2) Laboratory for Marine Mineral Resources, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3) College of Marine Life Science, Ocean University of China, Qingdao 266100, China
Ocean plays a significant role in global climate change since it is the largest carbon reservoir on the earth and hosts substantial hydrocarbons. These hydrocarbons (particularly methane) are recognized as 'greenhouse' gases and can absorb infrared radiation much more strongly than carbon dioxide, although their concentrations are two to six orders of magnitude lower than that of carbon dioxide in atmosphere (Lashof and Ahuja, 1990). Marine hydrocarbons can occur as dissolved and free gases in water and sediments, as well as crystalline solids (natural gas hydrates). Every year the leakage of oil and gas and decomposition of gas hydrates from their submarine reservoirs release substantial volumes of hydrocarbons (Etiope, 2015). Nonetheless, much of them may never reach the atmosphere. This is attributed to the submarine microbial community which consumes more than 90% of the generated hydrocarbons via anaerobic/aerobic oxidation processes (Valentine et al., 2001; Hinrichs and Boetius, 2002; Reeburgh, 2007). However, the microbial populations that contribute to such processes in submarine environments are still not well illustrated (Redmond et al., 2010).
Compared to microbial anaerobic oxidation (Wang et al., 2014), aerobic oxidation is more common and widespread in marine systems since oxygen is still abundant in the deep-sea below 3000 km (Boetius and Wenzhöfer, 2013). Some studies have been specially conducted to examine the features of microbes related to the aerobic oxidation of methane (e.g., Holmes et al., 1995; Ding and Valentine, 2008; Valentine, 2011; Cui et al., 2018). The known methanotrophic bacteria are separated into two assemblages, i.e., γ-proteobacteria (type Ⅰ) and α-proteobacteria (type Ⅱ) (Hanson and Hanson, 1996) and the key enzyme that they rely on for methane oxidation, methane monooxygenase (MMO), is found in two forms. The particulate MMO (pMMO) is associated with intracellular membranes and has been found in almost all described methanotrophs, whereas the soluble MMO (sMMO) is free in the cytoplasm and is only found in few genera (Inagaki et al., 2004; Op den Camp et al., 2009; Redmond et al., 2010). Although MMO is generally restricted to the oxidation of methane, recent studies found that it might also oxidize C2H6 – C4H10 alkanes, indicating that methanotrophic bacteria may not only grow on methane but also on multi-carbon compounds such as ethane and propane (Hazeu and de Bruyn, 1980; Berthe-Corti and Fetzner, 2002; Kinnaman et al., 2007). However, in submarine environments how methanotrophic bacteria behave in the co-existence of methane and higher hydrocarbons is not well established. This limits the quantitatively modelling of the microbial contributions in preventing marine hydrocarbon from entering atmosphere, as well as the understanding of the role of microbial community in global carbon circle.
In this study, we compared the aerobic microbial consumption of pure CH4 with that of mixed gases by incubating marine sediment samples collected from Bohai Bay, eastern China, which is a prolific gas- and oil-bearing region (and thus a natural seep of hydrocarbon gases). Specifically, we aim to characterize the assemblages of methanotrophic bacteria present in the natural seeps and determine how these microbial communities behave during the aerobic oxidation in the co-occurrence of methane and larger hydrocarbons. The results help to gain a better understanding of the effects and contributions of microbial activities in marine hydrocarbon seep ecosystems.
2 Materials and Methods 2.1 Sediment SampleThe marine sediment sample used in this study is the same as Li et al. (2019). This sample is used because Li et al. (2019) had demonstrated that it contains the microbial communities that can degrade hydrocarbons (including methane, ethane and propane) via aerobic oxidation. Petrographically, this marine sediment sample is mainly composed of clay and was collected from the surface sediment layer in the Shaleitian Coal Oil Point seep field in Bohai Bay, eastern China. The water depth of sampling position is 22.7 m and in situ temperature is 19℃. The composition of natural gas emitted from this area is characterized by 93% methane, 3% ethane and 4% propane (Zhang, 2016). The sample was collected by a remotely operated submersible box sampler which weighed nearly 2.0 kg. More details about the sediment sample and collection procedures can be found in Li et al. (2019).
2.2 Sediment IncubationsTo illustrate the response of microbial community structure to aerobic oxidation, microbial aerobic oxidation experiments were conducted by using the sample mentioned above. The detailed procedures for sediment incubations were given by Li et al. (2019) and are summarized here. Artificial seawater was added to the sediment at a ratio of 1:1 (v/v) with a pH of 7.0. The sediment sample was stirred thoroughly and fifty milliliters of the sediment slurry were added into a 120 mL serum bottle, which was then sealed with a butyl rubber septum and an aluminum crimp cap. The headspace of the bottle was subsequently purged with sterilized ultrahigh purity (UHP) N2 and then O2 and hydrocarbons were added. Both pure methane and mixed gases (methane: ethane: propane = 79.96:10.04:10.00) were used, corresponding to incubation ⅰ and incubation ⅱ, respectively. The ratio of gas mixtures was set according to the composition of hydrocarbon gases in marine seepages (methane: 62.30% – 100.00%; ethane: 0 – 7.70%; propane: 0 – 18.80%) (e.g., Milkov, 2005; Liu et al., 2015). The two incubations were performed with another one killed treatment sample as the control. The samples were incubated at 28℃ in the dark on an orbital shaker set at 120 r min−1. The headspace gases were subsampled for compositional measurement and isotope analyses at intervals of 3 – 7 days to monitor the process of the aerobic oxidation reaction. Once hydrocarbons had completely been consumed, the slurry sediments (15 mL) were sampled for lipid and DNA analyses.
2.3 Lipid Biomarker AnalysisThe freeze-dried, homogenized and powdered samples (including the original and incubated samples) were extracted twice with a mixture of dichloromethane (DCM)/ MeOH (3:1, v/v) by using a Dionex Accelerated Solvent Extraction system (ASE 200, Dionex Inc) at 110℃. The volume of the extract was reduced under a stream of N2 and then the extract was desulfurized by using copper sticks. After desulfurization, the total lipid extracts were saponified with 6% KOH in MeOH for 12 h and then separated into different fractions through silica gel flash column chromatography. The neutral lipid fraction was dried under a gentle N2 stream and derivatized by using N, O-bis (trimethylsilyl)-trifluoroacetamide (BSTFA) in acetonitrile to form TMS-ethers (Trimethylsilyl ethers) at 70℃ for 1 h before instrumental measurements. Hexane was subsequently added to extract fatty acids following the addition of HCl (pH < 2). Fatty acids were methylated with BF3-MeOH to form fatty acids methyl esters (FAMEs), then all lipids were analyzed by gas chromatography-mass spectrometry (GC-MS; Agilent 7890A/5975C). Separation was achieved on a HP-5MS column (30 m × 0.25 mm × 0.25 μm). The injector temperature was set at 290℃. We used He as a carrier gas at 1.0 mL min−1. For n-alkane analysis, the initial column temperature of 80℃ was held for 1 min, then programmed to 310℃ at 5℃ min−1 and held for 20 min. The temperature programs for fatty acids were employed as follows: 60℃ for 1 min, followed by 80 – 200℃ at 10℃ min−1 intervals, 200 – 250℃ at 5℃ min−1 intervals, and then 250 – 290℃ at 2℃ min−1 intervals and held at 290℃ for 10 min. Internal standards (C24D50 for neutral lipids and n-C19-COOH for fatty acids) were added to samples before GC-MS analysis to aid in quantification. Lipid concentrations were determined by ratios of the peak areas to those of the internal standards. Lipids were tentatively identified by comparing the retention times with that of the standard which periodically runs under same conditions, and by using the standard spectral library (NIST02L) and reported literature (Hu et al., 2006).
2.4 DNA ExtractionTotal DNA was extracted from the original and incubated samples by using the EZNA® Soil DNA kit (Omega Bio-Tek, Inc., Norcross, GA, USA) according to the manufacture's recommended protocol. Genomic DNA was eluted with 100 μL of elution buffer and its quality was verified by agarose gel electrophoresis. A negative control reaction (using DNA-free water to replace the test sample) was also included in the extraction step to verify the absence of environmental contamination, as conducted by Sagheddu et al. (2017). Each DNA sample was divided into two portions for MiSeq PE300 sequencing and realtime PCR. Purified DNA was stored at −20℃ until used.
2.5 Real-Time Quantitative PCR AssayThe abundance of 16S rRNA and pmoA genes in the DNA extracts was determined by using a real-time quantitative PCR (qPCR) assay. The qPCR amplification was carried out by 9600 plus real-time PCR system (Applied Biosystems, USA). Fragments of bacterial 16S rRNA genes (468 bp, V3-V4 region) and pmoA genes (508 bp) were amplified by the primer pairs 338F-806R and A189F-mb661R, respectively. These primers were demonstrated to work effectively in previous studies (Shrestha et al., 2008; Armingohar et al., 2014). The reaction mixture (20 μL) consisted of 10 μL of SYBR® Premix Ex Tag TM (TaKaRa, Dalian, China), 0.2 μmol L−1 of each primer, and 2 μL of the template DNA. Data are means ± SD of three technical replicates.
2.6 High-Throughput Sequencing and Data ProcessingPCR amplification and Illumina MiSeq sequencing of the DNA extracts were conducted in Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China. The bacterial and methanotrophic community were analyzed by using the primer pairs 338F-806R and A189F-mb661R targeting the 16S rRNA and pmoA genes for PCR amplification. The community structure, richness, and diversity of bacteria (and methanotrophs in particular) were studied through Illumina high-throughput sequencing of bacterial 16S rRNA and pmoA genes. The amplicons were paired-end sequenced on an Illumina MiSeq PE300 platform. The raw reads have been submitted to the NCBI Sequence Read Archive (SRA) database with the accession number of SRP189409. Paired-end reads (16S rRNA and pmoA genes) with no less than 20 bases were merged by the FLASH program, and truncated by removing low-quality fragments, barcodes and primers. The sequences were clustered against the database at 97% sequence similarity with UCLUST (Sengupta and Dick, 2017). Assembled 16S rRNA and pmoA gene sequences were taxonomically classified by using the SILVA SSU 128 and NCBI databases, respectively, with a confidence threshold of 70%. The phylogenetic tree was constructed by neighbor-joining method in the Mega 5.0 software (Saitou and Nei, 1987; Tamura et al., 2007).
3 Results 3.1 Lipid BiomarkerFigs. 1 and 2 show the chromatograms of three total lipid fractions (one initial sample and two incubated samples), with compounds listed in Tables 1 and 2. Both n-alkanes and fatty acids were determined in this study.
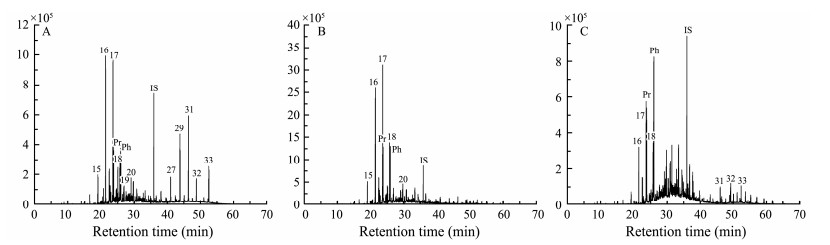
|
Fig. 1 Distribution patterns of n-alkanes in studied sediment samples (m/z = 57; IS, internal standard). A, original sample; B, incubation ⅰ sample; C, incubation ⅱ sample. |
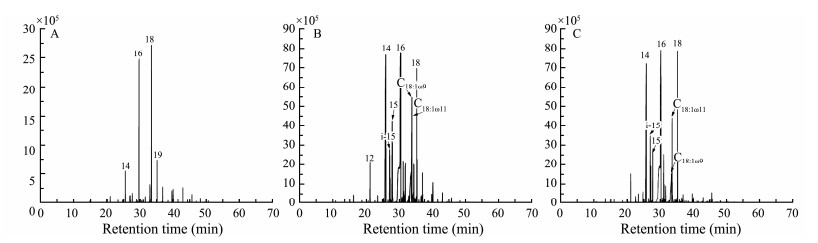
|
Fig. 2 Distribution patterns of fatty acids in studied sediment samples (m/z = 74; IS, internal standard). A, original sample; B, incubation ⅰ sample; C, incubation ⅱ sample. |
|
|
Table 1 Concentration of n-alkanes (%) for sediment samples used in this study |
|
|
Table 2 Concentration of free fatty acid (%) for sediment samples used in this study |
N-alkanes in all studied sediments range in carbon number from 11 to 40, with C15 – C20 n-alkanes as the most dominant homologues (accounting for 65.6% to 86.6% of total n-alkanes) (Table 1 and Fig.1). Compared to the original sample, concentrations of most n-alkanes in sediment samples from two incubations were elevated noticeably (Fig.1). Average chain lengths (ACL) for three samples were similar (30), and all values were within the range calculated from land-derived vegetation (Guillemot et al., 2019). A strong odd-over-even carbon number predominance, indicated by carbon preference index (CPI), was observed in the original sediment sample (CPI = 4.4). In contrast, a much lower CPI value was observed in the sample from incubation ⅰ (1.7). The CPI value in the sample from incubation ⅱ, however, is slightly high (2.6) but still much lower than that of the original sediment sample. Low CPI values of two incubated samples reflect degraded organic matter (Grewer et al., 2018) and similar values also have been reported by Bröder et al. (2016) and Fang et al. (2018).
Fatty acids in the sediments range in carbon number from C8 to C30. In the initial sediment sample, saturated fatty acids dominated in the record, with C14:0, C16:0 and C18:0 being the most abundant homologues (Table 2 and Fig.2). Compared to the original sample, the incubated samples displayed much higher proportions of C14:0 and lower proportions of C18:0. Besides, noticeably low ratios of longchain-length to short-chain-length lipids (TARFA) were observed in two incubated samples, which were approximately 6 to 9 times lower than that in the original sediment sample. Of note, there was no discernible trend in the concentration of n-alkanes between two incubated samples.
3.2 Bacterial 16S rRNA GeneThe high-throughput sequencing analysis was performed by targeting the bacterial 16S rRNA gene of the studied samples. The calculated Alpha-diversity indices are presented in Table 3. Good's coverage values (at the 97% similarity level) were greater than 0.99 for all samples, indicating that the majority of bacterial community was captured at this sequencing depth. A total 433 OTUs were obtained in the original sediment sample, whereas the number of OTUs in the enrichment samples after incubations decreased significantly. Correspondingly, compared to the original sample, the lower Shannon and Chao 1 indices were observed in experimental samples, indicating lower richness and diversity. In contrast with Incubation ⅰ, total OTUs of incubation ⅱ decreased (157 vs. 104) whereas Shannon and Chao 1 indices increased (2.1 vs. 2.4 and 197 vs. 119, respectively), demonstrating that hydrocarbon compositions influenced the microbial community activity in two incubations.
|
|
Table 3 Results of bacterial community diversity and richness based on the 16S rRNA and pmoA genes in sediments |
In this study 171 different bacterial families were detected for all samples, and the dominant families are shown in in Fig.3a. For the original sample Planococcaceae was the most abundant family accounting for 48.11% of all sequences, followed by Moraxellaceae (18.55%), Methylocystaceae (3.60%, type Ⅱ methanotroph) and Carnobacteriaceae (3.15%). After aerobic oxidation incubations, the bacterial composition changed drastically. Methylococcaceae, which belongs to type Ⅰ methanotrophs and only accounted for 0.05% in the original sample, became the most abundant family in the experimental samples (40.81% for incubation ⅰ and 41.38% for incubation ⅱ). The predominant family in the original sample, Planococcaceae, only accounted for 7.58% and < 1.00% in sediment samples from incubations ⅰ and ⅱ, respectively. Moraxellaceae, which was also abundant in the original sample, decreased significantly after incubations. A similar variation pattern was also seen for Methylocystaceae, which accounted for < 0.1% in incubated samples. Discrepancies between two incubations are also noticeable. Phyllobacteriaceae, Rhizobiaceae, and Rhodocyclaceae were more abundant in the sample from incubation ⅱ (each accounting for 10%), whereas Flavobacteriaceae was second only to Methylococcaceae in the sample from incubation ⅰ (Fig.3a).
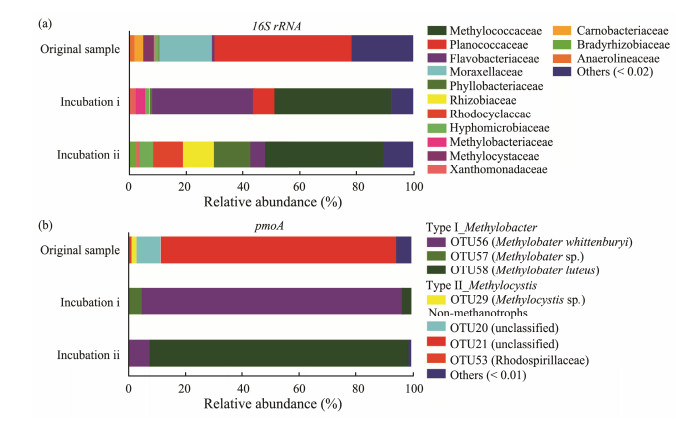
|
Fig. 3 Taxonomic composition of bacterial (a) and methanotrophic (b) communities in studied sediment samples. |
Illumina sequencing of pmoA gene was specially carried out to examine the methanotroph diversity in sediment samples. Chao 1 species richness and Shannon's diversity indexes were listed in Table 3. Good's coverage values of pmoA gene (at the 97% similarity level) were 1, suggesting that the obtained sequences could adequately reveal the diversity of methane-degrading bacteria in samples. 14020 high-quality sequences were obtained from the original sediment sample, which were classified into 55 OTUs by using a similarity cutoff of 97%. Compared to the original sample, the sediment samples from two incubations were similar and displayed much lower OTUs (3 for incubation ⅰ and 4 for incubation ⅱ), as well as lower Shannon (0.3) and Chao 1 indices (3.5).
The community structure of the pmoA genes in samples was analyzed based on the dominant OTUs and by constructing phylogenetic trees (Figs. 3b and 4). Phylogenetic analysis revealed the presence of both type Ⅰ methanotrophs (species Methylobacter whittenburyi, Methylobacter luteus and Methybacter sp. from the family Methylococcaceae) and type Ⅱ methanotrophs (species Methlocystis sp. from the family Methylocystaceae) in studied sediment samples. However, only a few of pmoA sequences (1.94%) affiliated to Methlocystis sp. (OTU29) were observed in the original sample, while the remainder were either nonmethanotrophs (i.e., Rhodospirillaceae bacteriums) or could not be assigned to any species that are deposited in public domain database. In contrast, all dominant pmoA sequences from two incubations were identical and affiliated to type Ⅰ methanotrophs. Nevertheless, in the sample from Incubation ⅰ, the number of pmoA sequences affiliated to Methylobacter whittenburyi (OTU56) was dominant (91.99%) among type Ⅰ methanotrophs, whereas those affiliated to Methybacter sp. (OTU57) and Methylobacter luteus (OTU 58) only accounted for 4.88% and 3.14% respectively. In the sample from incubation ⅱ, however, Methylobacter luteus was predominant, followed by Methylobacter whittenburyi, whereas Methybacter sp. was much less active, with their proportions being 91.53%, 7.64% and 0.01%, respectively.
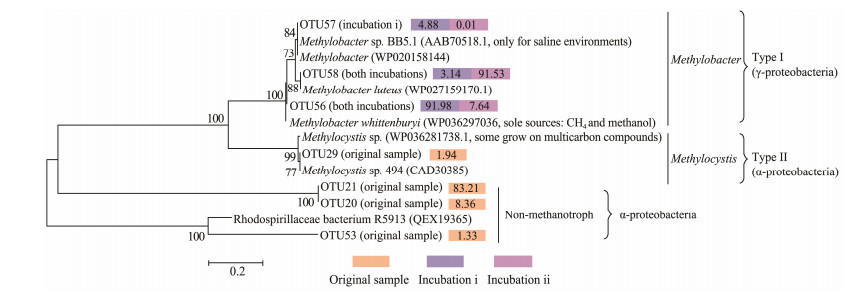
|
Fig. 4 Neighbor-joining tree of 7 most abundant OTUpmoA, with selected reference sequences. The relative abundances (%) for different samples are given to the right of each OTUpmoA. Bootstrap values > 50% (500 repetitions) are shown on nodes. The scale bar indicates sequence dissimilarity between nodes. |
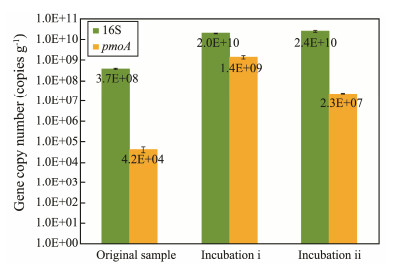
The abundances of bacterial 16S rRNA and pmoA genes were determined via qPCR (Fig.5). Compared to the original sediment sample, both of two incubations are characterized by much higher abundances of the bacterial 16S rRNA gene, with the average value being (2.2 ± 0.2)×1010 copies per gram soil. Although the abundances of pmoA gene also increased after aerobic oxidation incubation, different variation patterns were displayed between two incubations. The abundance of pmoA genes from incubation ⅰ is 1.4×109 copies per gram soil, which is much higher than that from incubation ⅱ (2.3×107 copies per gram soil).
|
Fig. 5 Bacterial 16S rRNA and pmoA genes copy numbers quantified by using qPCR for studied sediment samples. |
High concentrations of long chain n-alkanes and fatty acids, as well as high ACL values were generally regarded as indicators of land-derived input (Meyers, 2003; Guillemot et al., 2019). The CPI value is also an effective indicator of organic matter sources, with high values normally revealing terrestrial vegetation sources and low values (around 1) generally related to the significant bacterial activity or petroleum pollution (Zhu et al., 2005; Duncan et al., 2019; and references therein). Parkes and Taylor (1983) also suggested that the fatty acid analysis could be used to distinguish different types of microbes within sediments, which has been demonstrated by subsequently published studies (Sundh et al., 1995; Dedysh et al., 2007).
In this study, the high ACL value (30.4) and the strong odd-over-even carbon number predominance of n-alkanes (CPI = 4.4), as well as the dominance of long-chain fatty acids, were observed in the original sediment sample, collectively indicating the prevailingly terrigenous origin of organic matters in the sediments from Bohai Bay. These features are similar to those of n-alkanes and fatty acids from leaf waxes of higher plants (e.g., Bush and McInerney, 2013), in eolian dust samples (e.g., Schreuder et al., 2018) and in marine sediments (Sinninghe Damsté et al., 2001), all of which supported a terrigenous origin.
There was no significant variation between two samples treated with pure methane and mixed gas. Nevertheless, in contrast with the original sample, the n-alkanes and fatty acid data of the incubated samples indicate the mixture sources of organic matters and noticeable bacterial reworking. The CPI values of n-alkanes decreased in two samples that experienced aerobic oxidation, apparently related to the significant bacterial activity (Huang et al., 1999; Zhu et al., 2005). Similar values have also been reported by Bröder et al. (2016) and Fang et al. (2018). Higher concentrations of short chain acids (i.e., lower TARFA values, Table 2) in those two samples were also related to the presence of bacterial communities that utilize these fatty acids as their carbon sources. This utilization has been reported in studies from estuaries (Zhang et al., 2015), lakes (Arnold et al., 2018) and marine sediments (Parkes and Taylor, 1983). Although in this study high concentrations of cyclopropyl fatty acids (17 and 19), both of which were expected in the aerobic bacteria according to Parkes and Taylor (1983), were not detected in incubated samples, they are characterized by higher C18:1ω11/C18:1ω9 values (40.4 and 5.1 for incubations ⅰ and ⅱ respectively) than the original sample (1.5). According to Volkman et al. (1980), these high C18:1ω11 and low C18:1ω9 concentrations (and thus high C18:1ω11/C18:1ω9 ratios) are typical of the aerobic bacterial activity.
In conclusion, although the limited sample number of this study prevents any further implications from lipid analysis, the noticeable contrasts of n-alkanes and fatty acids between the original sample and two incubated samples demonstrated that microbial aerobic oxidation progressed in the two incubations. This is further demonstrated by results from the DNA analyses.
4.2 Hydrocarbon-Oxidizing Bacterial Activity in Response to Aerobic OxidationSignificant variations in the bacterial communities were seen between uninoculated and incubated samples, as well as between two incubated samples. Compared to incubated samples, the uninoculated sample was characterized by a high proportion of type Ⅱ methanotrophs (family Methylocystaceae) and a low proportion of type Ⅰ methanotrophs (family Methylococcaceae). Generally, the competition between types Ⅰand Ⅱ methanotrophs depends on the concentrations of CH4 and O2 and also on the presence of nitrogen (Bull et al., 2000; Siljanen et al., 2012; Sengupta and Dick, 2017). Previous studies suggested that type Ⅰ methanotrophs seem to prefer environments with plentiful O2 and limited CH4, whereas type Ⅱ methanotrophs dominate in environments with high concentrations of CH4 and limited O2 (Graham et al., 1993; Amaral and Knowles, 1995; Shrestha et al., 2008). The results from this study thus agree with this finding since the sediment samples here were collected from the seafloor of the gas- and oil-rich Bohai Bay, which corresponds to an environment with plentiful CH4 and limited O2, whereas the incubations were conducted with adequate O2 and limited hydrocarbons.
Monitoring the gas composition during the incubation ⅱ showed that both ethane and propane were completely consumed after the incubation, which indicated the occurrence of ethane- and propane-oxidizing bacteria in the study samples. However, currently less is known about the organisms that oxidize ethane or propane in marine environments. The known such organisms, which are primarily high G + C Gram-positive bacteria (Nocardia, Pseudonocardia, Gordonia, Mycobacterium, and Rhodococcus) or Pseudomonas species (Redmond et al., 2010), were not observed in our samples. Nevertheless, the comparison between two incubations shows that the amounts of families Phyllobacteriaceae, Rhizobiaceae, and Rhodocyclaceae increased significantly in incubation ⅱ, seemingly proposing that these organisms might consume ethane and/or propane. To test whether this is correct, pure cultures of these organisms should be conducted in the following work. It should be noted that Redmond et al. (2010) suggested that some species from the family Methylococcacea, which had previously been thought to be obligate methanotrophs, might be capable of growing on ethane or propane. Our study seems to support this since the proportion of Methylococcacea noticeable increase in samples from incubations ⅰ to ⅱ.
Interestingly, although nearly all methanotrophs found in two incubations were type Ⅰ, these species are quite different. The Methylobacter luteus was active when pure methane acts as carbon source, whereas Methylobacter whittenburyi took the predominant proportion when mixed gases are available. The observed discrepancies could result from the higher methane concentrations in incubations ⅰ than ⅱ, but more likely indicated that some methanotrophs could utilize ethane and propane, as suggested by Berthe-Corti and Fetzner (2002), Kinnaman et al. (2007) and Redmond et al. (2010). Whatever the case, these results indicate that the bacterial community in marine sediments may give the information regarding marine hydrocarbon seepages, especially for the leakage of trace amounts of hydrocarbons (Okita et al., 2020). Characteristic methanotrophic communities could be used not only to determine whether hydrocarbon leakage occurred, but also to know about the hydrocarbon composition.
4.3 Application to Natural SystemsThe distributions of microbial communities in marine sediments and overlying water have previously been applied to approximate the occurrence and the extent of hydrocarbon (especially methane) oxidation. This approach is well established for methanotrophs under pure methane condition, but less so under mixed gas condition. This study identifies several methanotroph lineages as participants in the oxidation of gaseous hydrocarbons in marine seeps. The fact that the methanotroph community structure is quite different under pure methane and mixed gas conditions is promising. Once justified by following studies, this can be used to determine the gas compositions in hydrocarbon seeps by sequencing pmoA genes, especially in places where the direct analyses of gas composition are hard. The characteristics of methanotrophs presented in this work are specific to oxic marine sediments at 28℃ to facilitate the rapid growth of bacteria, which is slightly higher than the in situ temperature in the sampling place. Besides, in a given environment, the expression of methanotrophs will depend on a variety of environmental factors including pH, salinity, nutrient level, etc. Thus, care must be taken in applying the results obtained in this study to the natural environmental systems.
5 ConclusionsIn this study, we have combined both lipid and DNA analyses to elucidate the role of methanotrophs in the marine sediments collected from Bohai Bay, with different hydrocarbons as carbon source over the incubation period. Although both incubations demonstrated that type Ⅰ methanotrophic populations might play a particularly important role in the marine ecosystem, they exhibited the pronounced variations with respect to their activities and population sizes. Methylobacter whittenburyi was predominant when pure methane acts as carbon source, whereas Methylobacter luteus are much more active when ethane and propane were also involved. The results collectively suggest that sequencing of 16S rRNA and pmoA genes may be used to identify the occurrence and/or extent of aerobic oxidation, as well as to determine the gas compositions in hydrocarbon seeps.
AcknowledgementsThis work was supported by the Natural Science Foundation of Shandong Province (No. ZR2020QD070), the National Natural Science Foundation of China (No. 4187 6051), and the China Geological Survey Project (No. DD 20190221).
Amaral, J. A. and Knowles, R., 1995. Growth of methanotrophs in methane and oxygen counter gradients. FEMS Microbiology Letters, 126: 215-220. DOI:10.1111/j.1574-6968.1995.tb07421.x (  0) 0) |
Armingohar, Z., Jørgensen, J. J., Kristoffersen, A. K., Abesha-Belay, E. and Olsen, I., 2014. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. Journal of Oral Microbiology, 6: 23408. DOI:10.3402/jom.v6.23408 (  0) 0) |
Arnold, T. E., Kenney, W. F., Curtis, J. H., Bianchi, T. S. and Brenner, M., 2018. Sediment biomarkers elucidate the Holocene ontogeny of a shallow lake. PLoS One, 13: e0191073. DOI:10.1371/journal.pone.0191073 (  0) 0) |
Berthe-Corti, L. and Fetzner, S., 2002. Bacterial metabolism of n-alkanes and ammonia under oxic, suboxic and anoxic conditions. Acta Biotechnologica, 22: 299-336. DOI:10.1002/1521-3846(200207)22:3/4<299::AID-ABIO299>3.0.CO;2-F (  0) 0) |
Boetius, A. and Wenzhöfer, F., 2013. Seafloor oxygen consumption fuelled by methane from cold seeps. Nature Geoscience, 6: 725-734. DOI:10.1038/ngeo1926 (  0) 0) |
Bröder, L., Tesi, T., Andersson, A., Eglinton, T. I., Semiletov, I. P., Dudarev, O. V., Roos, P. and Gustafasson, Ö., 2016. Historical records of organic matter supply and degradation status in the East Siberian Sea. Organic Geochemistry, 91: 16-30. DOI:10.1016/j.orggeochem.2015.10.008 (  0) 0) |
Bull, I. D., Parekh, N. R., Hall, G. H., Ineson, P. and Evershed, R. P., 2000. Detection and classification of atmospheric methane oxidizing bacteria in soil. Nature, 405: 175-178. DOI:10.1038/35012061 (  0) 0) |
Bush, R. T. and McInerney, F. A., 2013. Leaf wax n-alkane distributions in and across modern plants: Implications for paleoecology and chemotaxonomy. Geochimica et Cosmochimica Acta, 117: 161-179. DOI:10.1016/j.gca.2013.04.016 (  0) 0) |
Cui, H. P., Su, X., Wei, S. P., Zhu, Y. H., Lu, Z. Q., Wang, Y. F., Li, Y., Liu, H., Zhang, S. and Pang, S., 2018. Comparative analyses of methanogenic and methanotrophic communities between two different water regimes in controlled wetlands on the Qinghai-Tibetan Plateau, China. Current Microbiology, 75: 484-491. DOI:10.1007/s00284-017-1407-7 (  0) 0) |
Dedysh, S. N., Belova, S. E., Bodelier, P. L., Smirnova, K. V., Khmelenina, V. N., Chidthaisong, A., Trotsenko, Y. A., Liesack, W. and Dunfield, P. F., 2007. Methylocystis heyeri sp. nov., a novel type Ⅱ methanotrophic bacterium possessing 'signature' fatty acids of type Ⅰ methanotrophs. International Journal of Systematic and Evolutionary Microbiology, 57: 472-479. (  0) 0) |
Ding, H. and Valentine, D. L., 2008. Methanotrophic bacteria occupy benthic microbial mats in shallow marine hydrocarbon seeps, Coal Oil Point, California. Journal of Geophysical Research: Biogeosciences, 113: G01015. (  0) 0) |
Duncan, B., McKay, R., Bendle, J., Naish, T., Inglis, G. N., Moos-sen, H., Levy, R., Ventura, G., Lewis, A. and Chamberlain, B., 2019. Lipid biomarker distributions in Oligocene and Miocene sediments from the Ross Sea region, Antarctica: Implications for use of biomarker proxies in glacially-influenced settings. Palaeogeography, Palaeoclimatology, Palaeoecology, 516: 71-89. DOI:10.1016/j.palaeo.2018.11.028 (  0) 0) |
Etiope, G., 2015. Natural Gas Seepage. The Earth's Hydrocarbon Degassing. Springer, Switzerland, 118-130.
(  0) 0) |
Fang, J., Wu, F., Xiong, Y., Li, F., Yang, H., Wang, S. and Xie, Y., 2018. Sources of organic matter in the surface sediments from Lake Sihailongwan Maar and Lake Zhanjiang Maar (Lake Huguangyan Maar) in China. Limnologica, 69: 18-23. DOI:10.1016/j.limno.2017.08.004 (  0) 0) |
Graham, D. W., Chaudhary, J. A., Hanson, R. S. and Arnold, R. G., 1993. Factors affecting competition between type-Ⅰ and type-Ⅱ methanotrophs in 2-organism, continuous-flow reactors. Microbial Ecology, 25: 1-17. (  0) 0) |
Grewer, D. M., Lafrenière, M. J., Lamoureux, S. F. and Simpson, M. J., 2018. Spatial and temporal shifts in fluvial sedimentary organic matter composition from a high Arctic watershed impacted by localized slope disturbances. Organic Geochemistry, 123: 113-125. DOI:10.1016/j.orggeochem.2018.07.004 (  0) 0) |
Guillemot, T., Stockhecke, M., Bechtel, A., Ladd, S. N., Nelson, D. B. and Schubert, C. J., 2019. Paleoenvironmental and paleoclimatic variations around Lake Van (eastern Turkey) recorded by sedimentary source specific biomarkers 250 – 130 ka (MIS7 and MIS6). Quaternary Science Reviews, 225: 105997. DOI:10.1016/j.quascirev.2019.105997 (  0) 0) |
Hanson, R. S. and Hanson, T. E., 1996. Methanotrophic bacteria. Microbiological Reviews, 60: 439-471. DOI:10.1128/mr.60.2.439-471.1996 (  0) 0) |
Hazeu, W. and and, de Bruyn J. C., 1980. Ethane oxidation by methane-oxidizing bacteria. Antonie Van Eeuwenhoek, 46: 443-455. DOI:10.1007/BF00395825 (  0) 0) |
Hinrichs, K. U., and Boetius, A., 2002. The anaerobic oxidation of methane: New insights in microbial ecology and biogeochemistry. In: Ocean Margin Systems. Wefer et al., eds., Springer, Berlin, 457-477.
(  0) 0) |
Holmes, A. J., Owens, N. J. P. and Murrell, J. C., 1995. Detection of novel marine methanotrophs using phylogenetic and functional gene probes after methane enrichment. Microbiology, 141: 1947-1955. DOI:10.1099/13500872-141-8-1947 (  0) 0) |
Huang, Y., Street-Perrott, F. A., Perrott, R. A., Metzger, P. and Eglinton, G., 1999. Glacial-interglacial environmental changes inferred from molecular and compound-specific δ13C analyses of sediments from Sacred Lake, Mt. Kenya. Geochimica et Cosmochimica Acta, 63: 1383-1404. DOI:10.1016/S0016-7037(99)00074-5 (  0) 0) |
Hu, J. F., Zhang, H. B. and Peng, P. A., 2006. Fatty acid composition of surface sediments in the subtropical Pearl River Estuary and adjacent shelf, southern China. Estuarine, Coastal and Shelf Science, 66: 346-356. DOI:10.1016/j.ecss.2005.09.009 (  0) 0) |
Inagaki, F., Tsunogai, U., Suzuki, M., Kosaka, A., Machiyama, H., Takai, K., Nunoura, T., Nealson, K. H. and Horikoshi, K., 2004. Characterization of C1-metabolizing prokaryotic communities in methane seep habitats at the Kuroshima Knoll, southern Ryukyu Arc, by analyzing pmoA, mmoX, mxaF, mcrA, and 16S rRNA genes. Applied Environmental Microbiology, 70: 7445-7455. DOI:10.1128/AEM.70.12.7445-7455.2004 (  0) 0) |
Kinnaman, F. S., Valentine, D. L. and Tyler, S. C., 2007. Carbon and hydrogen isotope fractionation associated with the aerobic microbial oxidation of methane, ethane, propane and butane. Geochimica et Cosmochimica Acta, 71: 271-283. DOI:10.1016/j.gca.2006.09.007 (  0) 0) |
Lashof, D. A. and Ahuja, D. R., 1990. Relative contributions of greenhouse gas emissions to global warming. Nature, 344: 529-531. DOI:10.1038/344529a0 (  0) 0) |
Li, J., Liu, C. L., He, X. L., Santosh, M., Hu, G. W., Sun, Z. L., Li, Y., Meng, Q. and Ning, F., 2019. Aerobic microbial oxidation of hydrocarbon gases: Implications for oil and gas exploration. Marine and Petroleum Geology, 103: 76-86. DOI:10.1016/j.marpetgeo.2019.02.013 (  0) 0) |
Liu, C. L., Meng, Q. G., He, X. L., Li, C. F., Ye, Y. G., Zhang, G. X. and Liang, J. Q., 2015. Characterization of natural gas hydrate recovered from Pearl River Mouth Basin in South China Sea. Marine and Petroleum Geology, 61: 14-21. DOI:10.1016/j.marpetgeo.2014.11.006 (  0) 0) |
Meyers, P. A., 2003. Applications of organic geochemistry to paleolimnological reconstructions: A summary of examples from the Laurentian Great Lakes. Organic Geochemistry, 34: 261-289. DOI:10.1016/S0146-6380(02)00168-7 (  0) 0) |
Milkov, A. V., 2005. Molecular and stable isotope compositions of natural gas hydrates: A revised global dataset and basic interpretations in the context of geological settings. Organic Geochemistry, 36: 681-702. DOI:10.1016/j.orggeochem.2005.01.010 (  0) 0) |
Okita, N., Hoaki, T., Suzuki, S. and Hatamoto, M., 2020. Characteristics of aerobic methane-oxidising bacterial community at the sea-floor surface of the Nankai Trough. Marine and Freshwater Research, 71: 1252-1258. DOI:10.1071/MF19317 (  0) 0) |
Op den Camp, H. J., Islam, T., Stott, M. B., Harhangi, H. R., Hynes, A., Schouten, S., Jetten, M., Birkeland, N., Pol, A. and Dunfield, P., 2009. Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environmental Microbiology Reports, 1: 293-306. DOI:10.1111/j.1758-2229.2009.00022.x (  0) 0) |
Parkes, R. J. and Taylor, J., 1983. The relationship between fatty acid distributions and bacterial respiratory types in contemporary marine sediments. Estuarine, Coastal and Shelf Science, 16: 173-189. DOI:10.1016/0272-7714(83)90139-7 (  0) 0) |
Redmond, M. C., Valentine, D. L. and Sessions, A. L., 2010. Identification of novel methane-, ethane-, and propane-oxidizing bacteria at marine hydrocarbon seeps by stable isotope probing. Applied and Environmental Microbiology, 76: 6412-6422. DOI:10.1128/AEM.00271-10 (  0) 0) |
Reeburgh, W. S., 2007. Oceanic methane biogeochemistry. Chemical Reviews, 107: 486-513. DOI:10.1021/cr050362v (  0) 0) |
Sagheddu, V., Patrone, V., Miragoli, F. and Morelli, L., 2017. Abundance and diversity of hydrogenotrophic microorganisms in the infant gut before the weaning period assessed by denaturing gradient gel electrophoresis and quantitative PCR. Frontiers in Nutrition, 4: 29. DOI:10.3389/fnut.2017.00029 (  0) 0) |
Saitou, N. and Nei, M., 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4: 406-425. (  0) 0) |
Schreuder, L. T., Stuut, J. B. W., Korte, L. F., Damsté, J. S. S. and Schouten, S., 2018. Aeolian transport and deposition of plant wax n-alkanes across the tropical North Atlantic Ocean. Organic Geochemistry, 115: 113-123. DOI:10.1016/j.orggeochem.2017.10.010 (  0) 0) |
Sengupta, A. and Dick, W. A., 2017. Methanotrophic bacterial diversity in two diverse soils under varying land-use practices as determined by high-throughput sequencing of the pmoA gene. Applied Soil Ecology, 119: 35-45. DOI:10.1016/j.apsoil.2017.05.031 (  0) 0) |
Shrestha, M., Abraham, W. R., Shrestha, P. M., Noll, M. and Conrad, R., 2008. Activity and composition of methanotrophic bacterial communities in planted rice soil studied by flux measurements, analyses of pmoA gene and stable isotope probing of phospholipid fatty acids. Environmental Microbiology, 10: 400-412. DOI:10.1111/j.1462-2920.2007.01462.x (  0) 0) |
Siljanen, H. M., Saari, A., Bodrossy, L. and Martikainen, P. J., 2012. Seasonal variation in the function and diversity of methanotrophs in the littoral wetland of a boreal eutrophic lake. FEMS Microbiology Ecology, 80: 548-555. DOI:10.1111/j.1574-6941.2012.01321.x (  0) 0) |
Sinninghe Damsté, J. S., Schefuss, E., Versteegh, G. J. M., and Jansen, J. H. F., 2001. Marine and terrigenous lipids in southeast Atlantic sediments (Leg 175) as paleoenvironmental indicators: Initial results. In: Proceedings of the Ocean Drilling Program: Scientific Results. Ocean Drilling Program, Texas A & M University, 1-34.
(  0) 0) |
Sundh, I., Borgå, P., Nilsson, M. and Svensson, B. H., 1995. Estimation of cell numbers of methanotrophic bacteria in boreal peatlands based on analysis of specific phospholipid fatty acids. FEMS Microbiology Ecology, 18: 103-112. DOI:10.1111/j.1574-6941.1995.tb00167.x (  0) 0) |
Tamura, K., Dudley, J., Nei, M. and Kumar, S., 2007. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24: 1596-1599. DOI:10.1093/molbev/msm092 (  0) 0) |
Valentine, D. L., 2011. Emerging topics in marine methane biogeochemistry. Annual Review of Marine Science, 3: 147-171. DOI:10.1146/annurev-marine-120709-142734 (  0) 0) |
Valentine, D. L., Blanton, D. C., Reeburgh, W. S. and Kastner, M., 2001. Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel River Basin. Geochimica et Cosmochimica Acta, 65: 2633-2640. DOI:10.1016/S0016-7037(01)00625-1 (  0) 0) |
Volkman, J. K., Eglinton, G., Corner, E. D. and Forsberg, T. E. V., 1980. Long-chain alkenes and alkenones in the marine coccolithophorid Emiliania huxleyi. Phytochemistry, 19: 2619-2622. DOI:10.1016/S0031-9422(00)83930-8 (  0) 0) |
Wang, F. P., Zhang, Y., Chen, Y., He, Y., Qi, J., Hinrichs, K. U., Zhang, X., Xiao, X. and Boon, N., 2014. Methanotrophic archaea possessing diverging methane-oxidizing and electrontransporting pathways. The ISME Journal, 8: 1069-1078. DOI:10.1038/ismej.2013.212 (  0) 0) |
Zhang, N. L., 2016. Geochemical of hydrocarbon gas from the surface seabed sediment in Bohai Bay Basin. Master thesis. Jilin University, 1-70.
(  0) 0) |
Zhang, X., Bianchi, T. S. and Allison, M. A., 2015. Sources of matter in sediments of the Colville River Delta, a multi-proxy approach. Organic Geochemisty, 87: 96-106. DOI:10.1016/j.orggeochem.2015.07.002 (  0) 0) |
Zhu, C., Pan, J. M., Lu, B., Hu, C. Y., Liu, X. Y., Ye, X. R. and Xue, B., 2005. Source indication and accumulative effect of sedimentary organic matter in the Changjiang Estuary, the old Huanghe River subaqueous delta and the East China Sea shelf. Journal of Marine Sources, 23: 36-46 (in Chinese with English abstract). (  0) 0) |
 2021, Vol. 20
2021, Vol. 20


