Sexual dimorphism of vertebrate growth has received a lot of attentions. Sexual dimorphism is commonly defined as the morphological and physiological differences between females and males (Dean et al., 2014; Mei and Gui, 2015). Feeding and digestion and energy allocation during growth and reproduction, species heredity, genotype and phenotype, steroid hormone levels and gene expression of the somatotrophic axis are the main causes of sexual dimorphism of vertebrate growth (Ma et al., 2009). Many male mammals display a larger body size or greater weight, and higher growth rate than females, which can occur throughout the life cycle or during developmental stages (Gatford et al., 1998). Fish are the largest group of vertebrates, and a large number of species display evident sexual size dimorphism and growth differences. In rainbow trout Oncorhynchus mykiss (Sheehan et al., 1999), common carp Cyprinus carpio (Lv et al., 2008), yellow perch Perca flavescens (Headley and Lauer, 2008) and tongue sole Cynoglossus semilaevis (Ji et al., 2011b), females grow faster and larger than males. Conversely, in Nile tilapia Oreochromis niloticus (Beardmore et al., 2001) and yellow catfish Pelteobagrus fulvidraco (Wang et al., 2009), males grow faster and larger than females. Further studies on these fish species will help us to understand the mechanism underlining the sexual dimorphism of vertebrate growth.
Numerous studies have revealed that, like other vertebrates, the growth of fishes is regulated by the growth hormone (GH)/insulin-like growth factor 1 (IGF-1) (GH/ IGF) axis (Gatford et al., 1998; Moriyama et al., 2000; Reinecke et al., 2005; Hyun, 2013; Bergan-Roller and Sheridan, 2018). GH is a peptide hormone secreted mainly by the pituitary that is regulated by many neuroendocrine factors (Steyn et al., 2016). Growth hormone-releasing hormone (Ghrh) is the most important regulator of GH secretion and synthesis, which functions via a specific G-protein coupled receptor, growth hormone-releasing hormone receptor (Ghrhr). Mutation of either Ghrhr or Ghrh can decrease GH secretion and growth performance in mice (Gaylinn et al., 1999; Alba and Salvatori, 2004). Previous studies showed that GH/IGF biosynthesis amounts are related to the growth rate and are critical for sexual dimorphism of fish growth. In female European eel Anguilla anguilla, the faster growth rate of females has been attributed to significantly higher gh mRNA abundance in pituitary (Degani et al., 2003). Similarly, in tongue sole, fast-growing females have 6.4-fold higher gh mRNA abundance than slowgrowing males (Ji et al., 2011b). Conversely, species with faster male growth rate, such as Nile tilapia, had higher gh mRNA abundance in male pituitary than in female one (Ma et al., 2006). The sexual dimorphic modulation of the GH/IGF system depends on the integration of multiple factors. It is still unclear whether Ghrh/Ghrhr is linked to the sexually dimorphic expression of gh in fish. In tongue sole, ghrh expression is higher in male hypothalamus than in females, which is caused by GH deficiency in males (Ji et al., 2011a, b). Further comparative expression analysis of ghrh/ghrhr should be carried out in species with sexual dimorphism of growth.
Scatophagus argus is a euryhaline subtropical fish that is widely distributed in Indian-Pacific waters. S. argus is an important aquaculture fish that has an XY sex determination system (Mustapha et al., 2018). Female S. argus grow faster and larger than male, providing an excellent model to understand the mechanism underlining sexual dimorphism of growth (Cai et al., 2010; Deng et al., 2014). S. argus females have higher gh mRNA abundance than males in pituitary, which may explain the sexual dimorphism of growth of this species (Deng et al., 2014). In this study, ghrh and ghrhr cDNAs were isolated from S. argus. Among tissues and between sex, the expression differentials of ghrh, ghrhr and gh were examined. To better understand the role of Ghrh/Ghrhr in S. argus, females were injected Ghrh peptide. Additionally, the effects of steroid hormones on ghrh, ghrhr and gh expressions were also analyzed. Our results should aid to understand the mechanisms underlining the sexual dimorphism of S. argus growth.
2 Materials and Methods 2.1 Experimental Fish IndividualsS. argus (body weight, 250–300 g) used for cloning and tissue distribution studies were purchased from Dongfeng Market (Zhanjiang, Guangdong, China). Adult S. argus (body weight, 74–250 g) used in comparing growth and gene expression analysis between females and males were cultured at Donghai Island Marine Biology Research Base at Guangdong Ocean University (Zhanjiang, China). Adult female fish (body weight, 160–290 g) obtained from Zhuhai Yucheng Fry Cultivation Base (Zhuhai, Guangdong, China), were injected intraperitoneally with Ghrh or steroid hormones. All animal experiments were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals for the Science and Technology Bureau of China.
2.2 Chemical ReagentsS. argus Ghrh-27, HADAIFTNSYRKVLGPISARKFLQTIM in sequence with a c-terminal amide (Lee et al., 2007; Grey and Chang, 2013) were synthesized commercially (GL Biochem Ltd., Shanghai, China). Ghrh-27 was dissolved in sterile phosphate buffered saline (pH 7.4). 17α-methyltestosterone (17α-MT) (46444, 98% purity) and 17β-Estradiol (E2) (491187, 98% purity) were purchased from Sigma-Aldrich Corp. (St Louis, MO, USA). 17α-MT and E2 were dissolved in alcohol.
2.3 cDNA Isolation and Sequence AnalysisHighly conservative transcripts of ghrh and ghrhr were identified using cDNA alignment data from Oreochromis niloticus (as deposited in GenBank) and transcriptome data from S. argus mixed tissue (Yang et al., 2017) and gonads (He et al., 2019). Forward and reverse primers were designed for the 5' and 3' UTR of ghrh and ghrhr cDNAs of S. argus. The cDNA sequences containing the open reading frame (ORF) of ghrh and ghrhr were confirmed by cloning. All primers used in this study are listed in Table 1. S. argus cDNA were used as templates. The PCR protocol was as follows: initial denaturation at 94℃ for 3 min, 37 cycles of denaturation at 94℃ for 30 s, annealing at 60℃ for 30 s and extension at 72℃ for 2 min, followed by a final extension at 72℃ for 10 min. The PCR products were separated by electrophoresis on a 1.5% agarose gel, and the gel was stained with ethidium bromide (EB). Selected fragments were extracted and cloned into a p-Easy-T3 vector (TransGen Biotech, China). Positive clones were sequenced by an ABI 377 sequencer.
|
|
Table 1 Primer sequences used in this study (–, not detected) |
Full-length cDNA sequences of ghrh and ghrhr were analyzed using DNASTAR (http://www.dnastar.com/). ORFs and amino acid sequence were predicted using ORF finder software from the NCBI website (https://www.ncbi.nlm.nih.gov/). Sequence homology alignment was performed by using MegAlign, part of the DNASTAR software package, and multiple alignments carried out by using ClustalX (http://www.clustal.org/). The phylogenetic tree was constructed by using the neighborjoining method and implemented in MEGA 6.06 (http://www.megasoftware.net/).
2.4 Tissue Expression of ghrh, ghrhr and gh in S. argusFemale (n =3) and male (n =3) adult fish were anesthetized with 100 mg L−1 tricaine methane sulfonate (MS 222, Sigma, Saint Louis, MO) and dissected. Hypothalamus, pituitary, gill, liver, heart, spleen, kidney, stomach, intestine, testis, ovary, muscle and head kidney tissues were collected, frozen with liquid nitrogen, and stored at −80℃ prior to RNA extraction. Total RNA was isolated using TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, China) with 1 μg total RNA according to the manufacturer's protocol. Briefly, total RNA was treated with RNase-free DNase I, then reverse transcriptase was used for cDNA synthesis with oligo-dT and Random 6-mer primers. Tissue distribution studies were performed using reverse transcription PCR (RT-PCR). Gene β-actin was used as an internal control (Table 1). The amplification regime consisted of 35 cycles (28 cycles for β-actin) at 95℃ for 30 s, 60℃ for 30 s, and 72℃ for 30 s; followed by further amplification at 72℃ for 10 min. PCR products were separated on a 2.0% agarose gel and visualized with EB.
2.5 Analyses of ghrh, ghrhr and gh ExpressionsThe hypothalamus and pituitary were collected from adult male and female fish cultured under the same conditions for 1.5 years. The body and gonad weights were recorded. The total RNA was extracted from the hypothalamus and pituitary, and reverse transcription was carried out as described in Section 2.4. The real-time PCR primers are listed in Table 1. Real-time PCR was performed on a Light Cycler 480 (Roche) using SYBR Green Realtime PCR Master Mix (Toyobo, Japan) according to the manufacturer's protocol. Real-time PCR conditions were as follows: denaturation at 95℃ for 1 min followed by 40 cycles of 95℃ for 15 s, 60℃ for 30 s and 72℃ for 30 s (fluorescent data collection). The relative abundances of ghrh, ghrhr and gh transcripts were evaluated using the formula: R = 2−∆∆Ct. The reference gene β-actin was used to normalize expression values.
2.6 Injection of Ghrh-27 into Female S. argusGhrh-27 (0, 0.001, 0.1, and 0.1 mg kg−1 body weight) was injected intraperitoneally into adult female spotted scats (n = 40). Five fish each treatment were sampled at 3 and 6 h post-injection. The hypothalamus and pituitary were dissected, frozen with liquid nitrogen, and stored at −80℃ for gene expression assays.
2.7 Injection of 17α-MT or E2 into Female S. argus17α-MT (4 mg kg−1 body weight) or E2 (4 mg kg−1 body weight) were injected intraperitoneally into adult female S. argus. The negative control was administered with alcohol only. The injection volume was restricted (1 μL per gram body weight) to avoid any possible influence on the electrolyte balance. Five fish individuals each treatment were sampled at 6 h post-injection. The injection dose of hormone and sampling time were chosen following what we have done in our previous study (Deng et al., 2018). According to the serum androgen and estrogen concentrations reported in S. argus (Zhang et al., 2018), the injected dose of steroid hormones should be higher than the physiological concentration in present study. The relative higher dose can ensure that enough exogenous steroid hormones are transported to the target tissues in a short time. The hypothalamus and pituitary were stored at −80℃ for gene expression analysis through real-time PCR.
2.8 Statistical AnalysisAll data are expressed as means ± SD. Significant difference among groups was tested with one-way ANOVA with Duncan's post-hoc test or independent-samples T test. The significance level was set at P < 0.05, and all statistical tests were performed using SPSS 16.0 (SPSS, Chicago, IL, USA).
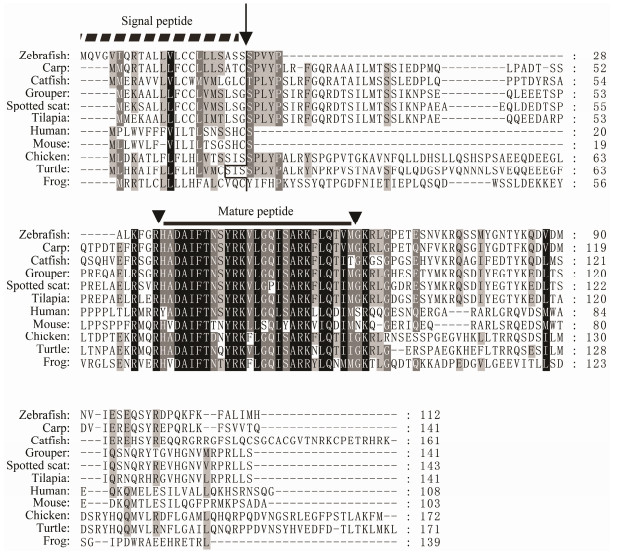
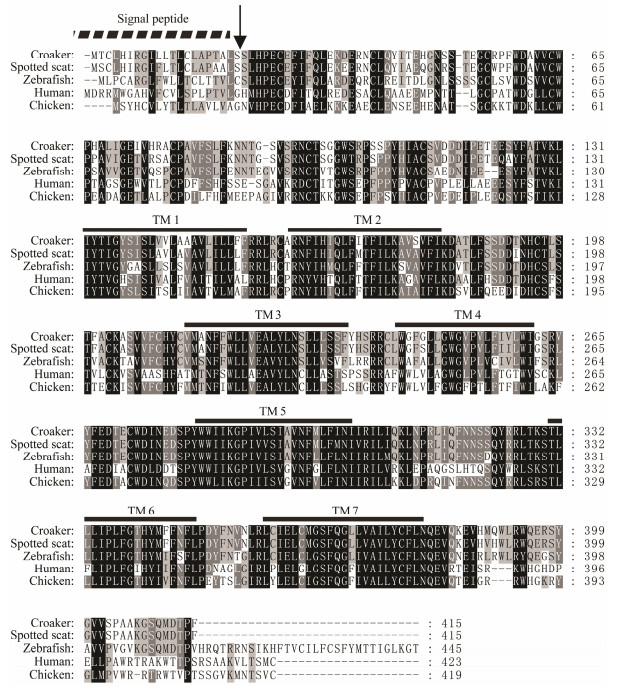
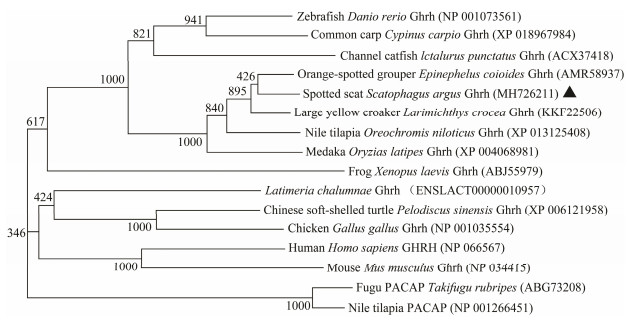
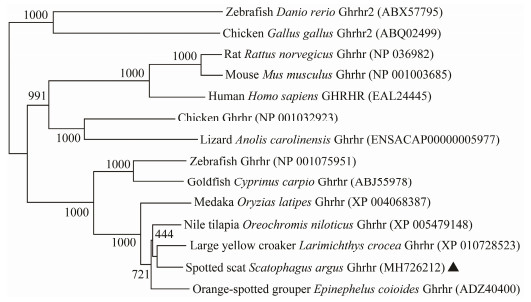
3 Results 3.1 cDNA Isolation and Sequence AnalysisBoth ghrh and ghrhr cDNA of S. argus were isolated and analyzed. The partial cDNA of ghrh consisted of 498 nucleotides with a 432 bp ORF encoding 143 amino acid residues (NCBI accession no. MH726211). The cDNA of ghrhr was 1393 bp in length, and contained a 1248 bp ORF encoding 415 amino acid residues (NCBI accession no. MH726212). The signal peptide located at the Nterminal of Ghrh and Ghrhr. The conserved mature peptide region was found in the middle of Ghrh. As a typical G-protein coupling receptor, Ghrhr has seven conserved transmembrane domains (Figs. 1 and 2). The overall S. argus Ghrh was 90.1% similar to (Calculated by using MegAlign software) that of orange-spotted grouper Epinephelus coioides, 23% to that of mouse. The mature peptide region of S. argus Ghrh was 96% and 64% similar to fish and mouse Ghrhs, respectively. S. argus Ghrhr was 92.3% similar to that of large yellow croaker Larimichthys crocea, 73.5% to that of zebrafish and 51% to that of human beings. S. argus Ghrh and Ghrhr clustered closely to those of perciform fish species (Figs. 3 and 4). S. argus Ghrh grouped closely with that of orange-spotted grouper while S. argus Ghrhr grouped closely with that of large yellow croaker. S. argus Ghrh and Ghrhr are close to that of Perciformes species which is consistent with taxonomy.
|
Fig. 1 Alignment of the deduced amino acid sequence of S. argus growth hormone-releasing hormone (Ghrh) with those of other vertebrates. Signal peptide and mature peptide are indicated by the dotted and full line, respectively. The arrow indicates the cleavage site of the signal peptide. The arrowhead indicates the cleavage site of the mature peptide. The number on the right column represents the numbers of the amino acids. GenBank accession numbers of the sequences are as follows: human Homo sapiens (NP 066567), mouse Mus musculus (NP 034415), chicken Gallus gallus (NP 001035554), Chinese soft-shelled turtle Pelodiscus sinensis (XP 006121958), frog Xenopus laevis (ABJ55979), zebrafish Danio rerio (NP 001073561), common carp Cyprinus carpio (XP 018967984), channel catfish Ictalurus punctatus (ACX37418), Nile tilapia Oreochromis niloticus (XP 013125408), orange-spotted grouper Epinephelus coioides (AMR58937), and spotted scat Scatophagus argus (MH726211). |
|
Fig. 2 Alignment of the deduced amino acid sequence of S. argus growth hormone-releasing hormone receptor (Ghrhr) with those of other vertebrates. Signal peptide and transmembrane domain are indicated by the dotted and full lines, respectively. The arrow indicates the cleavage site of the signal peptide. The number on the right column represents the numbers of the amino acids. GenBank accession numbers of the sequences are as follows: human Homo sapiens (EAL24445), chicken Gallus gallus (NP 001032923), zebrafish Danio rerio (NP 001075951), large yellow croaker Larimichthys crocea (XP 010728523) and spotted scat Scatophagus argus (MH726212). |
|
Fig. 3 Phylogenetic analysis of vertebrate Ghrh. The neighbor-joining method was used to construct the phylogenetic tree with MEGA5.0 using the Nile tilapia and fugu PACAP as an out-group. The credibility of the branching was tested by using bootstrap resampling with 1000 pseudo replicates. |
|
Fig. 4 Phylogenetic analysis of vertebrate Ghrhr. The neighbor-joining method was used to construct the phylogenetic tree with MEGA5.0 using the chicken and zebrafish Ghrhr2 as an out-group. The credibility of the branching was tested by using bootstrap resampling with 1000 pseudo replicates. |
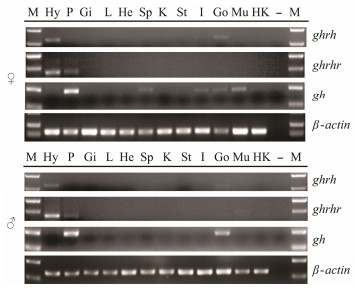
The ghrh highly expressed in the hypothalamus of adult males and females but lowly expressed in the ovary and testis (Fig.5). The ghrhr highly expressed in the hypothalamus and pituitary of adult males and females. In addition, ghrhr weakly expressed in the gonads (ovary and testis), muscle and head kidney while gh highly expressed in the pituitary of males and females. The gh expressed lowly in various female fish tissues including spleen, intestine, ovary and muscle tissues. In male, gh also expressed highly in the testis and weakly in muscle.
|
Fig. 5 Tissue expression patterns of ghrh, ghrhr and gh in adult Scatophagus argus. β-actin was used as the internal control. Each gel figure represents three independent results from three tails of fish. Hy, hypothalamus; P, pituitary; Gi, gill; L, liver; He, heart; Sp, spleen; K, kidney; St, stomach; I, intestine; Go, gonad (ovary or testis); Mu, muscle; Hk, head kidney; M, DNA marker; –, negative control. |
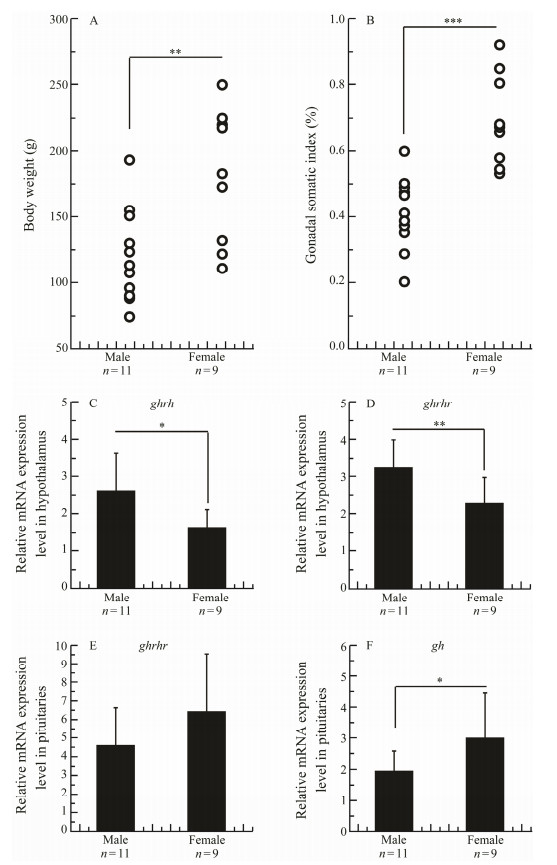
The growth performance of male and female fish individuals was compared among randomly sampled 20 1.5-year-old fish individuals, 11 male and 9 female, which were reared in the same tank containing 90 tons of water under a natural light cycle. The average body weight (181.3 g) was significantly heavier than that of males (120.0 g) (Fig.6A). The gonadal somatic index (GSI) of females was significantly greater than that of males (Fig.6B). The hypothalamus and pituitary of these fish individuals were sampled for gene expression analysis. In the hypothalamus, the expressions of ghrh and ghrhr were significantly higher in males than in females (Figs. 6C, D). In the pituitary, the expression of ghrhr was aimilar between males and females while that of gh was significantly higher in females than in males (Figs. 6E, F).
|
Fig. 6 Difference in body weight (A), gonadal somatic index (B), ghrh (C) and ghrhr (D) expressions in the hypothalamus, and ghrhr (E) and gh (F) expressions in the pituitary between male and female Scatophagus argus. Data are expressed as the mean ± SD. * indicates means with significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Independent-Samples T test, SPSS). |
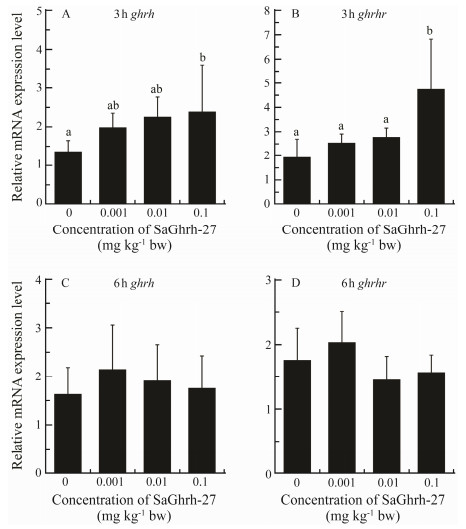
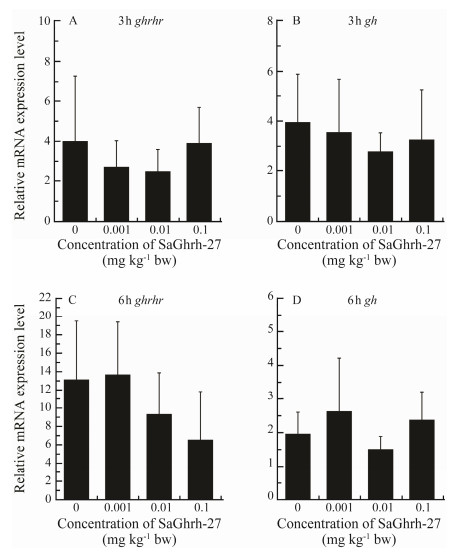
The ghrh and ghrhr expressions were significantly upregulated in the hypothalamus 3 h after injection with Ghrh-27 at 0.1 mg kg−1 body weight (Figs. 7A, B). Expressions of ghrh and ghrhr were not significantly different between the treated and control groups at 6h postinjection (Figs. 7C, D). Ghrh-27 did not affect the expression of ghrhr and gh in the pituitary at both 3 and 6 h after injection (Fig.8).
|
Fig. 7 Effects of Ghrh on ghrh and ghrhr mRNA abundances in the hypothalamus of S. argus. The expression levels are shown as the ratio to the expression of β-actin. Data were means ± SD (n = 5). Different letters above the error bars indicate statistical differences at P < 0.05 as determined by one-way ANOVA followed by Duncan's post hoc test. |
|
Fig. 8 Effects of Ghrh on ghrhr and gh mRNA expressions in the pituitary of Scatophagus argus. The expression levels are shown as the ratio to the expression of β-actin. Data are presented as means ± SD (n = 5). |
The expressions of ghrh and ghrhr in hypothalamus were similar between control and the fish injected 17α-MT or E2 at 4 mg kg−1 body weight after 6 h (Figs. 9A, B). Compared to the control, pituitary abundance of ghrhr mRNA was significantly lower in 17α-MT-treated fish whereas no significant difference was found between control and E2-treated fish. Conversely, gh mRNA abundance was significantly up-regulated by E2 but not affected by 17α-MT (Figs. 9C, D).
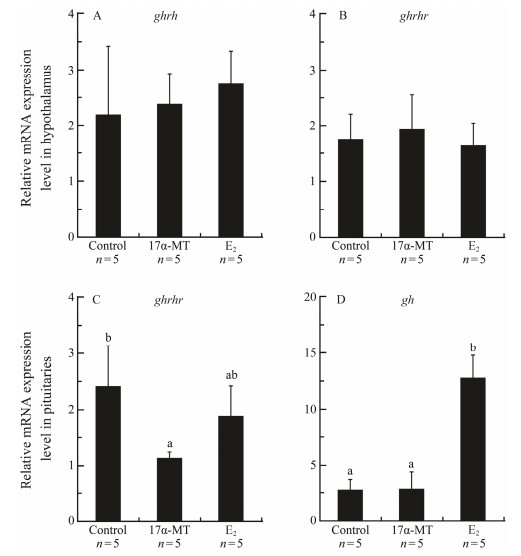
|
Fig. 9 Effects of 17α-methyltestosterone (17α-MT) and 17β-Estradiol (E2) on the expressions of ghrh (A), ghrhr (B and C) and gh (D) in the hypothalamus or pituitary of Scatophagus argus. Data are presented as mean ± SD (n = 5). Different letters above the error bars indicate statistical differences at P < 0.05 as determined by one-way ANOVA followed by Duncan's post hoc test. |
The GH/IGF axis plays a regulatory role in the growth process of vertebrates (Bergan-Roller and Sheridan, 2018). GHs are critical proteins for sexual growth dimorphism in several fish species while the mechanism underlining differential gh expression and GH biosynthesis between male and female individuals has not been understood well. In goldfish Carassius auratus, Ghrh stimulates the release of GH (Lee et al., 2007; Grey and Chang, 2013). In olive flounder Paralichthys olivaceus and orangespotted grouper, Ghrh stimulates gh expression (Nam et al., 2011; Qian et al., 2012). In this study, we tried to describe possible roles of Ghrh and Ghrhr in sexual growth dimorphism in S. argus.
In this study, ghrh and ghrhr cDNA were successfully isolated from S. argus, as in other species. The length of predicated mature Ghrh of S. argus is 27 amino acids, which is the same as goldfish Ghrh (Grey and Chang, 2013). Compared with S. argus Ghrh, S. argus Ghrhr amino acid sequence was more similar to vertebrate Ghrhr, which may be due to the higher similarity of Ghrhr adaptor (mature Ghrh peptide). Sequence analysis revealed that the Ghrh amino acid sequence deduced possessed the conserved mature peptide domain of vertebrate Ghrh (Ling et al., 1984). Howerver, another ghrhr, ghrhr2, which has only been identified in chicken, xenopus and zebrafish, was not found in S. argus and most fish species, indicating that ghrhr2 was the duplicated gene in few species (Tam et al., 2013). Tissue expression analysis revealed that ghrh was expressed predominantly in S. argus hypothalamus, which agreed with the findings in olive flounder and orange-spotted (Nam et al., 2011; Qian et al., 2012). In S. argus and orangespotted grouper, ghrhr expression was higher in hypothalamus than in pituitary (Qian et al., 2012). The similar expression pattern of Ghrh and Ghrhr among different fish species indicated a conserved function of fish Ghrh and Ghrhr.
Female S. argus grows faster than male does (Cai et al., 2010; Deng et al., 2014). Previous expression analyses of ghrh, ghrhr and gh indicated that GH, not Ghrh and Ghrhr, involved in sexual growth dimorphism of S. argus (Deng et al., 2014). A relative higher expression of ghrh was observed in male S. argus. A relative lower ghrh expression in the hypothalamus of tongue sole female has been reported previously, which has been due to the higher GH concentration (Ji et al., 2011a, b). In addition, Ghrh was not linked to the up-regulation of gh expression in the present study, indicating that Ghrh and Ghrhr are not directly relevant for sexual dimorphic expression of gh in S. argus. As previously mentioned, Ghrh has been linked to gh expression (Nam et al., 2011; Qian et al., 2012). The inconsistencies between these observations may be due to the influence of intrinsic factors on gh expression. On the other hand, it is impossible to exclude that Ghrh is able to up-regulate gh expression in the pituitary of male S. argus. An interesting observation in this study is that Ghrh was linked to the stimulation of ghrh and ghrhr expressions in the hypothalamus of female S. argus (Figs. 7A, B). To the best of our knowledge, this is the first report of auto-regulation of Ghrh in fish. The regulation mechanisms for this regulation need to be studied more in the future.
After we excluded the functions of Ghrh and Ghrhr in the sexual dimorphic expression of gh in S. argus, we evaluated the possible role of steroid hormones. There are numerous reports of the effects of sex steroid hormone in growth regulation-related genes in fish (Jiao et al., 2006; Ma et al., 2015; Hanson et al., 2017). In female Nile tilapia, E2 injection up-regulated gh expression in the female pituitary, while 17α-MT injection showed no such effect (Ma et al., 2015). Our results also suggested that gh expression was up-regulated by E2 in female S. argus, while it was not affected by 17α-MT. Zhang (2018) found that E2 injection is able to up-regulate the pituitary gh expression in female S. argus, while pituitary gh expression is not affected in the male fish. The serum E2 is significantly higher in female than that in male S. argus (Zhang et al., 2018; He et al., 2019). These results suggested that E2 level and pituitary gh expression are positively correlated in female S. argus, while the relative lower gh expression in the male pituitary should be attributed to some unknown reasons instead of the lack of estrogen. Three estrogen receptor (Er) subtypes, including er, er 1 and er 2, were previously isolated from S. argus, and they were all highly expressed in the pituitary of males and females (Cui et al., 2017). The critical subtype of Er for gh transcriptional regulation will be investigated in our future studies. On the other hand, the serum androgen (11-ketotestosterone, 11-KT) is significantly higher in male than that in female S. argus (Zhang et al., 2018; He et al., 2019). In male Nile tilapia, 17α-MT injection up-regulated gh expression in the male pituitary, while no up-regulation of gh expression was observed in the female pituitary (Ma et al., 2015). Although 17α-MT did not affect the gh expression in female pituitary, the exogenous or endogenous androgen (17α-MT or 11-KT) might be able to influence the gh expression in the male pituitary of S. argus, which needs to be elucidated in the future.
In summary, ghrh and ghrhr cDNA were isolated from S. argus, and the expressions of ghrh, ghrhr and gh were analyzed. In the hypothalamus, the expressions of ghrh and ghrhr were higher in males than in females. In the pituitary, expression of gh was significantly higher in females than in males. Ghrh injection was associated with the up-regulation of ghrh and ghrhr expressions in the hypothalamus, while it was not linked to the upregulation of ghrhr and gh expressions in the pituitary. Additionally, E2 injection was linked to the up-regulation of gh expression in the pituitary. Therefore, the sexual dimorphism of growth performance and gh expression could be regulated by estrogen instead of Ghrh in S. argus. Our study has provided useful data to understand the effects of growth-related genes on sexual growth dimorphism in fish.
AcknowledgementsThis work was funded by grants from the Key Project of 'Blue Granary Science and Technology Innovation' of the Ministry of Science and Technology (No. SQ2018 YFD090006), the National Natural Science Foundation of China (Nos. 31702326 and 41706174), the Natural Science Foundation of Guangdong Province (Nos. 2016A03 0313743, 2017A030313101 and 2018B030311050), the Department of Education of Guangdong Province (Nos. 2018KTSCX090 and 2018KQNCX106), the Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams (No. 2019KJ149), the Zhanjiang Science and Technology Bureau (No. 2016A03017), Guangdong Ocean University Natural Science Research Program (2015 and 2016), the Project of Provincial Key Platform and Major Scientific Research of Colleges and Universities in Guangdong (No. 2015KTSCX058), the Sail Projects of Guangdong (2014.1), the Marine Fishery Science and Technology Extension Projects of Guangdong (Nos. A201408A06 and A201608B01), and the Program for Scientific Research Start-Up Funds of Guangdong Ocean University. We are grateful to Mr. Mustapha from Fisheries College, Guangdong Ocean University, China for his proofreading of the manuscript.
Alba, M. and Salvatori, R., 2004. A mouse with targeted ablation of the growth hormone-releasing hormone gene: A new model of isolated growth hormone deficiency. Endocrinology, 145(9): 4134-4143. DOI:10.1210/en.2004-0119 (  0) 0) |
Beardmore, J. A., Lewis, R. I. and Mair, G. C., 2001. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. Aquaculture, 197: 283-301. DOI:10.1016/S0044-8486(01)00590-7 (  0) 0) |
Bergan-Roller, H. E. and Sheridan, M. A., 2018. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. General and Comparative Endocrinology, 258: 119-133. DOI:10.1016/j.ygcen.2017.07.028 (  0) 0) |
Cai, Z. P., Wang, Y., Hu, J. W., Zhang, J. B. and Lin, Y. G., 2010. Reproductive biology of Scatophagus argus and artificial induction of spawning. Journal of Tropical Oceanography, 29: 180-185 (in Chinese with English abstract). (  0) 0) |
Cui, X. F., Zhao, Y., Chen, H. P., Deng, S. P., Jiang, D. N., Wu, T. L., Zhu, C. H. and Li, G. L., 2017. Cloning, expression and functional characterization on vitellogenesis of estrogen receptors in Scatophagus argus. General and Comparative Endocrinology, 246: 37-45. DOI:10.1016/j.ygcen.2017.03.002 (  0) 0) |
Dean, R. and Mank, J. E., 2014. The role of sex chromosomes in sexual dimorphism: Discordance between molecular and phenotypic data. Journal of Evolutionary Biology, 27(7): 1443-1453. (  0) 0) |
Degani, G., Tzchori, I., Yom-Din, S., Goldberg, D. and Jackson, K., 2003. Growth differences and growth hormone expression in male and female European eels Anguilla anguilla (L.). General and Comparative Endocrinology, 134(1): 88-93. (  0) 0) |
Deng, S. P., Jiang, D. N., Liu, J. Y., Liang, Z. Q., Chen, H. P., Wu, T. L., Zhu, C. H. and Li, G. L., 2018. Thimet oligopeptidase and prolyl endopeptidase of spotted scat Scatophagus argus: Characterization, tissue distribution, expression at different ovarian stages and down-regulation by estradiol. Fisheries Science, 84: 825-835. DOI:10.1007/s12562-018-1226-1 (  0) 0) |
Deng, S. P., Wu, B., Zhu, C. H. and Li, G. L., 2014. Molecular cloning and dimorphic expression of growth hormone (gh) in female and male spotted scat Scatophagus argus. Fisheries Science, 80(4): 715-723. DOI:10.1007/s12562-014-0763-5 (  0) 0) |
Gatford, K. L., Egan, A. R., Clarke, I. J. and Owens, P. C., 1998. Sexual dimorphism of the somatotrophic axis. Journal of Endocrinology, 157(3): 373-389. (  0) 0) |
Gaylinn, B. D., Dealmeida, V. I., Lyons, Jr., C. E., Wu, K. C., Mayo, K. E. and Thorner, M. O., 1999. The mutant growth hormone-releasing hormone (GHRH) receptor of the little mouse does not bind GHRH. Endocrinology, 140(11): 5066-5074. (  0) 0) |
Grey, C. L. and Chang, J. P., 2013. Growth hormone-releasing hormone stimulates GH release while inhibiting ghrelinand sGnRH-induced LH release from goldfish pituitary cells. General and Comparative Endocrinology, 186: 150-156. DOI:10.1016/j.ygcen.2013.02.037 (  0) 0) |
Hanson, A. M., Ickstadt, A. T., Marquart, D. J., Kittilson, J. D. and Sheridan, M. A., 2017. Environmental estrogens inhibit mRNA and functional expression of growth hormone receptors as well as growth hormone signaling pathways in vitro in rainbow trout (Oncorhynchus mykiss). General and Comparative Endocrinology, 246: 120-128. DOI:10.1016/j.ygcen.2016.07.002 (  0) 0) |
He, F. X., Jiang, D. N., Huang, Y. Q., Mustapha, U. F., Yang, W., Cui, X. F., Tian, C. X., Chen, H. P., Shi, H. J., Deng, S. P., Li, G. L. and Zhu, C. H., 2019. Comparative transcriptome analysis of male and female gonads reveals sex-biased genes in spotted scat (Scatophagus argus). Fish Physiology and Biochemistry. DOI:10.1007/s10695-019-00693-8 (  0) 0) |
Headley, H. C. and Lauer, T. E., 2008. Density-dependent growth of yellow perch in southern Lake Michigan, 1984–2004. North American Journal of Fisheries Management, 28: 57-69. DOI:10.1577/M06-097.1 (  0) 0) |
Hyun, S., 2013. Body size regulation and insulin-like growth factor signaling. Cellular & Molecular Life Sciences, 70(13): 2351-2365. (  0) 0) |
Ji, X. S., Chen, S. L., Jiang, Y. L., Xu, T. J., Yang, J. F. and Tian, Y. S., 2011a. Growth differences and differential expression analysis of pituitary adenylate cyclase activating polypeptide (PACAP) and growth hormone-releasing hormone (GHRH) between the sexes in half-smooth tongue sole Cynoglossus semilaevis. General and Comparative Endocrinology, 170(1): 99-109. (  0) 0) |
Ji, X. S., Liu, H. W., Chen, S. L., Jiang, Y. L. and Tian, Y. S., 2011b. Growth differences and dimorphic expression of growth hormone (GH) in female and male Cynoglossus semilaevis after male sexual maturation. Marine Genomics, 4(1): 9-16. DOI:10.1016/j.margen.2010.11.002 (  0) 0) |
Jiao, B., Huang, X., Chan, C. B., Zhang, L., Wang, D. and Cheng, C. H., 2006. The co-existence of two growth hormone receptors in teleost fish and their differential signal transduction, tissue distribution and hormonal regulation of expression in seabream. Journal of Molecular Endocrinology, 36(1): 23-40. (  0) 0) |
Lee, L. T., Siu, F. K., Tam, J. K., Lau, I. T., Wong, A. O., Lin, M. C., Vaudry, H. and Chow, B. K., 2007. Discovery of growth hormone-releasing hormones and receptors in nonmammalian vertebrates. Proceedings of the National Academy of Sciences of the United States of America, 104(7): 2133-2138. (  0) 0) |
Ling, N., Esch, F., Böhlen, P., Brazeau, P., Wehrenberg, W. B. and Guillemin, R., 1984. Isolation, primary structure, and synthesis of human hypothalamic somatocrinin: Growth hormone-releasing factor. Proceedings of the National Academy of Sciences of the United States of America, 81(14): 4302-4306. (  0) 0) |
Lv, Y. P., Lin, Z. H., Lei, H. Z., Wang, C. H. and Xiang, S. P., 2008. Sexual dimorphism in morphological traits and female individual fecundity of Oujiang color common carp, Cyprinus carpio var. Color. Journal of Huazhong Agricultural University, 27(2): 284-288 (in Chinese with English abstract). (  0) 0) |
Ma, X. L., Zhang, Y., Chen, Y. Z. and Zhou, L. B., 2015. Steroid hormones (E2 and MT) displayed difference in sex for Nile tilapia Oreochromis niloticus. Oceanologia et Limnologia Sinica, 46(6): 1487-1493 (in Chinese with English abstract). (  0) 0) |
Ma, X. L., Zhang, Y., Huang, W. R., Liu, X. C., Lin, H. R. and Ye, W., 2006. cDNA cloning of growth hormone, growth hormone receptor and the different expression between male and female Nile tilapia Oreochromis niloticus. Acta Zoologica Sinica, 52: 924-933 (in Chinese with English abstract). (  0) 0) |
Ma, X. L., Zhang, Y., Zhou, L. B., Liu, X. C. and Lin, H. R., 2009. Studies of growth sexual dimorphism in vertebrate. Chinese Journal of Zoology, 44: 141-146 (in Chinese with English abstract). (  0) 0) |
Mei, J. and Gui, J. F., 2015. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Science China Life Science, 58(2): 124-136. DOI:10.1007/s11427-014-4797-9 (  0) 0) |
Moriyama, S., Ayson, F. G. and Kawauchi, H., 2000. Growth regulation by insulin-like growth factor-I in fish. Journal of the Agricultural Chemical Society of Japan, 64(8): 10. (  0) 0) |
Mustapha, U. F., Jiang, D. N., Liang, Z. H., Gu, H. T., Yang, W., Chen, H. P., Deng, S. P., Wu, T. L., Tian, C. X., Zhu, C. H. and Li, G. L., 2018. Male-specific Dmrt1 is a candidate sex determination gene in spotted scat (Scatophagus argus). Aquaculture, 495: 351-358. DOI:10.1016/j.aquaculture.2018.06.009 (  0) 0) |
Nam, B. H., Moon, J. Y., Kim, Y. O., Kong, H. J., Kim, W. J., Kim, K. K. and Lee, S. J., 2011. Molecular and functional analyses of growth hormone-releasing hormone (GHRH) from olive flounder (Paralichthys olivaceus). Comparative Biochemistry and Physiology–Part B: Biochemistry & Molecular Biology, 159(2): 84-91. (  0) 0) |
Qian, Y., Yan, A., Lin, H. and Li, W., 2012. Molecular characterization of the GHRH/GHRH-R and its effect on GH synthesis and release in orange-spotted grouper (Epinephelus coioides). Comparative Biochemistry and Physiology–Part B: Biochemistry & Molecular Biology, 163(2): 229-237. (  0) 0) |
Reinecke, M., Björnsson, B. T., Dickhoff, W. W., McCormick, S. D., Navarro, I., Power, D. M. and Gutiérrez, J., 2005. Growth hormone and insulin-like growth factors in fish: Where we are and where to go. General and Comparative Endocrinology, 142(1-2): 20-24. DOI:10.1016/j.ygcen.2005.01.016 (  0) 0) |
Sheehan, R., Shasteen, S., Suresh, A., Kapuscinski, A. and Seeb, J., 1999. Better growth in all-female diploid and triploid rainbow trout. Transactions of the American Fisheries Society, 128(3): 8. (  0) 0) |
Steyn, F. J., Tolle, V., Chen, C. and Epelbaum, J., 2016. Neuroendocrine regulation of growth hormone secretion. Comprehensive Physiology, 6(2): 687-735. (  0) 0) |
Tam, J. K., Chow, B. K. and Lee, L. T., 2013. Structural and functional divergence of growth hormone-releasing hormone receptors in early sarcopterygians: Lungfish and Xenopus. PLoS One, 8(1): e53482. DOI:10.1371/journal.pone.0053482 (  0) 0) |
Wang, D., Mao, H. L., Chen, H. X., Liu, H. Q. and Gui, J. F., 2009. Isolation of Yand X-linked SCAR markers in yellow catfish and application in the production of all-male populations. Animal Genetics, 40(6): 978-981. DOI:10.1111/j.1365-2052.2009.01941.x (  0) 0) |
Yang, W., Chen, H., Cui, X., Zhang, K., Jiang, D., Deng, S., Zhu, C. and Li, G., 2017. Sequencing, de novo assembly and characterization of the spotted scat Scatophagus argus, (Linnaeus 1766) transcriptome for discovery of reproduction related genes and ssrs. Journal of Oceanology and Limnology, 36(4): 1329-1341. (  0) 0) |
Zhang, G., Wang, W., Su, M. and Zhang, J., 2018. Effects of recombinant gonadotropin hormones on the gonadal maturation in the spotted scat, Scatophagus argus. Aquaculture, 483: 263-272. DOI:10.1016/j.aquaculture.2017.10.017 (  0) 0) |
Zhang, K., 2018. Molecular cloning and gene expression of insulin-like growth factors 1 and 2 and regulation by estradiol-17β in spotted scat (Scatophagus argus). Master thesis. Guangdong Ocean University, Zhanjiang, 34-37 (in Chinese).
(  0) 0) |
 2019, Vol. 18
2019, Vol. 18


