me-dium. Although many trials used Leibovitz's L-15 (Poyer and Hartmann, 1992; Wen et al., 2008a, 2008b; Ku et al., 2009; Sun et al., 2015) and special neurobasal media to culture brain cell lines and murine neural stem/ progenitor cells (Corti et al., 2007), respectively. DMEM/ F12 me-dium was chosen in the present study on the basis of the culture condition of the NSC lines of A. leptorhynchus (Hinsch and Zupanc, 2006). No significant cell growth was observed when EAGB cell lines were incubated in M199, DMEM and RPIM1640 (Huang et al., 2011). In addition, no cytokines were used in the above cell cultures, expect for that of the adult brain of A. Leptorhynchus, wherein B27, bFGF and epidermal growth factor (EGF) were supplemented. Therefore, the cultivation and growth of brain neural cells required less nutrition, and almost all of the common culture media could facilitate satisfactory growth. In the present study, bFGF and IGF were initially present in all four brain cells, whereas FGF-9 was only supple-mented in the culture medium for PoB2. Thus, the different morphological and genetic features present in the four cell lines may share a certain relationship to these factors.
The cell lines grew well at 24℃. It has been reported that the FG cell lines exhibited optimal growth at the same temperature (Tong et al., 1997). Therefore, this temperature was used to maintain the cultures. The four cell lines showed different reactions to FBS (Fig. 3). In general, PoB1 and PoB2 were independent of FBS concentration during the growth period, whereas PoBf and PoBh showed partial independent to FBS. Karyotype analyses of the PoB1, PoBf, PoB2, and PoBh cell lines revealed that all four cell lines did not possess a diploid chromosome number of 2n = 48, which is identical to the modal of P. olivaceus (Tong et al., 1997; Wang et al., 2009). Their chromosome numbers ranged from less than 20 to more than 100. The rate of diploid chro-mosomes in the cell lines was low and the chromosome numbers of approximately 50% of the cell lines were 60 – 72. The distribution and change of chromosome of the four cell lines indicate that their genetic characteristics may have changed. We also checked the DNA content of these cell lines through flow cytometry. Results showed that the DNA contents of these cell lines were 1.5 – 2 times of that of normal cell lines. The presence of metacentric, sub-telocen-tric and spot chromosomes suggests the occurence of chro-mosome duplication, assembly and fracture in these cell lines. Alternative explanations to the aneuploidy have been elaborated as cell fusion, resulting from the possibility that the chromosomes simply failed to separate properly (Chen et al., 2006). Examination of karyotype and growth characteristics indicated that PoB1, PoB2, PoBf and PoBh were transformed (Frank, 1994) and were immortal similar to the reported FG cell lines (Wang et al., 2008). Even though these cell lines were all derived from the brain tissue, molecular identification indicated that they were different from each other (Fig. 5). All four cell lines significantly expressed nestin, a marker for the NSCs. This result suggests that the cell lines still exhibit some properties of NSCs. Nestin is an intermediate filament protein which is abundantly expressed during early embryogenesis in neuro-epithelial stem cells and then is downregulated in mature cells. On the basis of its expression and immunochemistry, nestin has been recognized as a marker for NSCs in brain tissues and many brain cells (Strojnik et al., 2007; Wei et al., 2008). In the present study, the PoB2 and PoBf lines shared similar expression patterns, while only nestin expressed in all of the tested cells. This result indicates that these PoB2 and PoBf are still neural stem cells or progenitor cells with a limited differentiation towards neurons. The PoB1 cell line also expressed slc6a4 mRNA. Slc6a4, encoded by the slc6a4 gene, is also known as a serotonin transporter (SERT or 5-HTT). SERT is located in axons, where it is concentrated in varicosities and terminal boutons. Thus it is an exquisite marker for serotonergic synapses (Hummerich et al., 2004). In recent years, several reports have provided com-pelling evidences for the expression of functionally active SERT in glial cells lines and primary astrocytes (Hirst et al., 1998; Kubota et al., 2001; Malynn et al., 2010). Therefore, the PoB1 cell line was recognized to comprise neural stem cells or progenitor cells with some differentiation towards serotonergic neurons. The PoBh line expressed not only nestin but also weak sox2 and significant aldh1l1 mRNA. In addition to nestin, sox2 has also been recognized as a marker gene for NSCs (Archer et al., 2011). Aldh1L1 is ro-bustly expressed in brain astrocytes and cells in NSC cul-ture conditions could promote the activity of Aldh1L1 (Ca-hoy et al., 2008; Yang et al., 2011; Foo and Dougherty, 2013). Moreover, another intermediate filament protein GFAP also expresses in differentiated astrocytes (Lendahl et al., 1990; Zendra, 1991; Strojnik et al., 2007). GFAP-/-astrocytes pro-duce both nestin and vimentin, and retain the ability to form neurons (Pekny et al., 1998). Therefore, we recognized PoBh cells as primary astrocytes with no or limited differentiation. The deficiency of GFAP suggests that the as-trocytes have not differentiated or GFAP gene loss has oc-curred during transformation as GBC1 cells (Wen et al., 2008b). Furthermore, the four cell lines showed a similar morphology to previously reported NSC lines of other fishes (Hinsch and Zupanc 2006; Wen et al., 2008a, 2008b; Ser-vili et al., 2009; Huang et al., 2011). Neurosphere formation was also observed in the four cell lines (Fig. 2). This observation further validates that these cell cultures are NSCs or progenitor cells (Servili et al., 2009).
We also tested the PoB1, PoBf, PoB2 and PoBh cell lines for potential use in transfection and have observed successful signals (Fig. 6). The transfection capability of the cells suggests their potential use as models in vitro for studies on neurogenesis and fish nerv-related genes' functions, which can elucidate the mechanisms of neurophysiology, neuro-toxicity and neuroendocrinology.
In summary, four brain cell lines designated as PoB1, PoBf, PoB2 and PoBh were established from the brain of P. olivaceus. These cell lines grew well and were characterized as NSCs or progenitor cells. The four cell lines may serve as useful tools for the study of genetic manipulation, cytotoxicity and virus pathogenesis in relation to brain de-velopment.
AcknowledgementsThis work was supported by the National High-Tech Re-search and Development Program of China (Nos. 2012AA10A402, 2012AA10A408).
Archer, T. C., Jin, J. and Casey, E. S., 2011. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Develop-mental Biology, 350(2): 429-440. (  0) 0) |
Cahoy, J. D., Emery, B., Kaushal, A., Foo, L. C., Jennifer, L. Z., Karen, S. C., Yi, X., Jane, L. L., Paul, A. K., Sergey, A. K., Wesley, J. T. and Ben, A. B., 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 28(1): 264-278. DOI:10.1523/JNEUROSCI.4178-07.2008 (  0) 0) |
Cai, J., 2002. Properties of a fetal multipotent neural stem cell (NEP cell). Developmental Biology, 251(2): 221-240. DOI:10.1006/dbio.2002.0828 (  0) 0) |
Charrier, C., Coronas, V., Fombonne, J., Roger, M., Jean, A. and Krantic, S., 2006. Characterization of neural stem cells in the dorsal vagal complex of adult rat by in vivo proliferation labeling and in vitro neurosphere assay. Neuroscience, 138(1): 5-16. (  0) 0) |
Chen, K. A., Laywell, E. D., Marshall, G., Walton, N., Zheng, T. and Steindler, D. A., 2006. Fusion of neural stem cells in culture. Experimental Neurology, 198(1): 129-135. (  0) 0) |
Corti, S., Nizzardo, M., Nardini, M., Donadoni, C., Locatelli, F. and Papadimitriou, D., 2007. Isolation and characterization of murine neural stem/progenitor cells based on prominin-1 expression. Experimental Neurology, 205(2): 547-562. (  0) 0) |
Duan, X., Kang, E. K., Liu, Y. C., Ming, G. L. and Song, H. J., 2008. Development of neural stem cell in the adult brain. Current Opinion in Neurobiology, 18(1): 108-115. DOI:10.1016/j.conb.2008.04.001 (  0) 0) |
Foo, L. C. and Dougherty, J. D., 2013. Aldh1L1 is expressed by postnatal neural stem cells in vivo. Glia, 61(9): 1533-1541. DOI:10.1002/glia.22539 (  0) 0) |
Frank, D. A., 1994. Culture of animal cells: A manual of basic technique. Journal of the Royal Society of Medicine, 57(2): 247-248. (  0) 0) |
Grandel, H., Kaslin, J., Ganz, J., Wenzel, I. and Brand, M., 2006. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Deve-lopmental Biology, 295(1): 263-277. (  0) 0) |
Hasoon, M. F., Daud, H. M., Abdullah, A. A., Arshad, S. S. and Bejo, H. M., 2011. Development and partial characterization of new marine cell line from brain of Asian sea bass Lates cal-carifer for virus isolation. Vitro Cellular & Developmental Bio-logy Animal, 47(1): 16-25. (  0) 0) |
Hinsch, K. and Zupanc, G. K., 2006. Isolation, cultivation, and dif-ferentiation of neural stem cells from adult fish brain. Journal of Neuroscience Methods, 158(1): 75-88. DOI:10.1016/j.jneumeth.2006.05.020 (  0) 0) |
Hirst, W. D., Price, G. W., Rattray, M. and Wilkin, G. P., 1998. Serotonin transporters in adult rat brain astrocytes revealed by[3H]5-HT uptake into glial plasmalemmal vesicles. Neuroche-mistry International, 33(1): 11-22. DOI:10.1016/S0197-0186(05)80003-8 (  0) 0) |
Huang, X., Huang, Y., Ouyang, Z. and Qin, Q., 2011. Establish-ment of a cell line from the brain of grouper (Epinephelus akaara) for cytotoxicity testing and virus pathogenesis. Aqua-culture, 311(1-4): 65-73. DOI:10.1016/j.aquaculture.2010.11.037 (  0) 0) |
Hummerich, R., Reischl, G., Ehrlichmann, W., Machulla, H. J., Heinz, A. and Schloss, P., 2004. DASB in vitro binding cha-racteristics on human recombinant monoamine transporters with regard to its potential as positron emission tomography (PET) tracer. Journal of Neurochemistry, 90(5): 1226. (  0) 0) |
Ku, C. C., Teng, Y. C., Wang, C. S. and Lu, C. H., 2009. Establishment and characterization of three cell lines derived from the rockfish grouper Epinephelus quoyanus: Use for transge-nic studies and cytotoxicity testing. Aquaculture, 294(1-2): 147-151. DOI:10.1016/j.aquaculture.2009.05.010 (  0) 0) |
Kubota, N., Kiuchi, Y., Nemoto, M., Oyamada, H., Ohno, M. and Funahashi, H., 2001. Regulation of serotonin transporter gene expression in human glial cells by growth factors. Euro-pean Journal of Pharmacology, 417(1-2): 69-76. DOI:10.1016/S0014-2999(01)00906-2 (  0) 0) |
Lendahl, U., Zimmerman, L. B. and Mckay, R. D. G., 1990. CNS stem cells express a new class of intermediate filament protein. Cell, 60(4): 585-595. DOI:10.1016/0092-8674(90)90662-X (  0) 0) |
Malynn, S., Torres, A. C., Moynagh, P. and Haase, J., 2010. The pro-inflammatory cytokine TNF-α regulates the expression of the serotonin transporter (SERT) gene in primary astrocytes and the C6 glioma cell line. Brain Behavior & Immunity, 24: S70-S71. DOI:10.1007/s11064-012-0967-y (  0) 0) |
Pekny, M., Eliasson, C., Chien, C. L., Kindblom, L. G., Liem, R. and Hamberger, A., 1998. GFAP-deficient astrocytes are capa-ble of stellation in vitro when cocultured with neurons and ex-hibit a reduced amount of intermediate filaments and an increased cell saturation density. Experimental Cell Research, 239(2): 332-343. DOI:10.1006/excr.1997.3922 (  0) 0) |
Poyer, J. and Hartmann, J., 1992. Establishment of a cell line from brain tissue of the silky shark, Carcharhinus falciformis. In Vitro Cellular & Developmental Biology-Animal, 28(11-12): 682-684. (  0) 0) |
Servili, A., Bufalino, M. R., Nishikawa, R., Melo, I. S. D., Muo-Oz-Cueto, J. A. and Lee, L. E. J., 2009. Establishment of long term cultures of neural stem cells from adult sea bass, Dicentrarchus labrax. Comparative Biochemistry & Physiology Part A: Molecular & Integrative Physiology, 152(2): 245-254. (  0) 0) |
Song, H., He, Y., Ma, L., Zhou, X., Liu, X. and Qi, J., 2015. Cha-racterisation of kisspeptin system genes in an ovoviviparous teleost: Sebastes schlegeli. General & Comparative Endocri-nology, 214: 114-125. (  0) 0) |
Strojnik, T., RoSland, G. V., Sakariassen, P. O., Kavalar, R. and Lah, T., 2007. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: Correlation of nestin with prognosis of patient survival. Surgical Neurology, 68(2): 133-143. (  0) 0) |
Sun, A., Wang, T. Z., Wang, N., Liu, X. F., Sha, Z. X. and Chen, S. L., 2015. Establishment and characterization of an ovarian cell line from half-smooth tongue Solecynoglossus semilaevis. Journal of Fish Biology, 86(1): 46-59. DOI:10.1111/jfb.12535 (  0) 0) |
Taupin, P., 2006. Neurogenesis in the adult central nervous system. Comptes Rendus Biologies, 329(7): 465-475. DOI:10.1016/j.crvi.2006.04.001 (  0) 0) |
Tong, S. L., Li, H. and Miao, H. Z., 1997. The establishment and partial characterization of a continuous fish cell line FG-9307 from the gill of flounder Paralichthys olivaceus. Aquaculture, 156(3-4): 327-333. DOI:10.1016/S0044-8486(97)00070-7 (  0) 0) |
Tsai, C. L., Wang, L. H. and Lin, Y. H., 2001. Effects of estrogen and neurotransmitters on the primary cultures of tilapia brain from different ages. Developmental Brain Research, 129(1): 111-113. (  0) 0) |
Wang, X. B., Zhang, Q. Q., Chen, Y. J., Qi, J., Wang, Z. G. and Wang, X. L., 2009. Cytogenetic characterization of olive flounder Paralichthys olivaceus: DNA content, karyotype, agnors and location of major ribosomal genes. Acta Oceanologica Sinica, 28(4): 72-77. (  0) 0) |
Wang, X. B., Zhang, Q. Q., Qi, J., Wang, Z. G. and Wang, X. L., 2008. Variation analysis of karyotype of flounder gill cell line. Journal of Fishery Sciences of China, 15(3): 483-487. (  0) 0) |
Wei, L. C., Shi, M., Cao, R., Chen, L. W. and Chan, Y. S., 2008. Nestin small interfering RNA (siRNA) reduces cell growth in cultured astrocytoma cells. Brain Research, 1196: 103-112. DOI:10.1016/j.brainres.2007.11.026 (  0) 0) |
Wen, C. M., Cheng, Y. H., Huang, Y. F. and Wang, C. S., 2008a. Isolation and characterization of a neural progenitor cell line from tilapia brain. Comparative Biochemistry & Physiology Part A: Molecular & Integrative Physiology, 149(2): 167-180. (  0) 0) |
Wen, C. M., Lee, C. W., Wang, C. S., Cheng, Y. H. and Huang, H. Y., 2008b. Development of two cell lines from Epinephelus coioides brain tissue for characterization of betanodavirus and megalocytivirus infectivity and propagation. Aquaculture, 278(1-4): 14-21. DOI:10.1016/j.aquaculture.2008.03.020 (  0) 0) |
Wen, C. M., Wang, C. S., Chin, T. C., Cheng, S. T. and Nan, F. H., 2010. Immunochemical and molecular characterization of a novel cell line derived from the brain of Trachinotus blochii (Teleostei, Perciformes): A fish cell line with oligodendrocyte progenitor cell and tanycyte characteristics. Comparative Bio-chemistry and Physiology Part A: Molecular & Integrative Phy- siology, 156(2): 224-231. (  0) 0) |
Wu, Y. C. and Chi, S. C., 2006. Persistence of betanodavirus in Barramundi brain (BB) cell line involves the induction of interferon response. Fish and Shellfish Immunology, 21(5): 540-547. DOI:10.1016/j.fsi.2006.03.002 (  0) 0) |
Yang, Y. J., Vidensky, S., Jin, L., Jie, C. F., Lorenzini, I., Frankl, M. and Rothstein, D. J., 2011. Molecular comparison of GLT1+ and ALDH1l1+ astrocytes in vivo in astroglial reporter mice. Glia, 59(2): 200-207. DOI:10.1002/glia.21089 (  0) 0) |
Yu, Z. N., Kong, X. Y. and Xie, Z. Y., 1995. Studies on karyotypes of fishes of economic importance in coastal waters of Shandong Peninsula. Journal of Fishery Sciences of China, 2(2): 1-6. (  0) 0) |
Zendra, E. Z., 1991. Regulation of intermediate filament gene ex-pression. Current Opinion in Cell Biology, 3(1): 67-74. DOI:10.1016/0955-0674(91)90167-W (  0) 0) |
Zupanc, G. K., 2008. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. Journal of Physiology-Paris, 102(4-6): 357-373. DOI:10.1016/j.jphysparis.2008.10.007 (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


