2) Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao 266237, China;
3) Key Laboratory of Tropical Medicinal Resource Chemistry of Ministry of Education, College of Chemistry and Chemical Engineering, Hainan Normal University, Haikou 571158, China;
4) Syngenta Jealott's Hill International Research Centre, Bracknell, Berkshire RG42 6EY, UK
The ocean occupies more than 70% of the surface of the earth and is a great resource of natural products. The marine invertebrates, such as corals, are very weak creatures in the ocean, and lack physical defense mechanisms. In order to evolve and survive, they produced functional metabolites with chemical defense functions that can defend against natural enemies, avoid attachment of marine organisms and floating debris, and transmit information between species (Burkhardt et al., 2022; Scesa et al., 2022). This is a chemical defense strategy, using toxic molecules to protect themselves from pathogens or predators, which are wide-spread in the marine environment (Zan et al., 2019). These metabolites with chemical defense functions have a variety of structural types, such as alkaloids, anthraquinones, peptides, polysaccharides, polyketides, and terpenes. Most of them displayed potent ecological activities, such as antifouling, antifeedant, fish poison, and algae inhibitor (Hou et al., 2019c; Hai et al., 2022a; Carroll et al., 2023, 2024; Chen et al., 2023; Guo et al., 2023b). Marine chemical ecology has led to the discovery of the so-called known (molecular structure) – unknown (mechanism of action) natural products (Yee et al., 2023). It is worth pointing out that it is a challenge to investigate the pharmacological activity of these MNPs that play important chemical defense functions in nature and elucidate their efficacy in drug development. The mechanism behind the function of known – unknown compounds has been reported. Antifouling compounds interfere with the homeostasis of cellular calcium ions (Ca2+) to restrain the attachment of fouling organisms (Yang et al., 2014, 2015). Antifouling compounds function by activating NO/cGMP pathways (Wang et al., 2016). Meanwhile, some compounds have been shown to prevent biofouling by decreasing the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2), or by interacting with multiple neurotransmitter systems (Kang et al., 2016; Chen and Qian, 2017). Notably, NO/cGMP, COX-2, and neurotransmitter are therapeutic targets for many diseases. It suggests that MNPs with chemical defense functions in marine chemical ecology are potential candidates for drug development.
The modern marine drugs research started in the 1930s, and entered a stage of rapid growth in the 1990s. Marin-Lit (http://pubs.rsc.org/marinlit) is one of the most famous and authoritative MNP databases. Up to April 29, 2024, it has contained over 41578 molecules and 41140 attached articles. A comprehensive chemoinformatic analysis of terrestrial natural products (TNPs), MNPs, and drugs showed that the halogenated molecules in MNPs occupy a high proportion. The proportion of overall halogenated MNPs is 5.24 times more than that of TNPs, and MNPs are the highest one in all kinds of halogenated compounds except for fluorine. This is consistent with the higher proportion of halogenated molecules in the drug. Meanwhile, the average quantities of nitrogen, sulfur, and nonfluorine halogens of MNPs are much larger than those of TNPs. Owing to the lack of oxygen in the ocean, the average quantity of oxygen in MNPs is much lower than that of TNPs. The structural difference between MNPs and TNPs is partially contributed to the different environments between land and ocean. MNPs with longer chains and larger rings, especially the 8- to 10-membered rings, can facilitate marine organisms to adapt to the water habitat conditions (Shang et al., 2018; Liu et al., 2021).
Two marine drugs have been developed in China and 15 drugs have been approved in other countries (https://www.marinepharmacology.org/approved, update on April 10, 2024) from 41578 discovered molecular entities (https://marinlit.rsc.org/, update on April 29, 2024). It means 1 drug has been developed from 3000 marine-derived products. It is approximately 1- to 3-fold higher than the industry ave- rage (1 in 5000 – 10000 tested compounds) (Gerwick and Moore, 2012). These drugs are used to treat infectious di- seases, inflammations, and tumor chemotherapy in clinical practice, including cytarabine (Cytosars®), omega-3-acid ethyl esters (Lovaza®), brentuximab vedotin (Adcetris®), trabectedin (Yondelis®), and eribulin mesylate (Halavens®) (Shinde et al., 2019; Ghareeb et al., 2020; Banerjee et al., 2022; Papon et al., 2022) (Fig.1).
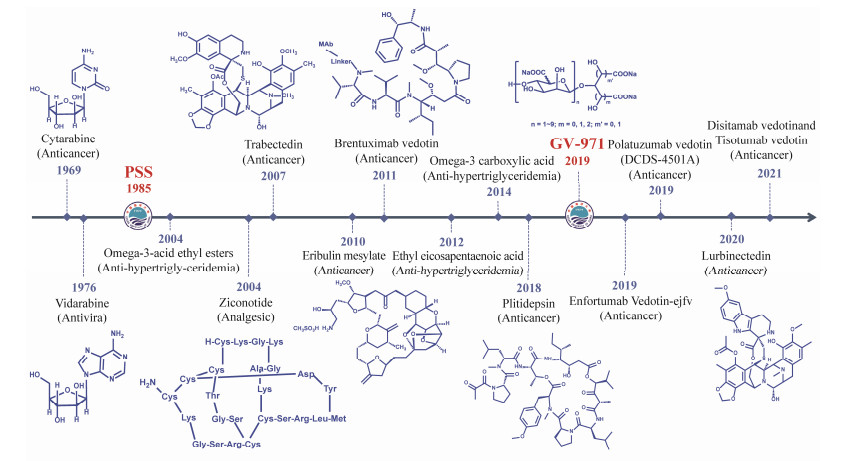
|
Fig. 1 Timeline of marine drug development. |
Furthermore, there are 33 drugs in phase III (6), II (17), and I (10) clinical trials (https://www.marinepharmacology.org/, update on April 10, 2024), primarily with functions not only in cancer treatment, but also in pain and virus infection treatments (Table 1) (https://clinicaltrials.gov/). Of these drugs, antibody drug conjugates (ADCs) are the dominated ones (21 in 33 compounds). Other compounds include diketopiperazine, alkaloid, mafodotin, β-lactone-gamma lactam, lectin, halichondrin, macrolide lactone and kinase inhibitor, respectively. In particular, China has independently developed two new marine drugs (Gao et al., 2019). Propylene glycol alginate sodium sulfate (PSS) is the first marine heparin drug in the world developed by Ocean University of China, for the prevention and treatment of hyperlipidemia and ischemic cardio-cerebrovascular diseases in China for 30 years (Shan et al., 2019). GV-971 is the first target β-amyloid protein oligosaccharide drug used for the treatment of Alzheimer's disease (AD) (Wang et al., 2019). Hence, China has made significant contributions in developing new marine drugs.
|
|
Table 1 33 compounds in clinical trial with details of their marine sources, chemical class, molecular target, main clinical area and number of clinical trials (III/II/I) |
In the past two decades, our group has conducted research on the discovery and optimization of drug-lead-compounds based on marine chemical ecology and has made a series of research achievements. A compound library of over 1500 organics isolated from marine-derived fungi has been constructed, including alkaloids, anthraquinones, peptides, polyketides, and terpenes. These MNPs exhibited a variety of potent biological activities, involving not only chemical ecology effects, but also pharmaceutical activities. The representative works of 83 bioactive compounds are briefly summarized in this review. We discussed the discovery of compounds, synthesis, structure-activity relationships (SARs), mechanisms of function, and druggability. We not only elucidated the antiviral mechanisms of QLA and antifibrotic mechanisms of CHNQD-00803, but also studied the antihepatocellular carcinoma (HCC) effects of two candidate drugs (CHNQD-01255 and CHNQD-01269), indicating that MNPs with different mechanisms of function are significant resources in the development of new drugs.
2 Marine Medicinal Microbial Resources and Marine Natural Products 2.1 Marine Medicinal Microbial ResourcesThe Paracel Islands, Nansha Islands, the South China Sea, and some special habitats, such as Mariana Trench, have
become highlights in searching for marine medicinal organism resources. In the recent two decades, we have developed a marine medicinal biological resource library. More than 1500 strains of fungi, including Penicillium and Aspergillus, have been obtained from marine organisms, such as coral and mangrove. In this regard, we have carried out many explorations for efficient utilization of these resources (Qin et al., 2015; Hou et al., 2019a; Wang et al., 2022).
2.2 Marine Natural ProductThe following section describes a series of bioactive compounds that were isolated to investigate the diversity of marine natural products.
2.2.1 Antifouling resorcylic acid lactonesMarine biofouling can cause significant economic losses, especially when it happens on ship hulls or aquaculture facilities. It is an ongoing focus of our laboratory to investigate novel bioactive MNPs and derivatives from marine-derived fungi. A number of 14-membered resorcylic acid lactones (RALs), cochliomycins A – G (1 – 7), were obtained from the fungus Cochliobolus lunatus derived from Dichotella gemmacea (Shao et al., 2011; Liu et al., 2014; Xu et al., 2019b) (Fig.2). Especially, compound 1 was effective against the barnacle Balanus Amphitrite with an EC50 value of 1.2 μg mL−1 (Shao et al., 2011). Compound 7 displayed potent antifouling activity against the microalgae Chlorella vulgaris, Chaetoceros socialis, and Navicula exigua, with EC50 values of 1.09, 0.92, and 0.611 μg mL−1, respectively (Xu et al., 2019b). In continuing efforts to investigate compounds with antifouling activity, a number of 14-membered RAL analogues were designed and semi-synthesized to construct a RALs library (Zhang et al., 2014; Wang et al., 2016; Zhang et al., 2017; Xu et al., 2022b; Jing et al., 2024). Compounds 8 and 9 were shown to have antifouling activity against the diatoms Navicula laevissima (EC50 = 6.67 μmol L−1) and Navicula exigua (EC50 = 8.55 μmol L−1), respectively, which had similar efficacy to those of the positive control SeaNine 211 (EC50 = 2.90 and 9.74 μmol L−1). These compounds represent potential promising antifouling agents (Xu et al., 2022b).
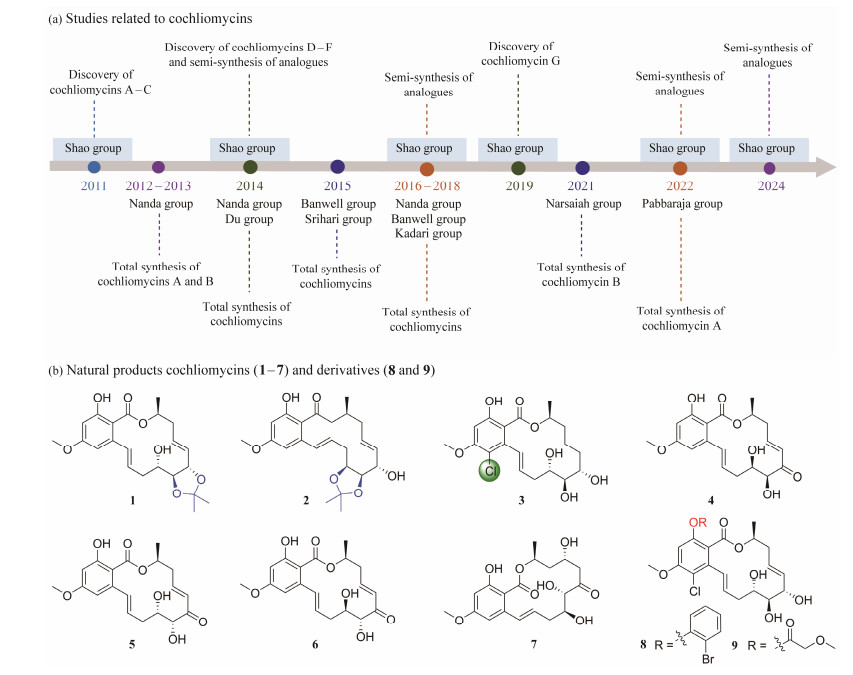
|
Fig. 2 Structures of representative 14-membered RALs. |
Noticeably, in addition to significant antifouling activity, some compounds exhibited inhibitory activity against Plasmodium falciparum with IC50 values of 3.54 – 9.72 μmol L−1 (Xu et al., 2022b). Worth mentioning is that these antiplasmodial derivatives showed nontoxic in the cytotoxicity tests and the zebrafish embryos model. SAR was observed that biphenyl substituent at C-2, acetonide at positions C-5′ and C-6′, and trior tetra-substituted of acyl groups increased biological activity (Xu et al., 2022b).
In recent years, the finding of biological activities of RALs has led to notably high interest in the chemical synthesis of cochliomycins, in which a crucial factor is that the method of the 14-membered lactone ring is formed (Banwell et al., 2017; Jana et al., 2018). To date, 6 research groups have reported the total synthesis of cochliomycins (Jana and Nanda, 2012; Gao et al., 2014; Wang et al., 2014; Bolte et al., 2015; Mahankali and Srihari, 2015; Ma et al., 2016; Pal et al., 2016; Banwell et al., 2017), among which Du's group and Banwell's group are especially notable. In 2014, the Du group (Gao et al., 2014; Wang et al., 2014) reported the synthesis of compounds 1 and 2 (Fig. 3a). Both of them started with L-arabinose, a base-promoted lactonisation reaction to close the ring of compound 1 (Wang et al., 2014), while a ring-closing metathesis reaction was used to construct the associated macrolide ring of compound 2 (Gao et al., 2014). Banwell group (Bolte et al., 2015) completed the total synthesis of the natural products cochliomycin A (1) and cochliomycin B (2) in 11 steps (Fig.3b). Olefin cross-metathesis, trans-esterification and Nozaki-Hiyama-Kishi (NHK) macrocyclization reactions were applied in the key steps. One year later, the group reported (Ma et al., 2016) the total syntheses of the cochliomycin C (3) using an intramolecular Loh-type α-allylation reaction for macrolide formation (Fig.3c). Meanwhile, Nanda group reported total synthesis of cochliomycin C (3) and other compounds (Pal et al., 2016). The highlight of the reported synthetic strategy mainly involves the successful application of the highly stereoselective Heck reaction (Fig.3d).
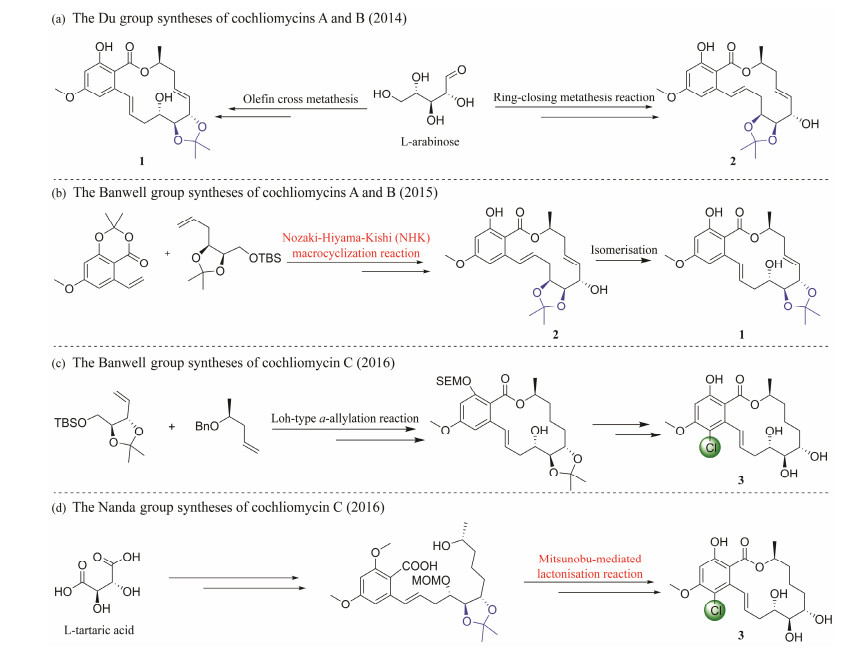
|
Fig. 3 Total synthesis of cochliomycins. |
In addition to marine invertebrates and their symbiotic microorganisms, macrolides were also isolated from marine-derived bacteria. Bastimolides are polyhydroxy macrolides. They feature a dense array of hydroxylated stereogenic centers. Bastimolides have always been used as attractive synthetic target compounds for synthesis of potential antimalarial agents (Quintard et al., 2018; Kumar et al., 2021; Cox et al., 2022; Fiorito et al., 2022; Molga et al., 2022; Celik et al., 2024) (Fig.4a). Bastimolide A (10), as the first example of these polyhydroxyl macrolides, was isolated from the tropical marine cyanobacterium Okeania hirsute, which has led to the discovery of a novel macrolide possessing a 40-membered ring. Compound 10 displayed highly potent and selective antimalarial activity against four resistant strains of Plasmodium falciparum (IC50 = 80 – 270 nmol L−1). Therefore, through X-ray diffraction analysis of the nona-p-nitrobenzoate derivative (11), its complete structure and absolute configuration have been determined (Shao et al., 2015a). Continued search of potent antimalarial macrolides from O. hirsuta resulted in the identification of a new analogue, bastimolide B (12), which is a 24-membered polyhydroxy macrolide with a long aliphatic chain and unique terminal tert-butyl group. Compound 12 was effective against chloroquine-sensitive Plasmodium falciparum strain HB3 (IC50 = 5.7 ± 0.7 µmol L−1) (Fig.4b). This study proposed the methanolysis mechanism for 10, and obtained an isomerization product of the C2 – C3 double bond, 2-(E)-bastimolide A (Shao et al., 2018). A SAR analysis based on six analogues indicated that the double bond as well as the 1, 3-diol and 1, 3, 5-triol functionalities are favorable for the expression of antimalarial activity (Shao et al., 2018). The discovery of this new polyhydroxy macrolide further suggests that the cyanobacterial genus Okeania should be a significant resource of molecules with interesting structure in the future (Shao et al., 2018).
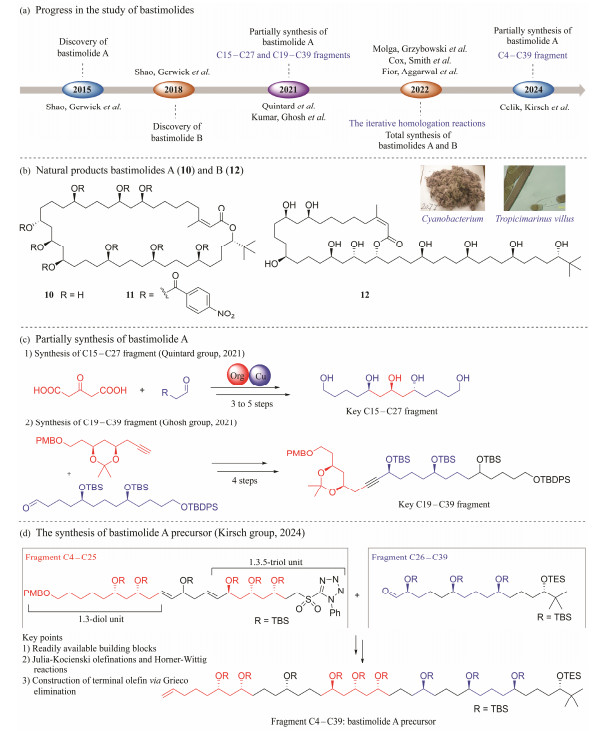
|
Fig. 4 Research progress of the novel polyhydroxy macrolide bastimolides. |
Quintard group (Quintard et al., 2018) and Ghosh group (Kumar et al., 2021) reported the synthesis of the key C15 – C27 fragment and the C19 – C39 fragment of 10, respectively (Fig.4c). In 2022, Grzybowski group (Molga et al., 2022) described the application of a computer algorithm to propose a plausible route to 10 in 43 steps (longest linear sequence (LLS)) by the method of the iterative homologation reactions (Fig.5a). Smith's group (Cox et al., 2022) reported the total synthesis of 11 with 0.7% yield and 20 steps of LLS. The key to this strategy was the preparation of linear polyol 2 in step 15 of LLS with a yield of 6% (Fig.5b). Shortly thereafter, the first total synthesis of 12 was reported by Aggarwal and co-workers (Fiorito et al., 2022), with 16 steps of LLS and 1.4% overall yield. Aggarwal's group proposed a stereocontrolled synthesis strategy for 1, 5-polyol based on iterative boronic ester homologation with enantiopure magnesium carbenoids (Fig.5c). After two years, Kirsch's group (Celik et al., 2024), using a HornerWittig reagent and an aldehyde derived from the same chiral-pool based intermediate as the key building block, reported a straight forward formal synthesis of 10 (Fig.4d).
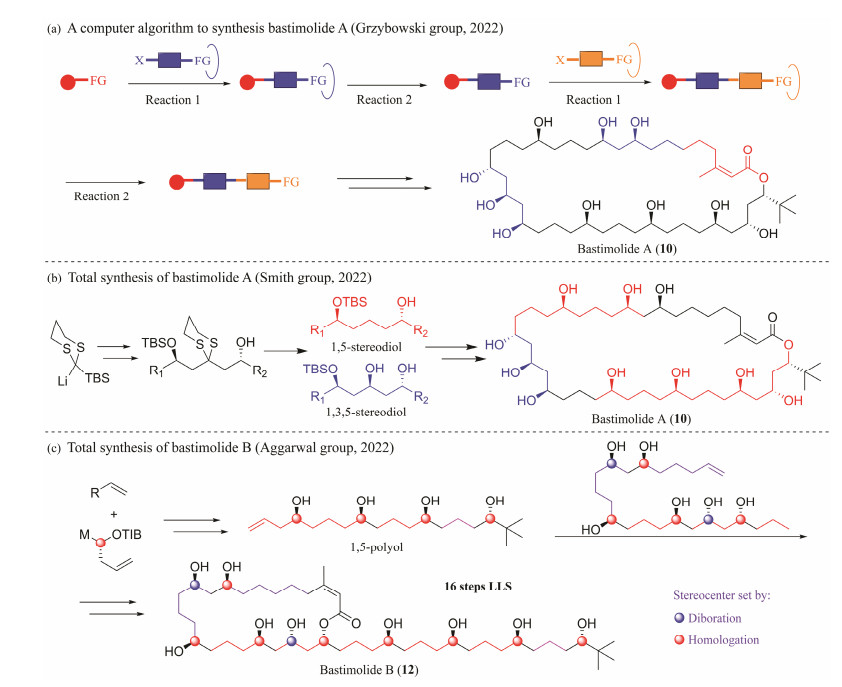
|
Fig. 5 Research progress of the synthesis of novel polyhydroxy macrolide bastimolides. |
In addition, a number of chemically diverse novel MNPs have been isolated from coral-derived fungi, exhibiting a variety of pharmacological activities such as antibacteria, anti-inflammation, antiviruses and anti-cancer (Fig.6). Chrysopiperazines A and B (13 and 14), and chrysopiperazine C (15) are three new diketopiperazine alkaloids isolated from the gorgonian-derived Penicillium chrysogenum fungus. Additionally, compounds 13 and 14 are the first reported natural oxepine-containing diketopiperazines from the genus Penicillium. By the vibrational circular dichroism (VCD) method, the absolute configurations of C-19 in 13 and 14 were successfully determined. Meanwhile, a series of methods to define the absolute configurations of complicated natural products was provided (Xu et al., 2019a).
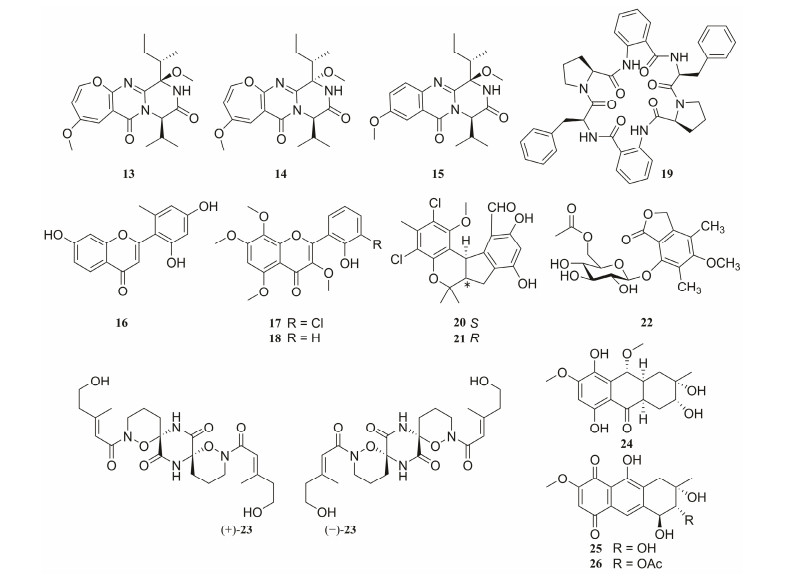
|
Fig. 6 Chemical structures of representative pharmacological active substances (13–26). |
Chemical investigation of the fungus Penicillium chry-sogenum isolated from a gorgonian Carijoa sp., led to the discovery of a new flavone analogues, penimethavone A (16), with a rare unique methyl group at ring-B. Compound 16 exhibited selective and moderate cytotoxicity against HeLa and rhabdomyosarcoma cell lines with IC50 values of 8.41 and 8.18 µmol L−1, respectively (Hou et al., 2016). Aspergivones A (17) and B (18) were isolated from the fungus Aspergillus candidus which was isolated from the gorgonian coral Anthogorgia ochracea obtained from the South China Sea. A biological assay against α-glucosidase showed that only compound 17 possesses slight inhibitory activity with an IC50 value of 244 μg mL−1 (Ma et al., 2017). Aspersymmetide A (19) was isolated from the fungus Aspergillus versicolor obtained from a gorgonian coral Carijoa sp.. It is the first compound of marine-derived centrosymmetric cyclohexapeptide. At the concentration of 10 µmol L−1, 19 displayed weak cytotoxicity against NCI-H292 and A431 cell lines (Hou et al., 2017).
Chemical investigation of the fungus Pestalotiopsis sp. from a soft coral Sarcophyton sp. led to the identification of (±)-pestalachlorides C and D (20 and 21), pestalotiolide A (22) and (±)-pestaloxazine A (23) (Wei et al., 2013; Jia et al., 2015a, 2015b). (±)-23 with an unprecedented symmetric spiro-[oxazinane-piperazinedione] skeleton is a pair of new enantiomeric alkaloid dimers (Jia et al., 2015b). In vitro, compounds 22 and (+)-23 showed significant anti-EV71 activities (IC50 = 27.7 and 14.2 μmol L−1, respectively) (Jia et al., 2015a, 2015b).
Two new hydroanthraquinone analogues, 4a-epi-9α-methoxydihydrodeoxybostrycin (24) and 10-deoxybostrycin (25), together with seven known anthraquinone derivatives were isolated from a sea anemone-derived fungus Nigrospora sp.. Compound 26, the acetylated derivative of a known anthraquinone (4-deoxybostrycin), displayed activity against Bacillus cereus (MIC = 48.8 nmol L−1), which has stronger effect than the positive control (ciprofloxacin, MIC = 1250 nmol L−1). The antibacterial screening data for the MNPs and their acetyl analogs indicated that the key feature is the cycloaliphatic ring (Yang et al., 2012).
In addition, chemical investigation of the marine-derived fungi led to the isolation of nigrodiquinone A, 5-acetyl-2-methoxy-1, 4, 6-trihydroxy-anthra-quinone, javanicin, anhydrofusarubin, 3-O-methylfusarubin, fusarubin, anhydrofusarubin, 8-(methoxycarbonyl)-1-hydroxy-9-oxo-9H-xanthene-3-carboxylic acid, and dimethyl 8-methoxy-9-oxo-9H-xanthene-1, 6-dicarboxylate (Shao et al., 2008, 2010; Xu et al., 2016). 3-O-methylfusarubin showed strong inhibitory effects on the growth of HepG2 and Hep2 cells, with IC50 values of 1.0 and 2.5 µg mL−1, respectively (Shao et al., 2010).
2.2.4 Discovery of new MNPs guided by molecular network and NMR-based technologiesDrug discovery based on bioactive natural product (NP) scaffolds will continue to be a major research area of NPs in the future. A challenging problem in natural product discovery is how to rapidly dereplicate known compounds and discover novel ones from complicated components. Recent advances in bioinformatics and the emergence of many powerful tools and platforms have reversed the laborious and time-consuming landscape of NP drug discovery. Molecular network strategies based on MS/MS cheminformatics could reveal structural relationships and accelerate the dereplication of molecules in crude extracts to mine new molecules (Zhang et al., 2023).
In our studies, under the direction of molecular networking techniques and 1H NMR technique, a total of 15 peptides were identified (Hou et al., 2019a, 2019b; Chao et al., 2021; Han et al., 2023). Investigation of biologically active metabolites from the coral-derived fungus Aspergillus versicolor (CHNSCLM-0063) enabled the discovery of eight new cycloheptapeptides, asperversiamides A – C (27 – 29) (Hou et al., 2019b), asperheptatides A – D (30, 31, 33, and 34) (Chao et al., 2021) and asperpyrroindotide A (32) (Han et al., 2023) (Figs.7a and 7b). Furthermore, compound 32 is the first pyrroloindoline-containing cyclopeptide isolated from marine fungi (Han et al., 2023). Their complete structures were elucidated by the comprehensive analysis of spectroscopic methods, Marfey's hydrolysis, and total synthesis. The complete structures of compounds 27 and 28 were further confirmed by total synthesis (Fig.7c). These compounds contained uncommon amino acids with D configurations, including D-Ala, D-Val, D-Ser, and D-Trp (Hou et al., 2019b). Asperheptatides C (33) and D (34) are trace metabolites, and have been characterized by ESI-MS/MS fragmentation methods (Chao et al., 2021).
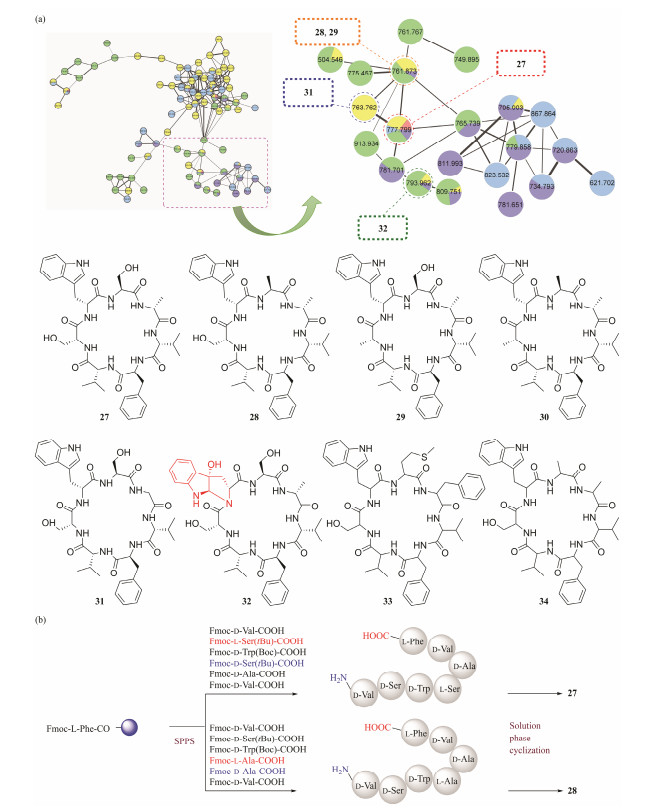
|
Fig. 7 Asperversiamides peptides discovered under the guidance of molecular networking. (a), structure of asperversiamides peptides; (b), total synthesis of asperversiamides A and B. |
The results of the activity assays indicated that compounds 27 – 29 displayed inhibitory activity against Mycobacterium marinum with minimum inhibitory concentrations (MICs) of 23.4, 81.2, and 87.5 µmol L−1, respectively, which were equivalent to those of the positive controls streptomycin (20.1 µmol L−1) and isoniazid (88.5 µmol L−1). Meanwhile, 30 exhibited weak activity against M. tuberculosis H37Rv with an MIC90 value of 100 µmol L−1 (Hou et al., 2019b). Furthermore, a number of new analogues of compound 27 were semisynthesized for SAR investigations (Chao et al., 2021; Han et al., 2023). Some compounds showed moderate activities against M. tuberculosis H37Ra with MIC90 values of 12.5 µmol L−1, having great potential to be developed as novel anti-tuberculosis drugs (Chao et al., 2021; Han et al., 2023). The SAR analysis suggested that the serine hydroxy groups and the tryptophan residue play an important role in its active expression (Chao et al., 2021; Han et al., 2023).
Seven new cyclohexadepsipeptides, chrysogeamides A – G (35 – 41), were isolated from the coral-derived fungus Penicillium chrysogenum (CHNSCLM-0003), under the guidance of the LC-MS/MS-based molecular networking and 1H NMR techniques (Hou et al., 2019a). The results of compound structure analysis indicated that compound 38 possessed a rare 3-hydroxy-4-methylhexanoic acid (HMHA) moiety, which was discovered for the first time from marine-derived organisms. Meanwhile, 13C1-L-Leu was transformed into 13C1-D-Leu moiety, which was confirmed by isotope-labeling feeding experiments, suggesting that L-Leu could isomerize into D-Leu. At the concentraion of 1.0 μg mL−1, compounds 35 and 36 obviously promoted angiogenesis in zebrafish with nontoxic effects to embryonic zebrafish at 100 μg mL−1 (Hou et al., 2019a). In addition, under the guidance of these technologies, two new alkaloid racemates featuring a rare o-hydroxyphenylalanine residue and an imide subunit, (±)-17-hydroxybrevianamide N (42) and (±)-N1-methyl-17-hydroxybrevia-namide N (43), were isolated from a soft-coral-derived Aspergillus sp. Fungus (Xu et al., 2021). The real natural products (+)-42 and (+)-43 were further monitored and obtained from the freshly prepared EtOAc extracts, while (−)-42 and (−)-43 were artifacts that were generated during extraction and purification processes. Interestingly, the basic solution promotes the racemization of (+)-42 and (−)-43, whereas acidic solution inhibits the transformation (Fig.8).
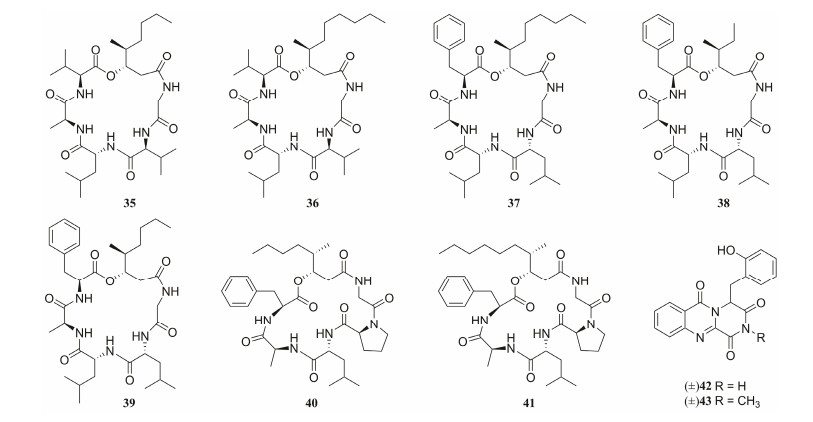
|
Fig. 8 Chemical structures of MNPs discovered under the guidance of molecular networking (35 – 43). |
The discovery and biological functional verification of bioactive compounds is important but challenging due to the low compound content, complex structure, and difficult preparation of marine natural products. Based on the goals of sustainability and environment-protection, significant progress has been made in the field of green chemical synthesis. However, it is still difficult to achieve synthesis with both high-level production and high-level efficiency and scalability.
The 3, 4-dioxygenated 5-hydroxy-4-aryl-quinolin-2(1H)-one alkaloids are promising lead structures with diverse bio-logical properties (He et al., 2005; Schmeda-Hirschmann et al., 2005; Scherlach and Hertweck, 2006; Simonetti et al., 2016b). Although these alkaloids display potential biological characteristics and synthetic attractive motifs, there are relatively few compounds prepared through total synthesis, especially those with C-6 side chains containing isoprene derived (C5 or C10) units (Li et al., 2009; Simonetti et al., 2016a; Schwan et al., 2018, 2020; Vece et al., 2018; Jia et al., 2021). A convenient method to construct a 6-propenyl side chain by Claisen rearrangement of 5-O-allyl in the heterocycle has been reported by Simonetti group. Similar reactions have also been applied by Vece group to install unsaturated pyran fragments in yaequinolones J1 and J2, as well as by the Christmann team to synthesize anidoquinolone C and peneprequinolone. In 2020, Christmann group developed the tandem Knoevenagel electrocyclization to further optimize the assembly of pyran fragments in yaequinolones J1 and J2 (Schwan et al., 2020). In 2021, Fernández-Ibáñez group reported the synthesis of yaequinolone-related compounds by introducing C-6 side chains through late-stage C-H olefinization (Jia et al., 2021). Despite the feasibility of generating the above members of yaequinolones over the past decade, the ultimate challenge of procuring large quantities and stereoselective synthesis of these compounds has not yet been overcome (Fig.9a). Aniduquinolone A (44) obtained from the marine-derived fungus Scopulariopsis sp., not only exhibits the potent antifouling activity with an EC50 value of 17.5 pmol L−1, but also has a very safe and high therapeutic ratio (LC50 /EC50 = 1200) (Shao et al., 2015b). In 2022, our group completed the first scalable total synthesis of 44 (Guo et al., 2022). The key step of the strategy was assembling isopropenyl substituted tetrahydrofuran onto the 3, 4-dioxygenated 5-hydroxy-4-aryl-quinolin-2(1H)-one core by E-stereoselective Horner-Wadsworth-Emmons (HWE) olefination (Fig. 9b). Surprisingly, by treating with trifluoroacetic acid, 44 could successfully transform into aflaquinolones A, C, and D (45 – 47) that feature different 2, 4-dimethyl cyclohexanone moieties (Fig.9c). The method described by our group can be widely applied to the synthesis of natural alkaloids including the core structure of 3, 4-dioxygenated 5-hydroxy-4-aryl-quino-lin-2(1H)-one (Guo et al., 2022).
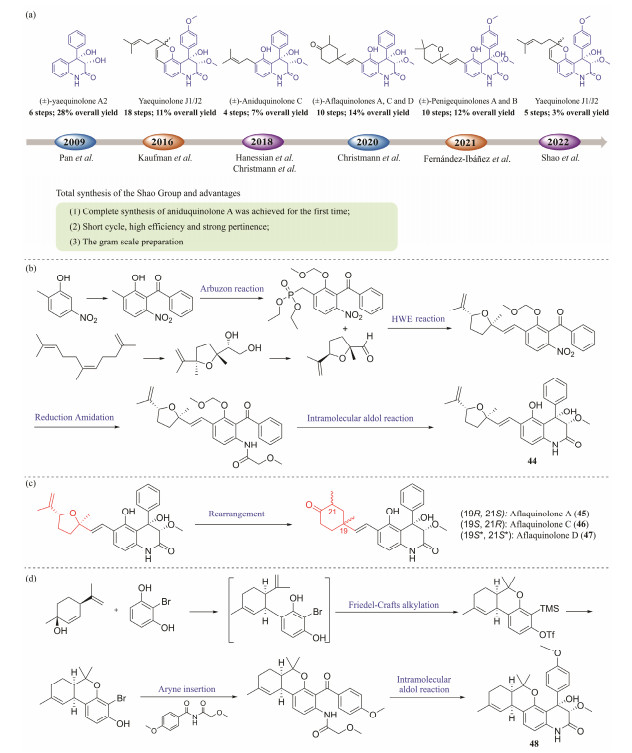
|
Fig. 9 Progress in total synthesis route and structures of active compounds. (a), progress in total synthesis of 3, 4-dioxy-genated 4-aryl-quinolin-2(1H)-one alkaloids; (b), total synthesis of (+)-aniduquinolone A; (c), the acid-catalyzed rearrangement from aniduquinolone A to 19-epi-aniduquinolone A and aflaquinolones; (d), total synthesis of pesimquinolone I. |
Pesimquinolone I (48) obtained from the fungus Penicillium simplicissimum, with a unique 6/6/6/6 tetracyclic core, displayed a potent anti-inflammatory (Dai et al., 2021). The highly efficient and scalable total synthesis of 48 was accomplished in 5 steps, starting from 2-bromoresorcinol and (1S, 4R)-menthadienol (Fig.9d). The key steps in this synthesis strategy include Lewis acid catalyzed Friedel Crafts alkylation reaction that can produce tricyclic intermediate, regioselective insertion of aromatic hydrocarbons into asymmetric imines, and diastereoselective alcohol cyclization reaction to construct a tetracyclic skeleton. Meanwhile, we have completed the synthesis and stereochemical allocation of the stereoisomer of 48 and its analogues. This strategy follows the ideal principle and introduces concepts such as atomc economiy, step economy, green chemistry, selectivity, and unprotected synthesis. Biological assay displayed that Δ3''-1''-epi-Pesimquinolone I showed potent NO inhibitory effects (IC50 = 0.44 μmol L−1), which has stronger inhibitory effect than positive control (indomethacin, IC50 = 50.00 μmol L−1). Thus, it provided potential prospects for the discovery of anti-inflammatory drugs (Guo et al., 2023c).
Aflaquinolone I, which is isolated from the marine fun-gus Metarhizium marquandii, is the first natural example of a 3, 4-dioxygenated 4-aryl-quinolin-2(1H)-one alkaloid with 3R, 4R configurations (El-Kashef et al., 2019). In 2023, our group reported the first total synthesis of aflaquinolone I, and it was completed through two parallel strategies with 9 and 4 steps, respectively. The key steps of the first method included a base-catalyzed coupling reaction to forge α-hydroxyanilide and a diastereoselective aldol cyclization to construct the 3, 4-dioxygenated-4-aryl-quinolin-2(1H)-one core, while the second approach included a regioselective insertion of aryne into unsymmetric imide to build the N-glycolated 2-amino-benzophenone (Guo et al., 2023a).
Investigation of the marine-derived Aspergillus candidus fungus led to the isolation of two pyrrolinone-fused benzoazepine alkaloids, (+)-asperazepanones A (49) and B (50), together with the (−)-asperazepanone A. The results of asperazepanone A isolated as a racemate and asperazepanone B isolated as an optically active reagent had piqued the interest of researchers. The short-term fermentation experiment was analyzed by chiral HPLC and showed that (+)-asperazepanone A was a true natural product, while compound (−)-asperazepanone A is an artifact (Fig.10a). In addition, we reported the total syntheses of 49 and 50 by starting from the commercial L-aspartic acid diethyl ester hydrochloride and monoethyl malonate with 7 and 8 steps, respectively (Fig.10b). Meanwhile, the intramolecular Friedel Crafts reaction, which constructs a unique tricyclic framework, is a crucial step in the synthesis. At the concentration of 0.1 μmol L−1, (+)-asperazepanone B not only inhibited NO production, but also inhibited LPS-induced expression of TNF-α and IL-6. This compound has the potential to be developed as an anti-inflammatory drug (Xu et al., 2022a).
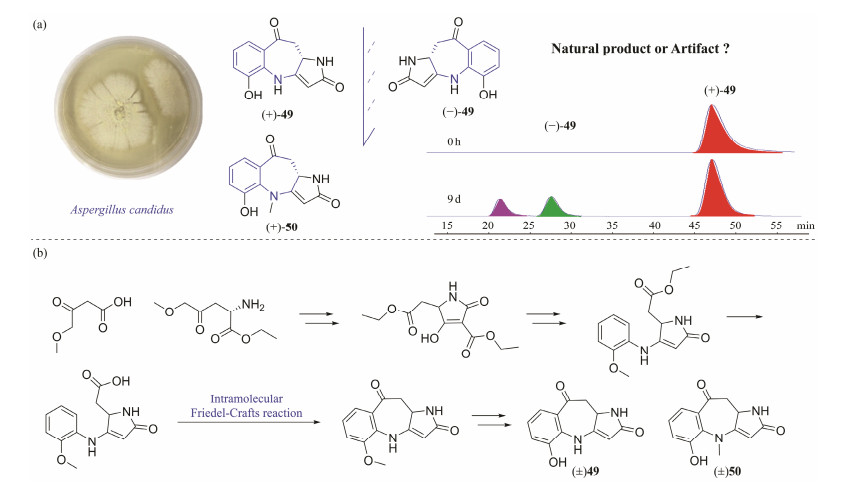
|
Fig. 10 Isolation and total synthesis of asperazepanones with a new skeleton. (a), Aspergillus candidus fungus and identification of natural product or artifact; (b), total syntheses of 49 and 50. |
In addition, aeries of derivatives of active metabolites have been semisynthesized to enhance their biological activity. Geodin (51), isolated from the marine-derived fungus Aspergillus sp., was shown to have insecticidal activity against Helicoverpa armigera Hübner with an IC50 value of 500 µmol L−1. A number of new ether analogues were semisynthesized to increase the biological activity of 51 (Fig.11a). The biological assay indicated that most of these derivatives exhibited stronger activities against H. armigera Hübner than 51. In particular, 52 displayed activity with an IC50 value of 89 µmol L−1, equivalent to the positive control (azadirachtin, IC50 = 70 µmol L−1). Additionally, some derivatives were shown to have activity against Staphylococcus aureus and Aeromonas salmonicida with MIC values in the range of 1.15 – 4.93 µmol L−1. The SAR indicated that key factors in increasing the insecticidal and antibacterial activities of 51 were the introduction of halogenated benzyl especially fluorobenzyl, into 51 and substitution of 4-OH (Chao et al., 2022).
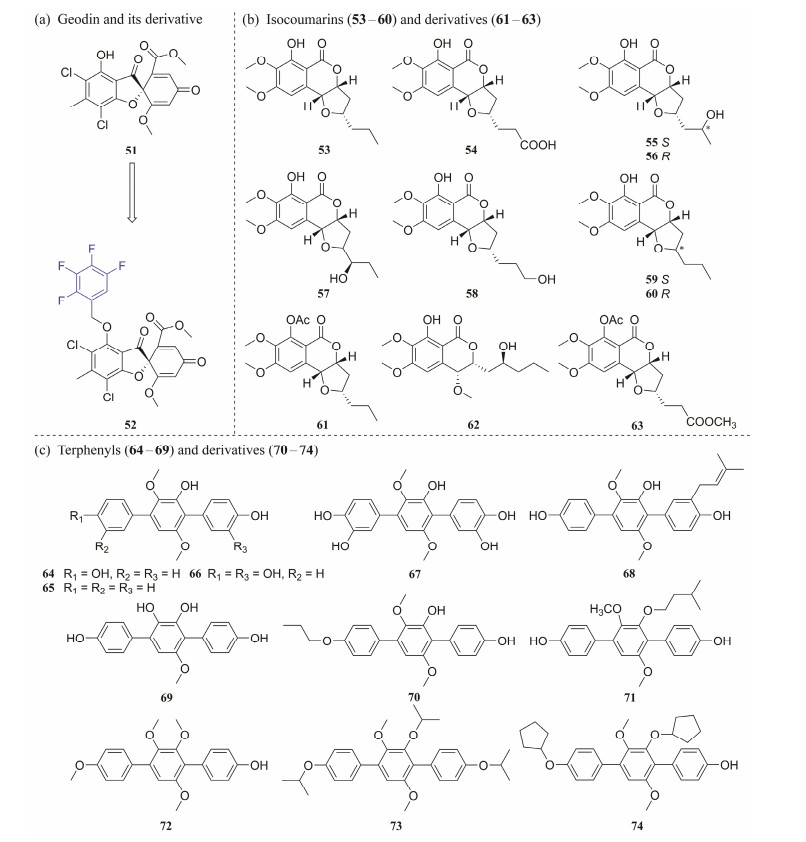
|
Fig. 11 Structure of MNPs and their derivatives. |
Investigation of the marine-derived fungus Exserohilum sp. (CHNSCLM-0008), led to the isolation of a series of isocoumarins (53 – 60). A small library of 22 new derivatives were established for SAR investigation and discovered potent antimalarial activity, with different substituents on the aromatic ring and the aliphatic side chains. Compound 53 and its derivatives (61 – 63) (Fig.11b) have an allcis stereochemistry, display potential antiplasmodial activity against P. falciparum HB3 with an IC50 value ranging from 0.4 to 2.6 μmol L−1. In addition, a SAR analysis indicated that the configurations of C-3/C-4/C-10 play a major role in antiplasmodial activity (Coronado et al., 2021).
Investigating the metabolites of the marine-derived fungus Aspergillus candidus led to the discovery of a naturally abundant p-terphenyl metabolite, terphenyllin (64), and four natural analogues 65 – 68. A small library of 115 derivatives of 64 was developed for evaluating their potency and selectivity (Zhang et al., 2018; Haider et al., 2020). In biological evaluation of inhibiting α-glucosidase, there are 15 derivatives that showed potent activity with an IC50 value ranging from 4.79 to 15 μmol L−1, which are stronger than positive controls (1-deoxynojirimycin IC50, 192.0 μmol L−1; acarbose IC50, 707.9 μmol L−1) (Fig.11c). Meanwhile, 69 and 70 had relatively high treatment indices with CC50 /IC50 values of 17 and 10, respectively. The enzyme kinetic studies of 64 and 71 displayed a non-competitive inhibition on α-glucosidase (Ki = 1.50 and 3.45 μmol L−1, respectively) (Zhang et al., 2018). In biological evaluation against BEL-7402, A549, SMMC-7721, and HepG2, 72 –74 exhibited strong inhibitory activities (IC50 = 0.13 – 5.51 μmol L−1), which were equivalent to those of the positive control, adriamycin. Investigation of SAR indicated that the introduction of alkyl substituents including ethyl, allyl, propargyl, isopropyl, bromopropyl, isopentenyl, cyclopropylmethyl, and cyclopentylmethyl are important for enhancing the cytotoxicity (Haider et al., 2020).
4 Mechanism of the Active Compounds Function 4.1 A Novel NP Inhibitor QLAIn addition to discovering novel MNPs and solving the problem of drug source, it is also important to clarify the mechanism of the function and evaluate the potency of bioactive compounds. A novel quinolone alkaloid (QLA, 75) with broad-spectrum anti-IAV activities and low toxicity was obtained from marine fungus (Zhang et al., 2023). The anti-IAV mechanisms of 75 might be blocking virus replication and viral RNA (vRNA) exporting from the nucleus by targeting virus nucleoprotein (NP), which was different from current drugs (Fig.12a). Further studies indicated that 75 could inhibit the nuclear output of NP and vRNP by blocking the binding of chromosome region maintenance 1 to nuclear export signal 3 of NP. In addition, 75 may also affect vRNP assembly by interfering with the binding of NP to RNA rather than NP oligomerization. Importantly, 75 can protect the mice against IAV-induced death and weight loss by oral administration, superior to the effects of oseltamivir. Hence, 75 has the potential to be developed as a novel anti-IAV agent in the future, targeting the virus NP protein (Zhang et al., 2023).
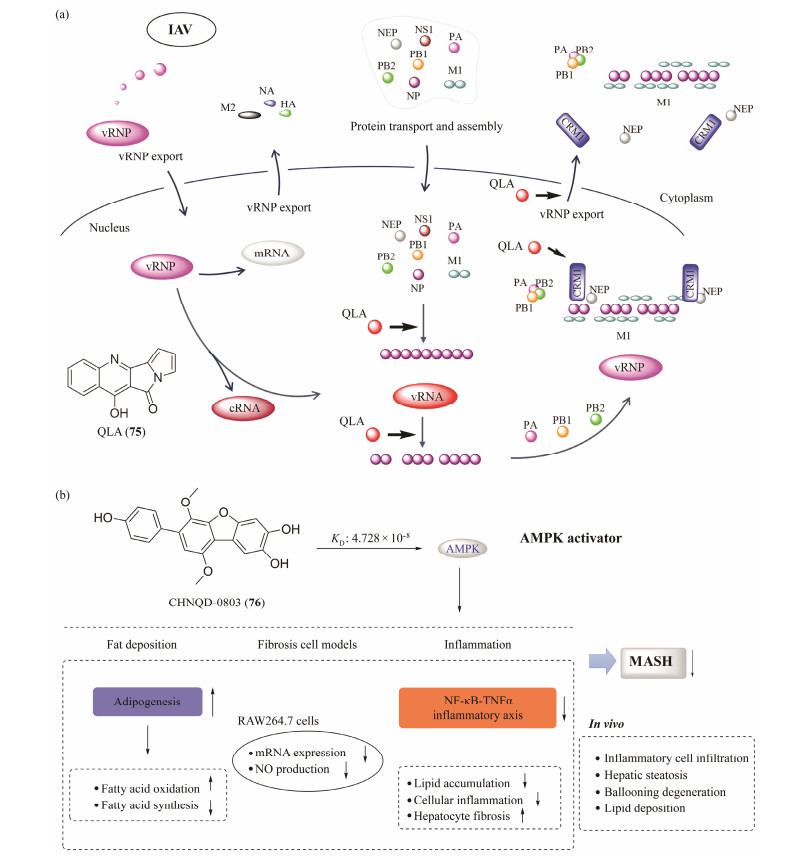
|
Fig. 12 Mechanism of the active compounds QLA (a) and CHNQD-00803 (b). |
It has been proposed that AMP-activated protein kinase (AMPK) activators could be used for treating metabolic diseases such as obesity, type 2 diabetes, and metabolic dysfunction-associated steatohepatitis (MASH). CHNQD-00803 (76) has been found as an effective AMPK activator by screening a MNPs library. The results of the activity assay showed that 76 directly binds with recombinant AMPK (KD = 4.728 × 10−8 mol L−1) and activates AMPK at both molecular and intracellular levels. Further research indicates that 76 inhibited the expression of adipogenesis genes and reduced fat deposition, negatively regulated the NF-κB-TNFα inflammatory axis to suppress inflammation, and ameliorated liver injury and fibrosis (Fig.12b). The results indicate that as an AMPK activator, 76 is a novel potential candidate for MASH treatment in the future (Chen et al., 2023).
5 Druggability of Active Compounds 5.1 Natural Arf-GEFs Inhibitor Prodrug CHNQD-01255 and Anti-HCC Agent CHNQD-01269In 2022, a total of 102 fungal strains were isolated from the medicinal mangrove Acanthus ilicifolius. The cytotoxic activity of fungi organic extracts was assessed against A549, HeLa, HepG2, and human acute lymphoblastic leukemia cell lines. In addition, at the concentration of 25 µg mL−1, the fungus Penicillium sp. (HS-N-27) exhibited significant cytotoxic activity. Guided by cytotoxic activity and fingerprint analysis (Wang et al., 2022), brefeldin A (77), a well-known natural Arf-GEFs inhibitor, was obtained from the target active fungus HS-N-27 (Fig.13a).
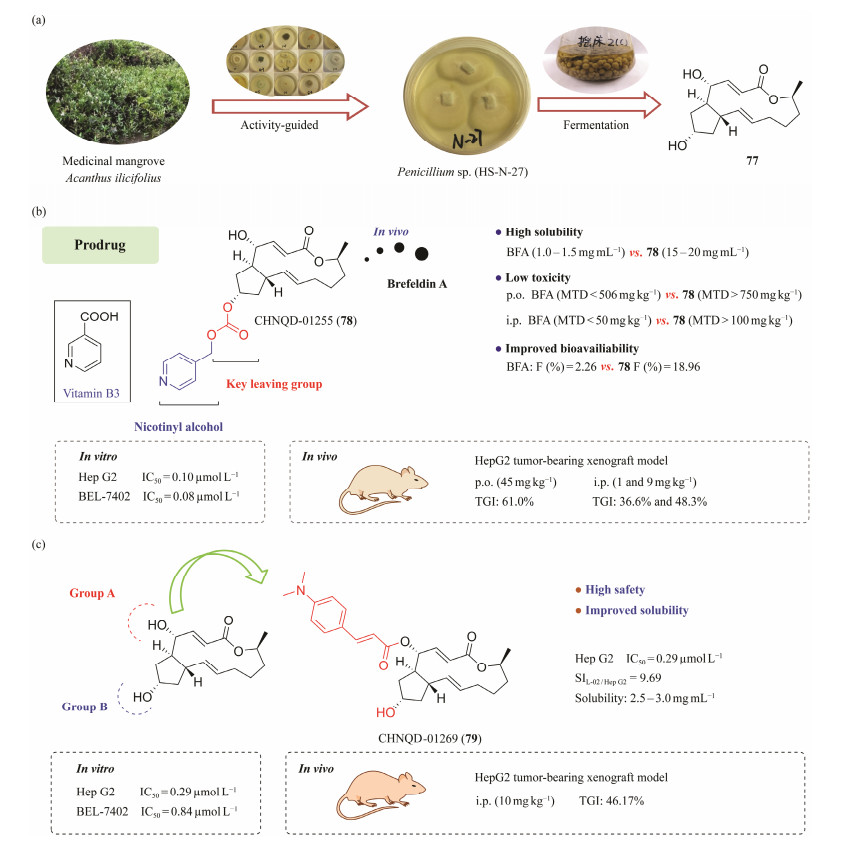
|
Fig. 13 Pharmacodynamic studies of bioactive molecules. (a), the discovery of brefeldin A; (b), structure and pharmacodynamic evaluation of prodrug CHNQD-01255; (c), structure and pharmacodynamic evaluation of CHNQD-01269. |
Previously, National Cancer Institute (NCI) has confirmed that compound 77 has significant anti-tumor activity (Sausville et al., 1996). However, its limited solubility, toxicity, and certain pharmacokinetic (PK) properties have severely hampered its clinical use (Phillips et al., 1993; Sausville et al., 1996). To address these issues, there has been increasing interest in synthesizing analogues as well as in improving the physicochemical properties of 77 to broaden their clinical applications (Phillips et al., 1998; Fox et al., 2001; Anadu et al., 2006). In our studies, a number of derivatives of 77 were prepared to improve the druggability of 77 (Jiang et al., 2022a, 2022b; Lu et al., 2022). The distinct corresponding prodrugs of 77, including esters, carbonates, cinnamic acid ester, and carbamates, were synthesized, and the growth inhibitory activity of their on HCC cells were evaluated. In particular, CHNQD-01255 (78) (Jiang et al., 2022a) possessed improved aqueous solubility (10-fold higher than 77), demonstrated favorable pharmacokinetic profiles, and enhanced bioavailability of 77 (F = 18.96%). Meanwhile, in the xenograft model, 78 significantly suppressed tumor growth (tumor growth inhibition rate, TGI = 61.0%) at a dose of 45 mg kg−1 (p.o.). Notably, it has been proved that the improved safety profile of 78 (maximum tolerated dose, MTD > 750 mg kg−1, p.o.) was superior to that of 77 (MTD < 506 mg kg−1). Evidence of antitumor activity against the HCC in vivo was confirmed in HepG2 tumor-bearing mice, with a TGI of 61.0% (Fig.13b). In conclusion, CHNQD-01255 (78), as a potent Arf-GEFs inhibitor, is qualified the capacity to be developed as a targeted candidate anticancer drug, which may be promised to apply for cancer immunotherapy.
Moreover, the 4-O-(4)-dimethylaminocinnamate group of CHNQD-01269 (79) (Fig.13c) enhanced aqueous solubility. It was confirmed that 79 showed strong cytotoxic activity against HepG2 and BEL-7402 cell lines (IC50 = 0.29 and 0.84 μmol L−1, respectively). More importantly, 79 performed low cytotoxicity against L-02 (normal liver cell line) with a selectivity index (SI) of 9.69 (17-fold higher than 77). Further mechanism studies showed that 79 arrested the cell cycle in the G1 phase, and induced apoptosis of HepG2 cells via elevating reactive oxygen species (ROS) production and increasing the expression of apoptosis-related proteins. Docking experiments also suggested that 79 was a promising inhibitor of Arf1, which was confirmed by the cellular thermal shift assay that 79 displayed a significant effect on the stability of Arf1 protein. Furthermore, 79 possessed high safety profile (MTD > 100 mg kg−1, i.p.) and favorable pharmacokinetic properties. Notably, the superior antiproliferative activity was verified in HepG2 tumor-bearing xenograft model in which 79 markedly suppressed the tumor growth (TGI = 46.17%) in nude mice at a dose of 10 mg kg−1 once a day for 16 days. The present study indicates that this series of highly efficient derivatives have the potential to be developed as drugs for the treatment of HCC (Jiang et al., 2022b). In addition, we also investigated the anti-leukemia potential of BFA derivatives (Zhang et al., 2021, 2022; Lu et al., 2022).
5.2 Anticancer Agent CHNQD-01829The MNP (+)-sclerotiorin (80) is a major secondary metabolite of the marine-derived Penicillium sclerotiorum fungus. A series of derivatives of 80 was prepared (Wei et al., 2017; Hai et al., 2022b). Compound 80 and its amine derivatives showed significant antifouling activities against the barnacle Balanus amphitrite larvae (EC50 = 0.47 – 18.2 μg mL−1). Moreover, the cytotoxic activities of compounds against six cancer cell lines were evaluated. The results showed that CHNQD-01829 (81) was the most effective compound against Bel-7402 cell line (IC50 = 1.15 µmol L−1). Compound 81 disrupted the mitochondrial membrane and induced apoptosis in a caspase-dependent manner. Additionally, 81 can affect the protein kinase B (AKT) and extracellular regulated protein kinases (ERK) signaling pathways and induce the degradation of AKT and ERK proteins through ubiquitin-proteasome system. Furthermore, 81 displayed significant anti-tumor effects in vivo in xenograft models with a 52.5% reduction in tumor mass. Finally, we conducted a comprehensive safety evaluation of 81 by acute toxicity and subchronic toxicity tests, paraffin sections of mice organs, and blood routine examination. In short, 81 has the potential to be developed as a therapeutic agent for HCC (Fig.14a) (Hai et al., 2022b).
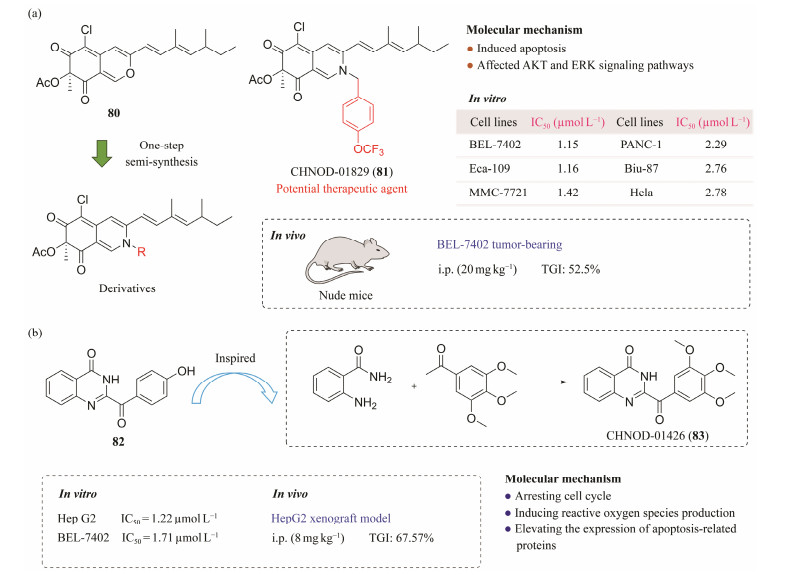
|
Fig. 14 Pharmacodynamic studies of bioactive molecules. (a), the semisynthesis and structure of CHNQD-01829 (81) and its activity against tumor cells; (b), the synthesis of CHNQD-01426 (83) and its activity against tumor cells. |
Penipanoid C (82) was first isolated from Penicillium paneum SD-44 (Li et al., 2011) and re-isolated from the organic crude extraction of the marine-derived Penicillum sp. CHNSCLM-0019 fungus in our laboratory. In the process of synthesizing and functionalizing the 2-benzoylqui-nazolin-4(3H)-one skeleton at gram-scale, a number of derivatives of 82 were synthesized and evaluated for their cytotoxic activities against four cancer cell lines (HepG2, Bel-7402, A549, and U251). Among these compounds, CHNQD-01426 (83) showed the strongest inhibitory effect on HepG2 and Bel-7402 cells with IC50 values of 1.22 and 1.71 µmol L−1, respectively. Mechanistic studies showed that 83 inhibited Bel-7402 proliferation via arresting the cell cycle. Additionally, 83 induced ROS production and increased the expression of apoptosis-related proteins to induce apoptosis in HepG2 cells. More importantly, in the HepG2 xenograft models, 83 displayed significant in vivo anticancer effects. The data indicate that 83 is a promising lead compound and may be developed as a chemotherapeutic agent for HCC (Fig.14b) (Wang et al., 2021).
'Asking for drugs from the sea' has become a key to find new sources of drugs and has made significant progress in the past few decades. At present, although a large number of active natural products have been obtained from the ocean, their active mechanism of action is not yet clear. It should be noted that the research on chemical defense matters not only aims to understand the ecological significance of these molecules, but also facilitates the discovery of more effective drug candidates. For example, conotoxins with significant cytotoxic and neuro-regulating effects are well studied, which not only resulted in the marketed drug Prialt, but also led to more than 10 ADC compounds at different stages of clinical trials (Wu et al., 2019; Fu et al., 2022).
6 ConclusionsIn this review, we briefly summarized our group's recent achievement on the discovery and optimization of marine natural drug lead compounds, basing on the chemical defense strategy of marine organisms. We discussed the discovery of compounds, total synthesis of bioactive compounds, SARs of 7 MNPs, mechanisms of QLA and CHNQD-00803, and the druggability of 4 compounds (CHNQD-01255, CHNQD-01269, CHNQD-01829 and CHNQD-01426). On the one hand, repeated separation of compounds greatly reduces the efficiency of marine drug discovery. In our study, the systematic construction of a chemical and function-oriented technical system provides the feasibility for improving the discovery rate of active MNPs. Particularly, LC-MS/MS-dependent molecular networking has been applied as a promising approach, not only for removing duplicate natural products, but also for promoting targeted separation of a range of new compounds. To improve the speed of identification of MNPs in extracts, metabolomics data combined with various chemometric methods is a powerful tool. On the other hand, another problem that restricts the efficiency of marine drug research and development is the drug source bottleneck of compounds. In our research, we use chemical synthesis and biosynthesis to solve the drug source problem. At the same time, the complex structure and mechanism of function, long development process and high capital investment have severely restricted the discovery efficiency and clinical application of marine drugs. Nowadays, advances in drug development technology can easily solve the problems of compound application. For instance, CHNQD-01255 (78) has well aqueous solubility and bioavailability through optimized modification by BFA (77) and is expected to become a drug for the treatment of HCC. Moreover, considering the rapid development of emerging technologies and methods, the potential of MNPs in drug development is immeasurable. For example, nano technology is helpful to surmount low stability and weak targeting ability, and artificial intelligence (AI) can be employed for drug target prediction and bioavailability prediction. Finally, the discovery and development of marine drugs need a multi-party, multi-sector, multi-disciplinary collaboration. We believe that the advances of MNPs discussed in this Review will provide a strong basis for marine drugs discovery and development.
AcknowledgementsOn the auspicious occasion of her 95th birthday, this paper is dedicated to Prof. Youyou Tu, the esteemed recipient of the 2015 Nobel Prize in Physiology or Medicine, in recognition of her groundbreaking discovery of Artemisinin, which has saved millions of lives worldwide. We thank Syngenta for the fellowship awarded to Qun Zhang. This work was supported by the Shandong Province Special Fund 'Frontier Technology and Free Exploration' from Laoshan Laboratory (No. 8-01), the National Natural Science Foundation of China (No. 42376116), the Special Funds of Shandong Province for Qingdao National Laboratory of Marine Science and Technology (No. 2022QN LM030003), the State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Guangxi Normal University (No. CMEMR2023-B16), the National Key Research and Development Program of China (No. 2022YFC2601305), the Innovation Center for Academicians of Hainan Province, and the Fundamental Research Funds for the Central Universities (No. 202461059).
Anadu, N. O., Davisson, V. J., and Cushman, M., 2006. Synthesis and anticancer activity of brefeldin A ester derivatives. Journal of Medicinal Chemistry, 49(13): 3897-3905. DOI:10.1021/jm0602817 (  0) 0) |
Banerjee, P., Mandhare, A., and Bagalkote, V., 2022. Marine na-tural products as source of new drugs: An updated patent review (July 2018 – July 2021). Expert Opinion on Therapeutic Patents, 32(3): 317-363. DOI:10.1080/13543776.2022.2012150 (  0) 0) |
Banwell, M. G., Ma, X., Bolte, B., Zhang, Y., and Dlugosch, M., 2017. Chemical syntheses of the cochliomycins and certain related resorcylic acid lactones. Tetrahedron Letters, 58(43): 4025-4038. DOI:10.1016/j.tetlet.2017.08.021 (  0) 0) |
Bolte, B., Basutto, J. A., Bryan, C. S., Garson, M. J., Banwell, M. G., and Ward, J. S., 2015. Modular total syntheses of the marine-derived resorcylic acid lactones cochliomycins A and B using a late-stage Nozaki-Hiyama-Kishi macrocyclization reaction. Journal of Organic Chemistry, 80(1): 460-470. DOI:10.1021/jo5024602 (  0) 0) |
Burkhardt, I., de Rond, T., Chen, P. Y. T., and Moore, B. S., 2022. Ancient plant-like terpene biosynthesis in corals. Nature Chemical Biology, 18(6): 664-669. DOI:10.1038/s41589-022-01026-2 (  0) 0) |
Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R., 2023. Marine natural products. Natural Product Reports, 40(2): 275-325. DOI:10.1039/d2np00083k (  0) 0) |
Carroll, A. R., Copp, B. R., Grkovic, T., Keyzers, R. A., and Prinsep, M. R., 2024. Marine natural products. Natural Product Reports, 41(2): 162-207. DOI:10.1039/d3np00061c (  0) 0) |
Celik, I. E., Mittendorf, F., Gomez-Suarez, A., and Kirsch, S. F., 2024. Formal synthesis of bastimolide A using a chiral Horner-Wittig reagent and a bifunctional aldehyde as key building blocks. Tetrahedron Chem, 9: 100065. DOI:10.1016/j.tchem.2024.100065 (  0) 0) |
Chao, R., Hou, X. M., Xu, W. F., Hai, Y., Wei, M. Y., Wang, C. Y., et al., 2021. Targeted isolation of asperheptatides from a coral-derived fungus using LC-MS/MS-based molecular networking and antitubercular activities of modified cinnamate derivatives. Journal of Natural Products, 84(1): 11-19. DOI:10.1021/acs.jnatprod.0c00804 (  0) 0) |
Chao, R., Said, G., Zhang, Q., Qi, Y. X., Hu, J., Zheng, C. J., et al., 2022. Design, semisynthesis, insecticidal and antibacterial activities of a series of marine-derived geodin derivatives and their preliminary structure-activity relationships. Marine Drugs, 20(2): 82. DOI:10.3390/md20020082 (  0) 0) |
Chen, J., Xu, L., Zhang, X. Q., Liu, X., Zhang, Z. X., Zhu, Q. M., et al., 2023. Discovery of a natural small-molecule AMP-activated kinase activator that alleviates nonalcoholic steatohepatitis. Marine Life Science & Technology, 5(2): 196-210. DOI:10.1007/s42995-023-00168-z (  0) 0) |
Chen, L., and Qian, P. Y., 2017. Review on molecular mechanisms of antifouling compounds: An update since 2012. Marine Drugs, 15(9): 264. DOI:10.3390/md15090264 (  0) 0) |
Chen, Z. H., Guo, Y. W., and Li, X. W., 2023. Recent advances on marine mollusk-derived natural products: Chemistry, chemical ecology and therapeutical potential. Natural Product Reports, 40(3): 509-556. DOI:10.1039/d2np00021k (  0) 0) |
Coronado, L., Zhang, X. Q., Dorta, D., Escala, N., Pineda, L. M., Ng, M. G., et al., 2021. Semisynthesis, antiplasmodial activity, and mechanism of action studies of isocoumarin derivatives. Journal of Natural Products, 84(5): 1434-1441. DOI:10.1021/acs.jnatprod.0c01032 (  0) 0) |
Cox, J. B., Kellum, A. A., Zhang, Y., Li, B., and Smith III, A. B., 2022. Total synthesis of (+)-bastimolide A: A showcase for type I anion relay chemistry. Angewandte Chemie, International Edition, 61(28): e202204884. DOI:10.1002/anie.202204884 (  0) 0) |
Dai, C., Li, X., Zhang, K., Li, X. N., Wang, W., Zang, Y., et al., 2021. Pesimquinolones I – S, eleven new quinolone alkaloids produced by Penicillium simplicissimum and their inhibitory activity on NO production. Bioorganic Chemistry, 108: 104635. DOI:10.1016/j.bioorg.2021.104635 (  0) 0) |
El-Kashef, D. H., Daletos, G., Plenker, M., Hartmann, R., Mándi, A., Kurtán, T., et al., 2019. Polyketides and a dihydroquinolone alkaloid from a marine-derived strain of the fungus Metarhizium marquandii. Journal of Natural Products, 82(9): 2460-2469. DOI:10.1021/acs.jnatprod.9b00125 (  0) 0) |
Fiorito, D., Keskin, S., Bateman, J. M., George, M., Noble, A., and Aggarwal, V. K., 2022. Stereocontrolled total synthesis of bastimolide B using iterative homologation of boronic esters. Journal of the American Chemical Society, 144(18): 7995-8001. DOI:10.1021/jacs.2c03192 (  0) 0) |
Fox, B. M., Vroman, J. A., Fanwick, P. E., and Cushman, M., 2001. Preparation and evaluation of sulfide derivatives of the antibiotic brefeldin A as potential prodrug candidates with enhanced aqueous solubilities. Journal of Medicinal Chemistry, 44(23): 3915-3924. DOI:10.1021/jm010054z (  0) 0) |
Fu, Z., Li, S., Han, S., Shi, C., and Zhang, Y., 2022. Antibody drug conjugate: The 'biological missile' for targeted cancer therapy. Signal Transduction and Targeted Therapy, 71(1): 93-780. DOI:10.1038/s41392-022-00947-7 (  0) 0) |
Gao, Y., Liu, J., Wang, L., Xiao, M., and Du, Y., 2014. Total syntheses of cochliomycin B and zeaenol. European Journal of Organic Chemistry, 2014(10): 2092-2098. DOI:10.1002/ejoc.201301613 (  0) 0) |
Gao, Y., Zhang, L., and Jiao, W., 2019. Marine glycan-derived therapeutics in China. Progress in Molecular Biology and Translational Science, 163: 113-134. DOI:10.1016/bs.pmbts.2019.02.006 (  0) 0) |
Gerwick, W. H., and Moore, B. S., 2012. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chemistry & Biology, 19(1): 85-58. DOI:10.1016/j.chembiol.2011.12.014 (  0) 0) |
Ghareeb, M. A., Tammam, M. A., El-Demerdash, A., and Atanasov, A. G., 2020. Insights about clinically approved and preclinically investigated marine natural products. Current Research in Biotechnology, 2: 88-102. DOI:10.1016/j.crbiot.2020.09.001 (  0) 0) |
Guo, F. W., Gao, Y., Gu, Y. C., and Shao, C. L., 2023a. Scalable total synthesis of aflaquinolone I and confirmation of the absolute configuration. Tetrahedron, 134: 133299. DOI:10.1016/j.tet.2023.133299 (  0) 0) |
Guo, F. W., Mou, X. F., Qu, Y., Wei, M. Y., Chen, G. Y., Wang, C. Y., et al., 2022. Scalable total synthesis of (+)-aniduquinolone A and its acid-catalyzed rearrangement to aflaquinolones. Communications Chemistry, 5(1): 35. DOI:10.1038/s42004-022-00655-x (  0) 0) |
Guo, F. W., Zhang, Q., Gu, Y. C., and Shao, C. L., 2023b. Sulfurcontaining marine natural products as leads for drug discovery and development. Current Opinion in Chemical Biology, 75: 102330. DOI:10.1016/j.cbpa.2023.102330 (  0) 0) |
Guo, F. W., Zhou, T. Y., Qu, Y., Wei, M. Y., Chen, G. Y., Gu, Y. C., et al., 2023c. Highly efficient and scalable total syntheses and stereochemical assignment of potent anti-inflammatory pesimquinolones I and J. Organic Chemistry Frontiers, 10(4): 852-858. DOI:10.1039/d2qo01788a (  0) 0) |
Hai, Y., Cai, Z. M., Li, P. J., Wei, M. Y., Wang, C. Y., Gu, Y. C., et al., 2022a. Trends of antimalarial marine natural products: Progresses, challenges and opportunities. Natural Product Reports, 39(5): 969-990. DOI:10.1039/d1np00075f (  0) 0) |
Hai, Y., Geng, J. J., Li, P. J., Ma, W. P., Wang, C. F., Wei, M. Y., et al., 2022b. Semisynthesis and biological evaluation of (+)-sclerotiorin derivatives as antitumor agents for the treatment of hepatocellular carcinoma. European Journal of Medicinal Chemistry, 232: 114166. DOI:10.1016/j.ejmech.2022.114166 (  0) 0) |
Haider, W., Xu, W. F., Liu, M., Wu, Y. W., Tang, Y. F., Wei, M. Y., et al., 2020. Structure-activity relationships and potent cytotoxic activities of terphenyllin derivatives from a small compound library. Chemistry & Biodiversity, 17(7): e2000207. DOI:10.1002/cbdv.202000207 (  0) 0) |
Han, Y. Q., Zhang, Q., Xu, W. F., Hai, Y., Chao, R., Wang, C. F., et al., 2023. Targeted isolation of antitubercular cycloheptapeptides and an unusual pyrroloindoline-containing new analog, asperpyrroindotide A, using LC-MS/MS-based molecular networking. Marine Life Science & Technology, 5(1): 85-93. DOI:10.1007/s42995-022-00157-8 (  0) 0) |
He, J., Lion, U., Sattler, I., Gollmick, F. A., Grabley, S., Cai, J. M., et al., 2005. Diastereomeric quinolinone alkaloids from the marine-derived fungus Penicillium janczewskii. Journal of Natural Products, 68(9): 1397-1399. DOI:10.1021/np058018g (  0) 0) |
Hou, X. M., Li, Y. Y., Shi, Y. W., Fang, Y. W., Chao, R., Gu, Y. C., et al., 2019a. Integrating molecular networking and 1H NMR to target the isolation of chrysogeamides from a library of marine-derived Penicillium fungi. Journal of Organic Chemistry, 84(3): 1228-1237. DOI:10.1021/acs.joc.8b02614 (  0) 0) |
Hou, X. M., Liang, T. M., Guo, Z. Y., Wang, C. Y., and Shao, C. L., 2019b. Discovery, absolute assignments, and total synthesis of asperversiamides A – C and their potent activity against Mycobacterium marinum. Chemical Communications (Cambridge, United Kingdom), 55(8): 1104-1107. DOI:10.1039/c8cc09347d (  0) 0) |
Hou, X. M., Wang, C. Y., Gerwick, W. H., and Shao, C. L., 2019c. Marine natural products as potential anti-tubercular agents. European Journal of Medicinal Chemistry, 165: 273-292. DOI:10.1016/j.ejmech.2019.01.026 (  0) 0) |
Hou, X. M., Wang, C. Y., Gu, Y. C., and Shao, C. L., 2016. Penimethavone A, a flavone from a gorgonian-derived fungus Penicillium chrysogenum. Natural Product Research, 30(20): 2274-2277. DOI:10.1080/14786419.2016.1163695 (  0) 0) |
Hou, X. M., Zhang, Y. H., Hai, Y., Zheng, J. Y., Gu, Y. C., Wang, C. Y., et al., 2017. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Marine Drugs, 15(11): 363. DOI:10.3390/md15110363 (  0) 0) |
Jana, N., and Nanda, S., 2012. Asymmetric total syntheses of cochliomycin A and zeaenol. European Journal of Organic Chemistry, 2012(23): 4313-4320. DOI:10.1002/ejoc.201200241 (  0) 0) |
Jana, N., and Nanda, S., 2018. Resorcylic acid lactones (RALs) and their structural congeners: Recent advances in their biosynthesis, chemical synthesis and biology. New Journal of Chemistry, 44(22): 17803-17873. DOI:10.1039/c8nj02534g (  0) 0) |
Jia, W. L., Ces, S. V., and Fernández-Ibáñez, M. Á., 2021. Divergent total syntheses of yaequinolone-related natural products by late-stage C-H olefination. The Journal of Organic Chemistry, 86(9): 6259-6277. DOI:10.1021/acs.joc.1c00042 (  0) 0) |
Jia, Y. L., Guan, F. F., Ma, J., Wang, C. Y., and Shao, C. L., 2015a. Pestalotiolide A, a new antiviral phthalide derivative from a soft coral-derived fungus Pestalotiopsis sp. Natural Product Sciences, 21(4): 227-230. DOI:10.20307/nps.2015.21.4.227 (  0) 0) |
Jia, Y. L., Wei, M. Y., Chen, H. Y., Guan, F. F., Wang, C. Y., and Shao, C. L., 2015b. (+)- and (−)-pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro [oxazinane-piperazinedione] skeleton from Pestalotiopsis sp. Organic Letters, 17(17): 4216-4219. DOI:10.1021/acs.orglett.5b01995 (  0) 0) |
Jiang, Y. Y., Gao, Y., Liu, J. Y., Xu, Y., Wei, M. Y., Wang, C. Y., et al., 2022a. Design and characterization of a natural Arf-GEFs inhibitor prodrug CHNQD-01255 with potent anti-hepatocellular carcinoma efficacy in vivo. Journal of Medicinal Chemistry, 65(18): 11970-11984. DOI:10.1021/acs.jmedchem.2c00532 (  0) 0) |
Jiang, Y. Y., Wu, S., Wu, Y. W., Gao, Y., Chong, D., Sun, C., et al., 2022b. New brefeldin A-cinnamic acid ester derivatives as potential antitumor agents: Design, synthesis and biological evaluation. European Journal of Medicinal Chemistry, 240: 114598. DOI:10.1016/j.ejmech.2022.114598 (  0) 0) |
Jing, Q. Q., Yin, J. N., Cheng, Y. J., Zhang, Q., Cao, X. Z., Xu, W. F., et al., 2024. Study on the anti-Mycobacterium marinum activity of a series of marine-derived 14-membered resorcylic acid lactone derivatives. Marine Drugs, 22(3): 135. DOI:10.3390/md22030135 (  0) 0) |
Kang, J. Y., Bangoura, I., Cho, J. Y., Joo, J., Choi, Y. S., Hwang, D. S., et al., 2016. Antifouling effects of the periostracum on algal spore settlement in the mussel Mytilus edulis. Fisheries and Aquatic Sciences, 19: 7. DOI:10.1186/s41240-016-0007-y (  0) 0) |
Kumar, N. S., Ramulu, B. J., and Ghosh, S., 2021. Stereoselec-tive synthesis of the C19 – C39 fragment of bastimolide A. SynOpen, 5(4): 285-290. DOI:10.1055/a-1647-7202 (  0) 0) |
Li, C. S., An, C. Y., Li, X. M., Gao, S. S., Cui, C. M., Sun, H. F., et al., 2011. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. Journal of Natural Products, 74(5): 1331-1334. DOI:10.1021/np200037z (  0) 0) |
Li, X., Huo, X., Li, J., She, X., and Pan, X., 2009. A concise synthesis of (±)-yaequinolone A2. Chinese Journal of Chemistry, 27(7): 1379-1381. DOI:10.1002/cjoc.200990230 (  0) 0) |
Liu, N., Lai, J., Lyu, C., Qiang, B., Wang, H., Jin, H., et al., 2021. Chemical space, scaffolds, and halogenated compounds of CMNPD: A comprehensive chemoinformatic analysis. Journal of Chemical Information and Modeling, 61(7): 3323-3336. DOI:10.1021/acs.jcim.1c00162 (  0) 0) |
Liu, Q. A., Shao, C. L., Gu, Y. C., Blum, M., Gan, L. S., Wang, K. L., et al., 2014. Antifouling and fungicidal resorcylic acid lactones from the sea anemone-derived fungus Cochliobolus lunatus. Journal of Agricultural and Food Chemistry, 62(14): 3183-3191. DOI:10.1021/jf500248z (  0) 0) |
Lu, X. X., Jiang, Y. Y., Wu, Y. W., Chen, G. Y., Shao, C. L., Gu, Y. C., et al., 2022. Semi-synthesis, cytotoxic evaluation, and structure-activity relationships of brefeldin A derivatives with antileukemia activity. Marine Drugs, 20(1): 26. DOI:10.3390/md20010026 (  0) 0) |
Ma, J., Zhang, X. L., Wang, Y., Zheng, J. Y., Wang, C. Y., and Shao, C. L., 2017. Aspergivones A and B, two new flavones isolated from a gorgonian-derived Aspergillus candidus fungus. Natural Product Research, 31(1): 32-36. DOI:10.1080/14786419.2016.1207073 (  0) 0) |
Ma, X., Bolte, B., Banwell, M. G., and Willis, A. C., 2016. Total syntheses of the resorcylic acid lactones paecilomycin F and cochliomycin C using an intramolecular Loh-type α-allylation reaction for macrolide formation. Organic Letters, 18(17): 4226-4229. DOI:10.1021/acs.orglett.6b01963 (  0) 0) |
Mahankali, B., and Srihari, P., 2015. A carbohydrate approach for the first total synthesis of cochliomycin C: Stereoselective total synthesis of paecilomycin E, paecilomycin F and 6'-epi-cochliomycin C. European Journal of Organic Chemistry, 2015(18): 3983-3993. DOI:10.1002/ejoc.201500395 (  0) 0) |
Molga, K., Szymkuć, S., Gołębiowska, P., Popik, A., Dittwald, P., Moskal, M., et al., 2022. A computer algorithm to discover iterative sequences of organic reactions. Nature Synthesis, 1: 49-58. DOI:10.1038/s44160-021-00010-3 (  0) 0) |
Pal, P., Chakraborty, J., Mali, A., and Nanda, S., 2016. Asym-metric total synthesis of paecilomycin F, cochliomycin C, zeaenol, 5-bromo-zeaenol and 3, 5-dibromo-zeaenol by Heck coupling and late stage macrolactonization approach. Tetrahedron, 72(18): 2336-2348. DOI:10.1016/j.tet.2016.03.054 (  0) 0) |
Papon, N., Copp, B. R., and Courdavault, V., 2022. Marine drugs: Biology, pipelines, current and future prospects for production. Biotechnology Advances, 54: 107871. DOI:10.1016/j.biotechadv.2021.107871 (  0) 0) |
Phillips, L. R., Supko, J. G., and Malspeis, L., 1993. Analysis of brefeldin A in plasma by gas chromatography with electron capture detection. Analytical Biochemistry, 211(1): 16-22. DOI:10.1006/abio.1993.1225 (  0) 0) |
Phillips, L. R., Wolfe, T. L., Malspeis, L., and Supko, J. G., 1998. Analysis of brefeldin A and the prodrug breflate in plasma by gas chromatography with mass selective detection. Journal of Pharmaceutical and Biomedical Analysis, 16(8): 1301-1309. DOI:10.1016/s0731-7085(97)00142-8 (  0) 0) |
Qin, X. Y., Yang, K. L., Li, J., Wang, C. Y., and Shao, C. L., 2015. Phylogenetic diversity and antibacterial activity of culturable fungi derived from the zoanthid Palythoa haddoni in the South China Sea. Marine Biotechnology, 17(1): 99-109. DOI:10.1007/s10126-014-9598-4 (  0) 0) |
Quintard, A., Sperandio, C., and Rodriguez, J., 2018. Modular enantioselective synthesis of an advanced pentahydroxy intermediate of antimalarial bastimolide A and of fluorinated and chlorinated analogues. Organic Letter, 20(17): 5274-5277. DOI:10.1021/acs.orglett.8b02213 (  0) 0) |
Sausville, E. A., Duncan, K. L., Senderowicz, A., Plowman, J., Randazzo, P. A., Kahn, R., et al., 1996. Antiproliferative effect in vitro and antitumor activity in vivo of brefeldin A. The cancer Journal from Scientific American, 2(1): 52-58. (  0) 0) |
Scesa, P. D., Lin, Z., and Schmidt, E. W., 2022. Ancient defensive terpene biosynthetic gene clusters in the soft corals. Nature Chemical Biology, 18(6): 659-663. DOI:10.1038/s41589-022-01027-1 (  0) 0) |
Scherlach, K., and Hertweck, C., 2006. Discovery of aspoquino-lones A – D, prenylated quinoline-2-one alkaloids from Aspergillus nidulans, motivated by genome mining. Organic & Biomolecular Chemistry, 4(18): 3517-3520. DOI:10.1039/b607011f (  0) 0) |
Schmeda-Hirschmann, G., Hormazabal, E., Astudillo, L., Rodriguez, J., and Theoduloz, C., 2005. Secondary metabolites from endophytic fungi isolated from the Chilean gymnosperm Prumnopitys andina (Lleuque). World Journal of Microbiology & Biotechnology, 21(1): 27-32. DOI:10.1007/s11274-004-1552-6 (  0) 0) |
Schwan, J., Kleoff, M., Hartmayer, B., Heretsch, P., and Christmann, M., 2018. Synthesis of quinolinone alkaloids via aryne insertions into unsymmetric imides in flow. Organic Letters, 20(23): 7661-7664. DOI:10.1021/acs.orglett.8b03392 (  0) 0) |
Schwan, J., Kleoff, M., Heretsch, P., and Christmann, M., 2020. Five-step synthesis of yaequinolones J1 and J2. Organic Letters, 22(2): 675-678. DOI:10.1021/acs.orglett.9b04455 (  0) 0) |
Shan, M., Feng, N., and Zhang, L., 2019. Efficacy of heparinoid PSS in treating cardiovascular diseases and beyond – A review of 31 years clinical experiences in China. Progress in Molecular Biology and Translational Science, 163: 75-93. DOI:10.1016/bs.pmbts.2019.02.007 (  0) 0) |
Shang, J., Hu, B., Wang, J., Zhu, F., Kang, Y., Li, D., et al., 2018. Cheminformatic insight into the differences between terrestrial and marine originated natural products. Journal of Chemical Information and Modeling, 58(6): 1182-1193. DOI:10.1021/acs.jcim.8b00125 (  0) 0) |
Shao, C. L., Linington, R. G., Balunas, M. J., Centeno, A., Boudreau, P., Zhang, C., et al., 2015a. Bastimolide A, a potent antimalarial polyhydroxy macrolide from the marine cyanobacterium Okeania hirsuta. Journal of Organic Chemistry, 80(16): 7849-7855. DOI:10.1021/acs.joc.5b01264 (  0) 0) |
Shao, C. L., Mou, X. F., Cao, F., Spadafora, C., Glukhov, E., Gerwick, L., et al., 2018. Bastimolide B, an antimalarial 24-membered marine macrolide possessing a tert-butyl group. Journal of Natural Products, 81(1): 211-215. DOI:10.1021/acs.jnatprod.7b00917 (  0) 0) |
Shao, C. L., Wang, C. Y., Wei, M. Y., Gu, Y. C., Xia, X. K., She, Z. G., et al., 2008. Structure elucidation of two new xanthone derivatives from the marine fungus Penicillium sp. (ZZF 32#) from the South China Sea. Magnetic Resonance in Chemistry, 46(11): 1066-1069. DOI:10.1002/mrc.2293 (  0) 0) |
Shao, C. L., Wang, C. Y., Zheng, C. J., She, Z. G., Gu, Y. C., and Lin, Y. C., 2010. A new anthraquinone derivative from the marine endophytic fungus Fusarium sp. (No. b77). Natural Product Research, 24(1): 81-85. DOI:10.1080/14786410902836701 (  0) 0) |
Shao, C. L., Wu, H. X., Wang, C. Y., Liu, Q. A., Xu, Y., Wei, M. Y., et al., 2011. Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. Journal of Natural Products, 74(4): 629-633. DOI:10.1021/np400079t (  0) 0) |
Shao, C. L., Xu, R. F., Wang, C. Y., Qian, P. Y., Wang, K. L., and Wei, M. Y., 2015b. Potent antifouling marine dihydroquinolin-2(1H)-one-containing alkaloids from the gorgonian coral-derived fungus Scopulariopsis sp. Marine Biotechnology, 17(4): 408-415. DOI:10.1007/s10126-015-9628-x (  0) 0) |
Shinde, P., Banerjee, P., and Mandhare, A., 2019. Marine natural products as source of new drugs: A patent review (2015 – 2018). Expert Opinion on Therapeutic Patents, 29(4): 283-309. DOI:10.1080/13543776.2019.1598972 (  0) 0) |
Simonetti, S. O., Larghi, E. L., and Kaufman, T. S., 2016a. A convenient approach to an advanced intermediate toward the naturally occurring, bioactive 6-substituted 5-hydroxy-4-aryl-1H-quinolin-2-ones. Organic & Biomolecular Chemistry, 14(9): 2625-2636. DOI:10.1039/c5ob02680f (  0) 0) |
Simonetti, S. O., Larghi, E. L., and Kaufman, T. S., 2016b. The 3, 4-dioxygenated 5-hydroxy-4-aryl-quinolin-2(1H)-one alkaloids. Results of 20 years of research, uncovering a new family of natural products. Natural Product Reports, 33(12): 1425-1446. DOI:10.1039/c6np00064a (  0) 0) |
Vece, V., Jakkepally, S., and Hanessian, S., 2018. Total synthesis and absolute stereochemical assignment of the insecticidal metabolites yaequinolones J1 and J2. Organic Letters, 20(14): 4277-4280. DOI:10.1021/acs.orglett.8b01701 (  0) 0) |
Wang, C. F., Ma, J., Jing, Q. Q., Cao, X. Z., Chen, L., Chao, R., et al., 2022. Integrating activity-guided strategy and fingerprint analysis to target potent cytotoxic brefeldin A from a fungal library of the medicinal mangrove Acanthus ilicifolius. Marine Drugs, 20(7): 432. DOI:10.3390/md20070432 (  0) 0) |
Wang, C. J., Guo, X. X., Zhai, R. Q., Sun, C. N., Xiao, G. K., Chen, J., et al., 2021. Discovery of penipanoid C-inspired 2-(3, 4, 5-trimethoxybenzoyl) quinazolin-4(3H)-one derivatives as potential anticancer agents by inhibiting cell proliferation and inducing apoptosis in hepatocellular carcinoma cells. European Journal of Medicinal Chemistry, 224: 113671. DOI:10.1016/j.ejmech.2021.113671 (  0) 0) |
Wang, K. L., Zhang, G., Sun, J., Xu, Y., Han, Z., Liu, L. L., et al., 2016. Cochliomycin A inhibits the larval settlement of Amphibalanus amphitrite by activating the NO/cGMP pathway. Biofouling, 32(1): 35-44. DOI:10.1080/08927014.2015.1121245 (  0) 0) |
Wang, L. L., Gao, Y. G., Liu, J., Cai, C., and Du, Y. G., 2014. Stereoselective total synthesis of cochliomycin A. Tetrahedron, 70(16): 2616-2620. DOI:10.1016/j.tet.2014.03.001 (  0) 0) |
Wang, X., Sun, G., Feng, T., Zhang, J., Huang, X., Wang, T., et al., 2019. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Research, 29(10): 787-803. DOI:10.1038/s41422-019-0216-x (  0) 0) |
Wei, M. Y., Li, D., Shao, C. L., Deng, D. S., and Wang, C. Y., 2013. (±)-Pestalachloride D, an antibacterial racemate of chlorinated benzophenone derivative from a soft coral-derived fungus Pestalotiopsis sp. Marine Drugs, 11: 1050-1060. DOI:10.3390/md11041050 (  0) 0) |
Wei, M. Y., Wang, C. F., Wang, K. L., Qian, P. Y., Wang, C. Y., and Shao, C. L., 2017. Preparation, structure, and potent antifouling activity of sclerotioramine derivatives. Marine Biotechnology, 19(4): 372-378. DOI:10.1007/s10126-017-9760-x (  0) 0) |
Wu, Q., Nay, B., Yang, M., Ni, Y., Wang, H., Yao, L., et al., 2019. Marine sponges of the genus Stelletta as promising drug sources: Chemical and biological aspects. Acta Pharmaceutica Sinica B, 9(2): 237-257. DOI:10.1016/j.apsb.2018.10.003 (  0) 0) |
Xu, L., Guo, F. W., Zhang, X. Q., Zhou, T. Y., Wang, C. J., Wei, M. Y., et al., 2022a. Discovery, total syntheses and potent antiinflammatory activity of pyrrolinone-fused benzoazepine alkaloids Asperazepanones A and B from Aspergillus candidus. Communications Chemistry, 5(1): 80. DOI:10.1038/s42004-022-00696-2 (  0) 0) |
Xu, W. F., Chao, R., Hai, Y., Guo, Y. Y., Wei, M. Y., Wang, C. Y., et al., 2021. 17-hydroxybrevianamide N and its N1-methyl derivative, quinazolinones from a soft-coral-derived Aspergillus sp. fungus: 13S enantiomers as the true natural products. Journal of Natural Products, 84(4): 1353-1358. DOI:10.1021/acs.jnatprod.1c00098 (  0) 0) |
Xu, W. F., Hou, X. M., Yang, K. L., Cao, F., Yang, R. Y., Wang, C. Y., et al., 2016. Nigrodiquinone A, a hydroanthraquinone dimer containing a rare C-9 – C-7' linkage from a zoanthid-derived Nigrospora sp. fungus. Marine Drugs, 14(11): 51. DOI:10.3390/md14030051 (  0) 0) |
Xu, W. F., Mao, N., Xue, X. J., Qi, Y. X., Wei, M. Y., Wang, C. Y., et al., 2019a. Structures and absolute configurations of diketopiperazine alkaloids chrysopiperazines A – C from the gorgonian-derived Penicillium chrysogenum fungus. Marine Drugs, 17(5): 250. DOI:10.3390/md17050250 (  0) 0) |
Xu, W. F., Wu, N. N., Wu, Y. W., Qi, Y. X., Wei, M. Y., Pineda, L. M., et al., 2022b. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Marine Life Science & Technology, 4(1): 88-97. DOI:10.1007/s42995-021-00103-0 (  0) 0) |
Xu, W. F., Xue, X. J., Qi, Y. X., Wu, N. N., Wang, C. Y., and Shao, C. L., 2019b. Cochliomycin G, a 14-membered resorcylic acid lactone from a marine-derived fungus Cochliobolus lunatus. Natural Product Research, 35(3): 490-493. DOI:10.1080/14786419.2019.1633646 (  0) 0) |
Yang, C., Sun, W., Liu, S., and Xia, C., 2015. Comparative effects of indole derivatives as antifouling agents on the growth of two marine diatom species. Chemistry and Ecology, 31(4): 299-307. DOI:10.1080/02757540.2015.1022536 (  0) 0) |
Yang, C., Yu, Y., Sun, W., and Xia, C., 2014. Indole derivatives inhibited the formation of bacterial biofilm and modulated Ca2+ efflux in diatom. Marine Pollution Bulletin, 88(1-2): 62-69. DOI:10.1016/j.marpolbul.2014.09.027 (  0) 0) |
Yang, K. L., Wei, M. Y., Shao, C. L., Fu, X. M., Guo, Z. Y., Xu, R. F., et al., 2012. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. Journal of Natural Products, 75(5): 935-941. DOI:10.1021/np300103w (  0) 0) |
Yee, D. A., Niwa, K. J., Perlatti, B., Chen, M., Li, Y., and Tang, Y., 2023. Genome mining for unknown-unknown natural products. Nature Chemical Biology, 19(5): 633-640. DOI:10.1038/s41589-022-01246-6 (  0) 0) |
Zan, J., Li, Z., Tianero, M. D., Davis, J., Hill, R. T., and Donia, M. S., 2019. A microbial factory for defensive kahalalides in a tripartite marine symbiosis. Science, 364(6445): eaaw6732. DOI:10.1126/science.aaw6732 (  0) 0) |
Zhang, J. M., Jiang, Y. Y., Huang, Q. F., Lu, X. X., Wang, G. H., Shao, C. L., et al., 2021. Brefeldin A delivery nanomicelles in hepatocellular carcinoma therapy: Characterization, cytotoxic evaluation in vitro, and antitumor efficiency in vivo. European Journal of Medicinal Chemistry, 172: 105800. DOI:10.1016/j.phrs.2021.105800 (  0) 0) |
Zhang, J. M., Wang, C. F., Wei, M. Y., Dong, H., Gu, Y. C., Mo, X. M., et al., 2022. Brefeldin A induces apoptosis, inhibits BCR-ABL activation, and triggers BCRABL degradation in chronic myeloid leukemia K562 cells. Anti-Cancer Agents in Medicinal Chemistry, 22(6): 1091-1101. DOI:10.2174/1871520621666210608110435 (  0) 0) |
Zhang, M., Otsukia, K., and Li, W., 2023. Molecular networking as a natural products discovery strategy. Acta Materia Medica, 2(2): 126-141. DOI:10.15212/AMM-2023-0007 (  0) 0) |
Zhang, W., Shao, C. L., Chen, M., Liu, Q. A., and Wang, C. Y., 2014. Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Letters, 55(35): 4888-4891. DOI:10.1016/j.tetlet.2014.06.096 (  0) 0) |
Zhang, X. Q., Mou, X. F., Mao, N., Hao, J. J., Liu, M., Zheng, J. Y., et al., 2018. Design, semisynthesis, α-glucosidase inhibitory, cytotoxic, and antibacterial activities of p-terphenyl derivatives. European Journal of Medicinal Chemistry, 146: 232-244. DOI:10.1016/j.ejmech.2018.01.057 (  0) 0) |
Zhang, X. Q., Spadafora, C., Pineda, L. M., Ng, M. G., Sun, J. H., Wang, W., et al., 2017. Discovery, semisynthesis, antiparasitic and cytotoxic evaluation of 14-membered resorcylic acid lactones and their derivatives. Scientific Reports, 7(1): 1-10. DOI:10.1038/s41598-017-12336-0 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


