2) Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
The Pacific oyster, Crassostrea gigas, is cultivated in numerous countries around the world due to its fast growing and a wide tolerance to different environmental conditions, ranking the first in production among all other aquaculture species worldwide (FAO, 2014). In China, the production of cultured oyster was over 4 million tons in 2016 (DOF, 2017), and C. gigas was one of the most important species. However, the production of C. gigas is mainly based on wild and unimproved populations, likely limiting profitability and expanded production of this species due to the unrealized potential for genetic improvement (Gjedrem et al., 2012).
Selective breeding has been shown to be an effective method of improving the performance of many important bivalve species (Gjedrem et al., 2012). For C. gigas, it should be particularly amenable to selection due to its high fecundity, relatively short generation interval and high phenotypic variation in wild populations (Newkirk, 1983). Shortly after the development of efficient hatchery techniques of C. gigas, numerous selective breeding programs have been launched in many countries to improve commercially important traits with additive genetic variance. Recent studies have reported on the genetic improvement on disease resistance (Dégremont et al., 2015), growth rate (Wang et al., 2012), yield (Langdon et al., 2003) and shell color (Brake et al., 2004; Xu et al., 2017) of C. gigas.
The shell pigmentation of C. gigas presents a large polymorphism and it continuously distributes from near-white to near-black. Previous studies showed that a considerable additive genetic variation in shell color makes it feasible to produce genetic improvements in selective breeding program (Wan et al., 2017; Xu et al., 2017; Xing et al., 2018). The attractive and exclusive shell color of C. gigas is highly appreciated by consumers, hence driving high prices in the international market. Therefore, a selective breeding program for genetic improvement of C. gigas has been initiated in 2010. As a result, a new selected line with black shell coloration of C. gigas was obtained through four-generation family selection. Then mass selection for fast growth was carried out to improve the productivity of this line.
In this study, the black shell color was further purified and the realized heritability, selection response and genetic gains of shell height were evaluated in both sixth-generation and seventh-generation selections of the black shell line. Our results will contribute to evaluate the progress being made per generation and the potential for increased gains in future generations of black shell line.
2 Materials and Methods 2.1 Base Stock and SelectionIn 2010, a total of 100 adult oysters with dark pigmentation selected from wild stock (Rushan, Shandong, China, 36.4˚N, 121.3˚E) were used to establish the first-generation selected family (BS1). Then further selection on shell color was carried out in each of the three years from 2011 to 2013, and a total of 80, 60 and 24 adult oysters were used to establish the second-generation (BS2), third-generation (BS3) and fourth-generation (BS4) selected families, respectively. In 2014, five full-sib families with black shell coloration and significant growth advantage were chosen among BS4, and totally 75 individuals were selected from all five selected families and served as parents to produce the fifth-generation selected line (BS5).
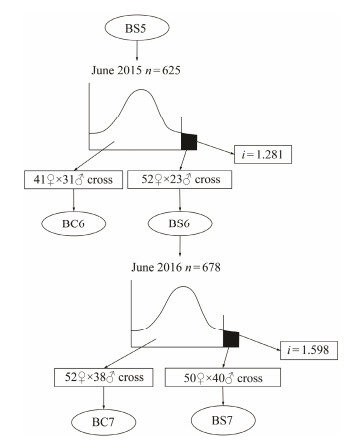
In this study, a stock of 625 oysters were randomly taken from BS5 and their size-frequency distribution of shell height was determined in 2015. Based on the shell height distribution, the top 12% of oysters were chosen as parents (23 sires and 52 dams) for producing the sixth-generation selected line (BS6). In addition, 72 individuals (31 sires and 41 dams) were randomly sampled to serve as parents for producing a control line (BC6). Following one year of culture, a total of 678 oysters were randomly taken from BS6 as base stock to produce the seventh-generation selected line (BS7). In 2016, 90 oysters (40 sires and 50 dams) with the top 13.3% of shell height distribution of the stock were used for producing BS7, and 90 oysters (38 sires and 52 dams) were randomly taken for producing control line (BC7).
2.2 Hatchery, Nursery, and Grow-OutIn June 2015, 75 individuals at the top end of shell height distribution of BS5 and 72 individuals randomly taken from BS5 were respectively stripped-spawned in two 20-m3 concrete tanks in the same day to create BS6 and BC6. For each line, oysters were sexed and males and females were separated. Gametes were then rinsed into separate buckets by stripping the gonad. Equal amounts of gametes were mixed well after estimating concentrations using a microscope. Fertilization was performed at a sperm: egg ratio of 50:1, with 107 oocytes being used for each female. After fertilization, embryos were put into a 20-m3 tank. After 24-h incubation, 100 million D-larvae were randomly collected from each hatching tank and stocked into two 20-m3 larval rearing tanks (Li et al., 2011).
The rearing of the larvae, spat, and adults were carried out using standard protocols described by Li et al. (2011), and the rearing conditions were maintained the same for control and selected lines. Briefly, larvae were raised for 18–22 days with an algae diet of Isochrysis galbana and Nitzschia closterium (30000–80000 cells mL−1) with an initial stocking density of approximately 5 larvae per mL. The water temperature was maintained at 24–25℃ and the salinity at 30. Small individuals were no culled to retain all genetic variability. Strings of scallop shells were used to collect the spat metamorphosed from eyed larvae. When shell height of reached 500–600 μm, the spats were transferred to an outdoor nursery tank (5000 m3). After 2-month culture, spats were transported to Sanggou Bay (Rongcheng, Shandong province, China, 37.1˚N, 122.5˚E) when the shell height reached 2–3 mm. They were placed on nylon ropes and cultured on suspended longlines. The density was about 15–20 spats per scallop shell.
In 2016, the BS7 (produced by 50 dams and 40 sires at top end of SH distribution of BS6) and BC7 (produced by 52 dams and 38 sires random from BS6) oysters were respectively produced with the same way as BS6 and BC6. The hatchery, nursery and grow-out procedures of BS7 and BC7 followed the description of BS6 and BC6.
2.3 Sampling and Growth MeasurementThirty larvae were randomly chosen from each line, and their shell heights were measured using a microscope equipped with an ocular micrometer on days 5, 10, 15 and 20 after fertilization. The shell heights of 40 spats per line were measured using an electronic Vernier caliper (0.01 mm accuracy) on days 80, 120 and 150. During grow-out, shell heights of 50 oysters per line were measured on days 270, 330 and 450.
2.4 Estimation of Genetic Parameters and Data AnalysisThe mean shell heights of both selected and unselected lines were calculated at different ages of both two generations. Similarly, the realized heritability (
| $h_R^2 = \frac{{{X_S} - {X_C}}}{{i{{\rm{ \mathit{ σ} }}_C}}}, $ |
| $ SR = \frac{{{X_S} - {X_C}}}{{{{\rm{ \mathit{ σ} }}_C}}}, $ |
| $ G G=\frac{X_{S}-X_{C}}{X_{C}} \times 100, $ |
where XS and XC are the mean size of offspring of selected and the control lines, respectively, σC is the standard deviation of control offspring, and i is the intensity of selection.
Differences in mean shell height, response to selection, realized heritability and current genetic gain between selected line and control line in each generation, as well as between sixth-generation selected line and seventh-generation selected line at different ages were analyzed with one-way analysis of variance (ANOVA). All data analyses were performed using the Statistical Package for the Social Sciences (SPSS) 22.0 software. Significance level for all analyses was P < 0.05.
3 Results 3.1 Base Stock and Selection IntensityThrough a four-generation family selection on shell color of C. gigas, a black shell line (BS5) was obtained with a genetically stable shell color (Fig. 1) and used for subsequent selection on shell height. A total of 625 adult oysters (mean shell height was 56.195 ± 8.29 mm) from BS5 were used as base stock to produce BS6 and BC6. Similarly, 678 adult oysters (mean shell height was 52.60 ± 9.27 mm) from BS6 were used as base stock to produce BS7 and BC7. The cutoff point for the truncation selection was determined as a shell height of 62.33 mm for the stock to produce BS6 and 63.04 mm for the stock to produce BS7. The mean shell heights of the stocks for producing BS6 and BS7 were 66.80 ± 4.13 mm and 67.41 ± 3.90 mm, respectively. Thus, the selection intensity was 1.281 and 1.598, respectively (Fig. 2).
|
Fig. 1 The left shell (A), the right shell (B) and the edible part with left shell (C) of black shell line of C. gigas. |
|
Fig. 2 Pedigree of selected lines indicating the size of base stock (n) and selection intensity (i) for a successive two-generation selection. |
Growth data of progeny from control and selected two generation lines at larval, spat and grow-out stages are presented in Table 1. In both generations, the selected line had a larger mean shell height than the control line at any stage of C. gigas. The differences in larval shell height between selected and control lines were significant (P < 0.05) only on day 21. In spat and grow-out stages, no significant difference (P > 0.05) was detected in shell height between selected and control lines on day 80 in each generation.
|
|
Table 1 Mean shell heights of two generations of C. gigas from selected and control lines at different developmental stages |
When harvested on day 450, mean shell heights of BS6 and BC6 reached 90.34 and 82.86 mm, respectively; oysters from BS7 and BC7 reached 78.04 and 70.04 mm, respectively. The increase in shell height of the selected line over the control was 9.03% in sixth-generation line and 11.42% in seventh-generation line, respectively.
Differences in mean shell height between BS6 and BS7 were compared at different oyster ages. The BS7 had a higher mean shell height than BS6 except at days 120 and 450, and a significant difference was displayed on day 450. On day 450, the average shell height of BS6 oysters was 13.62% higher than that of BS7 oysters.
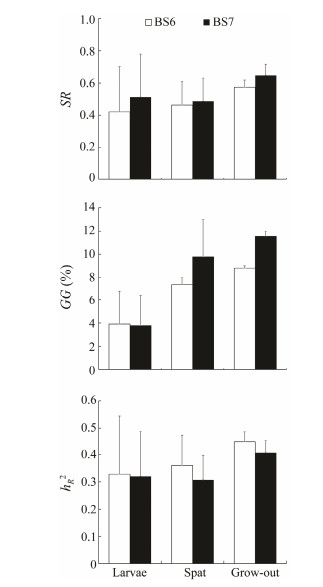
3.3 Genetic ParametersStandardized response to selection (SR), current genetic gain (GG) and realized heritability (
|
Fig. 3 Standardized response to selection (SR), current genetic gains (GG), and realized heritability ( |
In this study, genetic improvement on growth for the black shell line of C. gigas have achieved notable gains using a mass selection approach. At harvest day, the difference in shell height between the selected and control lines of each generation was significant. As all circumstances for parent-conditioning, larval-culture, spat-nursery, and grow-out were kept the same for the black shell lines, the significant differences between selected and control lines can be explained by the positive response to selection.
In the first twenty days of larval stage, there was no significant difference between the mean shell heights of selected lines and control lines for both two generations, which might be caused by the presence of maternal effects at the early development stage of C. gigas larvae. Maternal effects are also recognized as energy reserves on fitness traits at the early stage of shell species (Pirchner, 1969). Previous studies found that maternal effects usually play a significant role in early development of shell species and may obscure the effect of selection response at larval stages. For example, Cruz and Ibarra (1997) reported that maternal effects significantly affected larval growth and survival of the speckled scallop A. circularis. Wang et al. (2012) evaluated mass selection for fast growth in three stocks of C. gigas and found no significant differences on shell height between selected and control lines at the early larval stage. When the oysters grow for some time, the maternal effects on growth of C. gigas receded, and the effects of selection were enhanced gradually. It is well known the growth of larvae was mainly controlled by rearing conditions, such as the temperature, salinity, rearing density, food supply and so on. Hence the same rearing conditions of selected line and control line lead to similar growth of larvae with no significant difference. At the harvest day 450, mass selection led to an increase of 8.24% and 9.68% in shell height for sixth-generation and seventh-generation lines, respectively. The level of growth improvement is comparable to what has been reported in other bivalves. For instance, in some scallops, genetic gains for growth have been observed between 8% and 10% per generation (Ibarra et al., 1999; Zheng et al., 2004; Liang et al., 2010). Nell et al.(1996, 1999) found that the growth of Sydney rock oysters Saccostrea commercialis was 4% and 18% greater by mass selection after one and two generations of selection, respectively. In C. gigas, Langdon et al. (2003) observed 10%–20% gains per generation for general yield in different rearing environments by family-selection. de Melo et al. (2016) obtained an accumulated gain of 11.3% in the 5th generation from a family-based selective breeding program of C. gigas. Similarly, 7.2%–13.2% genetic gains per generation for shell height have been obtained in three stocks of C. gigas (Li et al., 2011; Wang et al., 2012).
Up to now, realized heritability (
In this study, it is notable that the mean shell height of BS7 was less than that of BS6 after day 270 onwards, and the same finding was observed for the control line. The reason might be the potential impact of genotype × environment interactions. Genotype × environment interactions are pervasive in natural and aquaculture environments. For instance, significant family × environment interactions have been observed for yield and survival in C. gigas (Langdon et al., 2003; Dégremont et al., 2005; Evans and Langdon, 2006).
In summary, the continuous selection of top individuals for shell height can achieve satisfactory growth improvement in black shell line of C. gigas, which lead to an average increase of growth for approximately 10% per generation. The moderate-to-high realized heritabilities in this study indicate that genetic variation exists in the black shell line, and mass selection for the genetic improvement can be successfully used for faster growth in black shell line of C. gigas.
AcknowledgementsThis work was supported by the grants from the National Natural Science Foundation of China (Nos. 3177 2843, 31741122), the Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province (No. 2017LZGC009), and the Fundamental Research Funds for the Central Universities (No. 201762014).
Brake, J., Evans, F. and Langdon, C., 2004. Evidence for genetic control of pigmentation of shell and mantle edge in selected families of Pacific oysters, Crassostrea gigas. Aquaculture, 229: 89-98. DOI:10.1016/S0044-8486(03)00325-9 (  0) 0) |
Cruz, P. and Ibarra, A. M., 1997. Larval growth and survival of two catarina scallop (Argopecten circularis, Sowerby, 1835) populations and their reciprocal crosses. Journal of Experimental Marine Biology & Ecology, 212: 95-110. (  0) 0) |
de Melo, C. M. R., Durland, E. and Langdon, C., 2016. Improvements in desirable traits of the Pacific oyster, Crassostrea gigas, as a result of five generations of selection on the West Coast, USA. Aquaculture, 460: 105-115. DOI:10.1016/j.aquaculture.2016.04.017 (  0) 0) |
Dégremont, L., Bédier, E., Soletchnik, P., Ropert, M., Huvet, A., Moal, J., Samain, J. F. and Boudry, P., 2005. Relative importance of family, site, and field placement timing on survival, growth, and yield of hatchery-produced Pacific oyster spat (Crassostrea gigas). Aquaculture, 249: 213-229. DOI:10.1016/j.aquaculture.2005.03.046 (  0) 0) |
Dégremont, L., Ernande, B., Bedier, E. and Boudry, P., 2007. Summer mortality of hatchery-produced Pacific oyster spat (Crassostrea gigas). I. Estimation of genetic parameters for survival and growth. Aquaculture, 262: 41-53. DOI:10.1016/j.aquaculture.2006.10.025 (  0) 0) |
Dégremont, L., Nourry, M. and Maurouard, E., 2015. Mass selection for survival and resistance to OsHV-1 infection in Crassostrea gigas spat in field conditions: Response to selection after four generations. Aquaculture, 446: 111-121. DOI:10.1016/j.aquaculture.2015.04.029 (  0) 0) |
Department of Fisheries, 2017. China Fisheries Statistic Yearbook 2016. China Agriculture Press, Beijing, 173pp (in Chinese).
(  0) 0) |
Evans, S. and Langdon, C., 2006. Effects of genotype × environment interactions on the selection of broadly adapted Pacific oysters (Crassostrea gigas). Aquaculture, 261: 522-534. DOI:10.1016/j.aquaculture.2006.07.022 (  0) 0) |
Falconer, D. S., and Mackay, T. F. C., 1981. Introduction to Quantitative Genetics. Wiley & Sons, New York, 350pp.
(  0) 0) |
FAO, 2016. FAO yearbook. Fishery and aquaculture statistics 2014: 238.
(  0) 0) |
Gjedrem, T., Robinson, N. and Rye, M., 2012. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture, 350-353: 117-129. DOI:10.1016/j.aquaculture.2012.04.008 (  0) 0) |
Hadley, N. H., Dillon, R. T. D. and Manzi, J. J., 1991. Realized heritability of growth rate in the hard clam Mercenaria mercenaria. Aquaculture, 93: 109-119. DOI:10.1016/0044-8486(91)90210-X (  0) 0) |
Ibarra, A. M., Ramirez, J. L., Ruiz, C. A., Cruz, P. and Avila, S., 1999. Realized heritabilities and genetic correlation after dual selection for total weight and shell width in catarina scallop (Argopecten ventricosus). Aquaculture, 175: 227-241. DOI:10.1016/S0044-8486(99)00100-3 (  0) 0) |
Jarayabhand, P. and Thavornyutikarn, M., 1995. Realized heritability estimation on growth rate of oyster, Saccostrea cucullata Born, 1778. Aquaculture, 138: 111-118. DOI:10.1016/0044-8486(95)01080-7 (  0) 0) |
Langdon, C., Evans, F., Jacobson, D. and Blouin, M., 2003. Yields of cultured Pacific oysters Crassostrea gigas Thunberg improved after one generation of selection. Aquaculture, 220: 227-244. DOI:10.1016/S0044-8486(02)00621-X (  0) 0) |
Li, Q., Wang, Q., Liu, S. and Kong, L., 2011. Selection response and realized heritability for growth in three stocks of the Pacific oyster Crassostrea gigas. Fisheries Science, 77: 643-648. DOI:10.1007/s12562-011-0369-0 (  0) 0) |
Liang, J., Zhang, G. and Zheng, H., 2010. Divergent selection and realized heritability for growth in the Japanese scallop, Patinopecten yessoensis Jay. Aquaculture Research, 41: 1315-1321. DOI:10.1111/j.1365-2109.2009.02419.x (  0) 0) |
Nell, J. A., Sheridan, A. K. and Smith, I. R., 1996. Progress in a Sydney rock oyster, Saccostrea commercialis (Iredale and Roughley), breeding program. Aquaculture, 144: 295-302. DOI:10.1016/0044-8486(96)01328-2 (  0) 0) |
Nell, J. A., Smith, I. R. and Sheridan, A. K., 1999. Third generation evaluation of Sydney rock oyster Saccostrea commercialis (Iredale and Roughley) breeding lines. Aquaculture, 170: 195-203. DOI:10.1016/S0044-8486(98)00408-6 (  0) 0) |
Newkirk, G. F., 1983. Applied breeding of commercially important molluscs: A summary of discussion. Aquaculture, 33: 415-422. DOI:10.1016/0044-8486(83)90419-2 (  0) 0) |
Newkirk, G. F., Haley, L. E., Waugh, D. L. and Doyle, R., 1977. Genetics of larvae and spat growth rate in the oyster Crassostrea virginica. Marine Biology, 41: 49-52. DOI:10.1007/BF00390580 (  0) 0) |
Newkirk, J. E. A. and Gary, F., 1991. Response to artificial selection and realized heritability estimate for shell height in the Chilean oyster Ostrea chilensis. Aquatic Living Resources, 4: 101-108. DOI:10.1051/alr:1991009 (  0) 0) |
Pirchner, F., 1969. Population genetics in animal breeding. Quarterly Review of Biology, 39: 535. (  0) 0) |
Toro, J. E. and Newkirk, G. F., 1990. Divergent selection for growth rate in the European oyster Ostrea edulis: Response to selection and estimation of genetic parameters. Marine Ecology Progress, 62: 219-227. DOI:10.3354/meps062219 (  0) 0) |
Wan, S., Li, Q., Liu, T., Yu, H. and Kong, L., 2017. Heritability estimates for shell color-related traits in the golden shell strain of Pacific oyster (Crassostrea gigas) using a molecular pedigree. Aquaculture, 476: 65-71. DOI:10.1016/j.aquaculture.2017.04.012 (  0) 0) |
Wang, Q., Li, Q., Kong, L. and Yu, R., 2012. Response to selection for fast growth in the second generation of Pacific oyster (Crassostrea gigas). Journal of Ocean University of China, 11: 413-418. DOI:10.1007/s11802-012-1909-7 (  0) 0) |
Xing, D., Li, Q., Kong, L. and Yu, H., 2018. Heritability estimate for mantle edge pigmentation and correlation with shell pigmentation in the white-shell strain of Pacific oyster, Crassostrea gigas. Aquaculture, 482: 73-77. DOI:10.1016/j.aquaculture.2017.09.026 (  0) 0) |
Xu, L., Li, Q., Yu, H. and Kong, L., 2017. Estimates of heritability for growth and shell color traits and their genetic correlations in the black shell strain of Pacific oyster Crassostrea gigas. Marine Biotechnology, 19: 421-429. DOI:10.1007/s10126-017-9772-6 (  0) 0) |
Zheng, H., Zhang, G., Liu, X. and Guo, X., 2006. Sustained response to selection in an introduced population of the hermaphroditic bay scallop Argopecten irradians irradians Lamarck (1819). Aquaculture, 255: 579-585. DOI:10.1016/j.aquaculture.2005.11.037 (  0) 0) |
Zheng, H., Zhang, G., Liu, X., Zhang, F. and Guo, X., 2004. Different responses to selection in two stocks of the bay scallop, Argopecten irradians irradians Lamarck (1819). Journal of Experimental Marine Biology & Ecology, 255: 213-223. (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


