With the development of coastal industrialization, marine heavy metal pollution have become a very prominent environmental problem (Santos et al., 2013; Narukawa et al., 2014). Studies have shown that the concentrations of many heavy metal ions, such as copper (Cu2+), lead (Pb2+), arsenic (As3+, As5+), mercury (Hg2+) and cadmium (Cd2+) in the central Bohai Sea, the southern Shandong Peninsula offshore, Shenzhen Bay waters, the Pearl River Estuary waters, Jiangsu offshore, Lianzhou Bay in Guangxi, and the South China Sea have increased year by year and have exceeded the first category of seawater quality standards (Huerga et al., 2005; Wu et al., 2013). High levels of arsenic may cause various health problems in humans, such as genetic damage, lack of hemoglobin and altered telomere length (Clowes and Francesconi, 2004; Peng et al., 2014). Arsenic is a kind of toxic and carcinogenic non-metal found in the biosphere (Liu et al., 2013; Wolle and Conklin, 2018). Among the elemental arsenic, the most physiologically toxic one is inorganic arsenic, which is significantly more toxic than organic arsenic because it can be completely and rapidly absorbed into the gastrointestinal tract. In addition, inorganic arsenic in the trivalent state enters cells at a much higher rate than inorganic arsenic in the pentavalent state, which can also cause a higher toxicity (Koch et al., 2007; Maher et al., 2015). Arsenic betaine, arsenic choline, arsenic sugar, arsenic lipid, etc., in organic arsenic will not be metabolically absorbed after ingestion, and will be completely eliminated from the body through the human excretory system, so they are not significantly toxic to humans (Ciardullo et al., 2010).
Arsenic compounds are present in high levels in aquatic products, mainly in the speciation of arsenic betaine and arsenic sugars (Hajeb and Jinap, 2012). The organisms change a portion of the inorganic arsenic into less toxic organic compounds such as methylated arsenic and arsenic-glutathione, thereby reducing the toxicity of arsenic. Arsenic is easily accumulated in the body after being absorbed by the organisms and can cause serious harm after a long-term overdose (Choi et al., 2015). Chlamys farreri is an edible shellfish that is commonly farmed in coastal areas of China. They are loved by customers because of their delicious taste, rich in protein, low fat with high concentration of unsaturated fatty acids (ω-3 and ω-6), and rich in many essential minerals and vitamins. Recently, bioaccumulation of heavy metals has been found in C. farreri (Wu et al., 2014).
The temporarily rearing method using purified seawater mainly refers to the method of placing aquatic products in clean seawater or adding certain substances to clean seawater, so that the heavy metals in the aquatic products can be excreted from the body through metabolism. Heavy metals in the water body can enter the shellfish body through respiration, feeding behavior, and osmotic exchange between the body surface and the water body, and can be enriched in the organism. If the concentration of heavy metals in the water exceeds the maximum heavy metal excretion capacity of the organism, the organism will accumulate heavy metals, and the heavy metal content in the organism is proportional to the entry rate at this time. It is related to the exposure concentration of free heavy metal ions in the water body (Sun et al., 2018). It suggests that the use of sodium acetate, sodium oxalate and sodium citrate can achieve a significant reduction of arsenic in the Perna viridis within 5 h (Azelee et al., 2014). In addition, the addition of metals and metal complexes can also remove heavy metals, and it was suggested that the removal was achieved by the replacement of the target heavy metals (Liu et al., 2021). Yang et al. (2020) showed that in the removal experiment of cadmium from C. farreri, the addition of zinc could achieve effective reduction of cadmium within 12 h. It was hypothesized that the removal of cadmium was achieved by zinc-cadmium substitution (Yang et al., 2020).
The aims of this work were to: 1) investigate the efficiency of sodium citrate, Fe3+ and changing the salinity of seawater on arsenic reduction during depuration; 2) examine the arsenic speciation in C. farreri from the experimental group with better removal effect. About 90% of the arsenic in people's daily diet comes from aquatic products, and C. farreri have certain enrichment capacity for arsenic, and the toxicity and physiological metabolism of arsenic are closely related to the arsenic speciation (John, 1994). The establishment of an accurate method for the determination of arsenic speciation compounds in C. farreri is of great importance for their food safety evaluation (Kösters et al., 2003; Mawia et al., 2021).
2 Materials and Methods 2.1 MaterialsThe C. farreri samples were purchased from an aquatic product market (Qingdao, China). We selected individuals with similar size, ranging from 6.0–7.0 cm in length and 7.0–8.0 cm in height. Before treatment, they were cultured in seawater, which was treated with sedimentation, aeration and sand filtration.
2.2 Total Arsenic Removal from C. farreriThe seawater containing different concentrations of sodium citrate (0.2, 0.4, 0.6, 0.8 g L−1) was prepared. Considering the high concentrations of ferric ions can cause significant turbidity in the seawater and reduce the survival rate of C. farreri, seawater containing 0.1, 0.2 g L−1 ferric ions was prepared for the experimental group. In the single-factor experiment with ferric ions, the probability of death of C. farreri was higher compared with other experimental groups, so appropriate concentrations of sodium citrate were added, which can chelate metal ions weakly (Anacleto et al., 2015; Mijošek et al., 2019). The two reagents were compounded with each other on the basis of singlefactor experiments. The salinity for the experimental groups included 17, 25 and 33 (natural seawater salinity). The sampling time points were 0, 12, 24, 36, and 48 h. The 0 h group was used as a control group. Six C. farreri were sampled each time; the shells were opened to remove the scallop flesh and the scallop flesh was temporarily stored in selfsealing bags in a low-temperature refrigerator (−20℃) for the next experiments (Papry et al., 2019). Oxygenation was maintained throughout the temporary rearing process. According to the single-factor experiment above, the appropriate concentration was selected for response surface experiment to optimize the removal effect.
2.3 Determination of Arsenic in C. farreriThe experimental samples of C. farreri were taken in digestion flasks, and nitric acid (5–10 mL) and perchloric acid (1–3 mL) were added according to the wet digestion method, depending on the number of samples. The samples were heated with an electric heater until the samples in the flasks were colorless and transparent or became yellowish liquid, then the solution was cooled and diluted to constant volume (Nam et al., 2010). The arsenic content was determined by inductively coupled plasma mass spectrometry (ICP-MS). The sample was passed through 0.22 μm water system cellulose membrane before testing (Dahl et al., 2010; Papry et al., 2019).
2.4 Effect of Removal Agent on Distribution of Arsenic Content in Various Organs of C. farreriSeawater was prepared according to the requirements of the experimental groups with better removal. C. farreri were temporarily reared and the rearing time was decided according to each single-factor experiment. After cultured for different periods, six samples were taken for analysis each time (Reid et al., 2020). The viscera, muscles and sides of C. farreri were separated, placed on filter paper and baked in an oven at 40℃ for 15 min to remove the moisture on the tissue surface. The tissues were then removed from the oven, cooled to room temperature, weighed, and then added to the digestion flasks for wet digestion (Ciardullo et al., 2010; Grotti et al., 2014).
2.5 Extraction of Different Speciation of Arsenic from C. farreriFor the experimental group with better removal effect, C. farreri individuals were temporarily reared, and then the scallop meat was put into an ultra-low temperature refrigerator (−80℃) for rapid freezing, followed by putting the frozen samples into a freeze-dryer for 2 days (Bacon et al., 2021). At the end of lyophilization, the samples were pounded into a uniform powder with a tissue masher, placed in self-sealing bags, wrapped in tin foil, packed in desiccators and stored away from light (Kösters et al., 2003; Maher et al., 2015). The polar arsenic (i.e., water-soluble arsenic) and non-polar arsenic were extracted from the samples in C. farreri by multi-step extraction method (Santos et al., 2013; Li and Gao, 2014).
2.6 Determination of Arsenic Speciation in Different Extracts of C. farreriThe determination of different speciation of arsenic was performed by high performance liquid chromatography-inductively coupled plasma mass spectrometry (HPLC-ICP-MS) (Li and Gao, 2014; Wolle and Conklin, 2018). Before the determination, the standard solution and mobile phase for different speciation of arsenic were prepared and the samples were passed through 0.22 μm organic microporous membrane (Hiraoka, 1991; Lin and Wu, 2021).
2.7 Statistical AnalysisAll data results were expressed as mean ± standard deviation (mean ± SD), and the significance analysis between data was performed by SPSS 22.0 statistical software. Figures were all produced using Origin 2017 for analysis. It is considered as significant when P < 0.05.
3 Results and Discussion 3.1 Arsenic Removal from C. farreriThe results of using seawater with different concentrations of sodium citrate to remove arsenic from C. farreri were shown in Fig.1A. The different concentrations of sodium citrate (0.2, 0.4 and 0.6 g L−1) had a significant reduction of arsenic in the C. farreri. 0.2 g L−1 sodium citrate had a significant effect on the removal of arsenic from the C. farreri at 48 h, from 11.07 mg per kg wet weight (mg kg −1) to 8.61 mg kg −1, with the removal rate of 22.2%; 0.4 g L−1 and 0.6 g L−1 sodium citrate showed significant removal effect on arsenic at 36 h, from the initial concentration of 11.07 mg kg −1 to 8.91 mg kg−1 and 8.40 mg kg−1, respectively, with the removal rate of 19.5% and 24.1% and further removal effect at 48 h. 0.8 g L−1 sodium citrate showed no significant removal effect on arsenic at 48 h. In general, sodium citrate showed good removal of arsenic from C. farreri within 48 h. The ability of sodium citrate to remove heavy metals from aquatic products has also been demonstrated in previous studies (Yang et al., 2020). The removal effect of sodium citrate was attributed to its chelating effect. The current circulation time of aquatic products in seafood markets does not exceed 48 h, thus the experiments were conducted within 48 h. In addition, the use of chelating agents without obvious toxic effects, such as sodium citrate, is not only less difficult in terms of practicality, but also less costly at the economic level, compared with the reduction of heavy metals in industrial waste water and adsorption of heavy metals from the water environment (Wu et al., 2013; Anacleto et al., 2015).
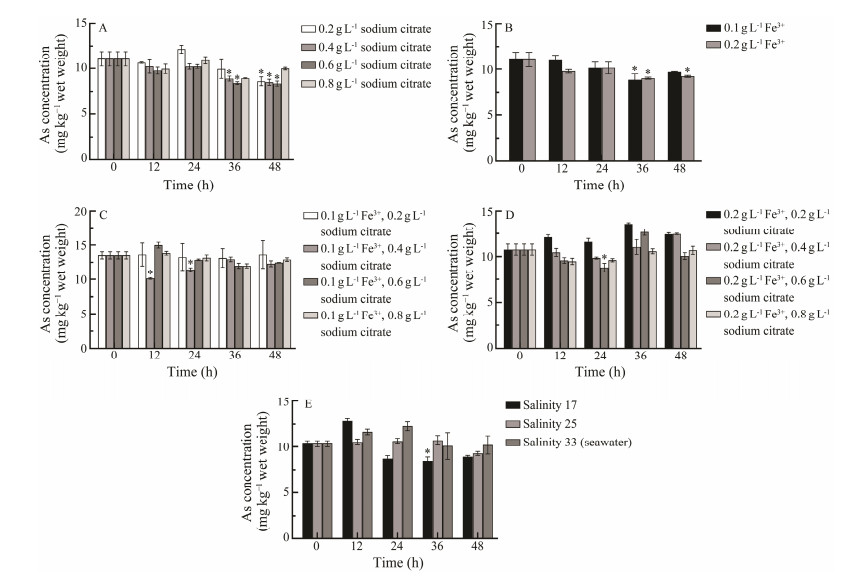
|
Fig. 1 A. Effect of sodium citrate on the removal of arsenic from C. farreri. B. Effect of Fe3+ on the removal of arsenic from C. farreri. C. Effect of 0.1 g L−1 Fe3+ and sodium citrate on the removal of arsenic from C. farreri. D. Effect of 0.2 g L−1 Fe3+ and sodium citrate on the removal of arsenic from C. farreri. E. Effect of salinity on the removal of arsenic from C. farreri. * represents significant differences (P < 0.05) compared with the group before treatment. |
The results of arsenic removal from C. farreri using different concentrations of Fe3+ were shown in Fig.1B. 0.1 g L−1 Fe3+ and 0.2 g L−1 Fe3+ could remove arsenic from C. farreri. At 36 h, the arsenic content decreased from 11.07 mg kg−1 to 8.86 mg kg−1 and 9.03 mg kg−1, and the removal rates reached 19.9% and 18.4%, respectively. However, it was found that 0.2 g L−1 Fe3+ caused more damage to the C. farreri during the temporary rearing process, and the mortality rate of C. farreri was higher, probably because the concentration of Fe3+ ions was too high and the seawater was weakly alkaline, which led to the increase of turbidity of the seawater. Considering that sodium citrate is a weak chelating agent with the ability to chelate metal ions, chelating ferric ions could reduce the turbidity of seawater and thus reduce the mortality of C. farreri (Hajeb and Jinap, 2012). The results of the experiment were as expected, the turbidity of seawater was reduced and the mortality of C. farreri was significantly reduced (almost no mortality). The results were shown in Fig.1C. The maximum removal effect was achieved at 12 h with the addition of 0.1 g L−1 Fe3+ and 0.4 g L−1 sodium citrate. The arsenic concentration decreased from 13.52 to 11.44 mg kg−1 at 24 h, and the removal rate was 15.4%. When Fe3+ increased to 0.2 g L−1 and 0.8 g L−1 sodium citrate was added, the arsenic content in the C. farreri at 24 h was significantly reduced, but the rest of the combinations had no significant effect on the removal of arsenic from the C. farreri. In the two experimental groups with 0.2 g L−1 Fe3+ and 0.2 g L−1 or 0.4 g L−1 sodium citrate, the arsenic content of C. farreri even tended to increase as shown in Fig.1D. The compound addition of sodium citrate and Fe3+ demonstrated a better removal effect, but the application of this method will change the color of seawater, which may affect the sale of seafood to some extent if used in seafood distribution.
Changing the salinity of seawater to take advantage of the change in osmotic pressure prompted the C. farreri to achieve the purpose of arsenic removal. The results are shown in Fig.1E. In addition, this experiment also investigated the effect of lower salinity (< 17) on the removal of arsenic from C. farreri, and found that a large number of C. farreri died during the temporary rearing process, presumably due to the difference in osmotic pressure between C. farreri and external seawater which exceeded the upper limit of tolerance of C. farreri.
Salinity is closely related to the survival of marine organisms. In this study, different salinities of seawater were found to correlate with the removal of arsenic, which was consistent with previous studies. Clowes and Francesconi (2004) adjusted the different salinity of seawater (32, 24, 16) to explore the uptake and elimination of arsenobetaine by the Mytilus edulis, and found it depended on the salinity of seawater. Low salinity was conducive to the elimination of arsenbetaine (Clowes and Francesconi, 2004). The effect is attributed to differences in osmotic pressure caused by changes in salinity. Sun et al. (2018) exposed the Crassostrea gigas to seawater containing 10 μg L−1 cadmium and investigated the effect of different salinities (13, 20, 27) on the uptake and elimination of arsenic in the Crassostrea gigas, and found that the uptake and elimination of arsenic betaine depended on the salinity of seawater. Although salinity can remove heavy metals, there is a problem of salinity tolerance as too much change in salinity is not conducive to the survival of organisms. This is a problem that needs to be taken into account when changing the salinity of seawater to remove arsenic from C. farreri (Anacleto et al., 2015; Sun et al., 2018).
3.2 Effect of Removal Agent on the Arsenic Contents in the TissuesBased on the experimental group with better removal effect, we investigated the effect of removal agent on the arsenic content in each tissue of C. farreri, which is helpful to understand from which part the arsenic was mainly removed. The results were shown in Fig.2. The distribution of arsenic in each tissue of C. farreri was as follows: muscle < shell side < viscera, with arsenic content of 1.61 mg kg−1, 2.64 mg kg−1 and 4.04 mg kg−1, respectively. The arsenic content of muscle did not change significantly before and after the removal. In the group treated with 0.6 g L−1 sodium citrate, the initial concentration of arsenic in the viscera of C. farreri was 4.04 mg kg−1, which decreased to 3.37 mg kg−1, 3.60 mg kg−1 and 3.49 mg kg−1 at 24 h, 36 h and 48 h, respectively. The arsenic content in the shell edge of C. farreri decreased from 2.68 mg kg−1 to 1.89 mg kg−1 at 24 h, 1.82 mg kg−1 at 36 h and 1.95 mg kg−1 at 48 h. In the group treated with 0.4 g L−1 sodium citrate and 0.1 g L−1 Fe3+, the arsenic content in the viscera decreased from 4.04 mg kg−1 at the beginning to 2.41 mg kg−1 at 12 h. The arsenic content in the shell edge of C. farreri decreased from 2.64 mg kg−1 to 2.43 mg kg−1 at 12 h, and 2.26 mg kg−1 at 24 h. In the muscle, there was no significant effect on the removal of arsenic in the two treatment groups. The organisms contain high levels of arsenic, and in order to cope with the toxicity caused by arsenic, marine organisms have evolved different detoxification mechanisms, which can convert inorganic arsenic to slightly toxic organic arsenic, and glutathione plays a great role in the conversion process. In addition, detoxification can also be achieved by methylation.
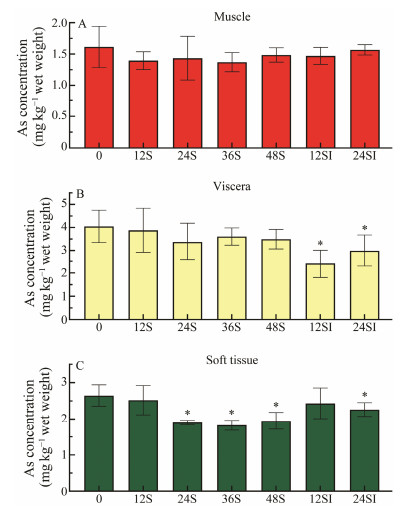
|
Fig. 2 Effect of the removal agent on the distribution of arsenic in the tissues of C. farreri. S refers to 0.6 g L−1 sodium citrate; 12S refers to temporary rearing in seawater containing 0.6 g L−1 sodium citrate for 12 h; the rest is with the same meaning. SI refers to 0.4 g L−1 sodium citrate and 0.1 g L−1 Fe3+; 12SI refers to temporary rearing in seawater containing 0.4 g L−1 sodium citrate and 0.1 g L−1 Fe3+ for 12 h; and the rest is with the same meaning. * indicates a significant difference (P < 0.05) compared to the concentration of arsenic in this tissue before delimitation. |
The C. farreri take up heavy metals from seawater through gills and then undergo a series of metabolic detoxification, and the heavy metals are finally deposited in the visceral parts containing detoxification organs such as liver and kidney, resulting in high heavy metal content in the visceral parts. The content of heavy metal in gills represents the temporary loading level of heavy metal and the content of heavy metal in viscera represents the long-term loading level of heavy metal. This also suggests that consumers should be aware of the intake of viscera when consuming C. farreri, especially to coastal residents who have a habit of consuming C. farreri as a whole.
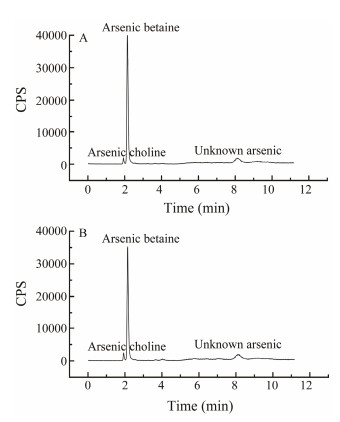
3.3 Effects of Removal Agent on Arsenic SpeciationBefore determining arsenic speciation in C. farreri, the peak profiles of six different speciation of arsenic were obtained by HPLC-ICP-MS, and the results were shown in Fig.3. Arsenical choline, arsenobetaine, arsenious acid, dimethyl arsenic, monomethyl arsenic, and arsenic acid were all detected in the samples.
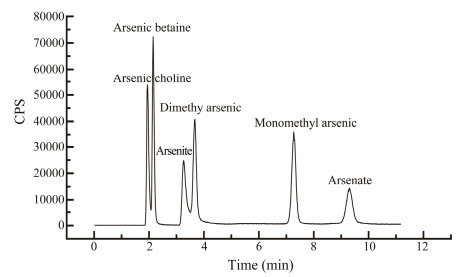
|
Fig. 3 Standard spectra of different speciation of arsenic at a concentration of 100 μg L−1. |
The total arsenic in the extracts of C. farreri samples was measured to analyze the distribution of polar (i.e., watersoluble arsenic) and non-polar (methanol-extracted arsenic) arsenic content and to understand which speciation the removal agent had the greatest effect on. The results were shown in Fig.4. In the treatment groups of 0.4 g L−1 sodium citrate and 0.1 g L−1 Fe3+, the arsenic removal from the C. farreri was mainly in the polar arsenic, which decreased from 5.71 mg kg−1 at the beginning to 4.83 mg kg−1 at 12 h. The remaining arsenic in the residue decreased from 2.47 mg kg−1 at the beginning to 2.29 mg kg−1 at 12 h, while the non-polar arsenic did not change significantly before and after the removal. In addition, it was found that the arsenic of the C. farreri was mainly polar arsenic with the highest percentage up to 63%, non-polar arsenic accounted for 15%, and unextracted arsenic (remaining arsenic in the residue) occupied 22% (Fig.4B). According to this result, both polar arsenic and non-polar arsenic were analyzed to further understand which speciation of arsenic was responsible for the decrease of the total arsenic content in C. farreri, and to analyze the safety of consumption from the different speciation of arsenic contained in C. farreri. The results were shown in Fig.5. In the treatment groups of 0.4 g L−1 sodium citrate and 0.1 g L−1 Fe3+, three speciation of arsenic were detected in the C. farreri, including arsenical choline, arsenobetaine and unknown speciation of arsenic, among which arsenobetaine was the main speciation of arsenic, which is also consistent with the previous conclusion that arsenic in seafood mainly exists in the speciation of arsenobetaine (Marcinkowska and Barałkiewicz, 2016). No any in nonpolar arsenic was detected. In addition, in the arsenic speciation analysis of C. farreri, no arsenious acid and arsenic acid with high toxicity, or even monomethyl arsenic and dimethyl arsenic with slight toxicity were detected, presumably caused by the different sources and individual differences of experimental samples (Reid et al., 2020). Although the highly toxic arsenious acid and arsenic acid were not detected in the C. farreri in this experiment, the high arsenic content in the C. farreri should not be ignored. Thus a suitable method need to be developed to control the total arsenic content in C. farreri (Anacleto et al., 2015; Fang and Zhang, 2020).
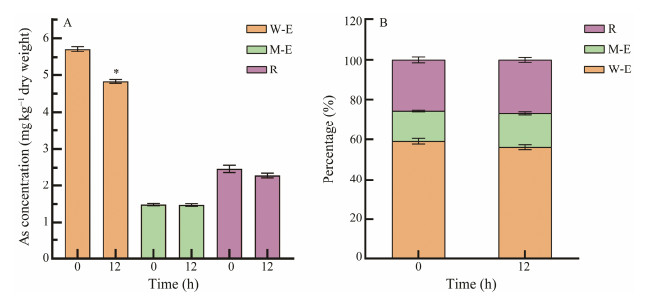
|
Fig. 4 Effect of removal agents on different arsenic from C. farreri. Temporary rearing process in seawater contained 0.4 g L−1 sodium citrate and 0.1 g L−1 Fe3+ for 12 h. W-E refers to the extraction of arsenic from C. farreri with ultrapure water; M-E refers to the extraction of arsenic from C. farreri with anhydrous methanol; R represents the residue remaining after the arsenic extraction of C. farreri. * indicates significant difference compared with before removal (P < 0.05). |
|
Fig. 5 Effects of removal agents on arsenic speciation from C. farreri. C. farreri were cultured in seawater containing 0.4 g L−1 sodium citrate and 0.1 g L−1 Fe3+ for 12 h. Graph A represents the arsenic speciation of C. farreri before removal, and graph B represents the arsenic speciation of C. farreri after 12 h removal. |
In this study, we investigated the method of removal of arsenic from live C. farreri in short time and the speciation of the arsenic removed. In order to deal with heavy metal contamination of aquatic products, we are looking for a simple and effective method to ensure food security. The sodium citrate and its combination with Fe3+ and the change of seawater salinity showed the ability to remove arsenic, and the arsenic speciation removed was mainly the non-toxic arsenic betaine. However, the use of Fe3+ can change the color of seawater and affect the consumption, and the C. farreri have poor salinity tolerance, so it does not have a large economic benefit in practical applications.
4 ConclusionsThe results of the study illustrate that the reduction of heavy metals in aquatic products can be achieved through temporary rearing process. Furthermore, the results showed that most of the arsenic in C. farreri is not toxic to humans. However, the greater the total arsenic level, the greater the risk to human health. Therefore, it is still necessary to control the total arsenic level in C. farreri through the temporary rearing process.
AcknowledgementThis work is supported by the National Key R & D Program of China (No. 2017YFC1600702).
Anacleto, P., Maulvault, A. L., Nunes, M. L., Carvalho, M. L., Rosa, R., and Marques, A., 2015. Effects of depuration on metal levels and health status of bivalve molluscs. Food Control, 47: 493-501. DOI:10.1016/j.foodcont.2014.07.055 (  0) 0) |
Azelee, I. W., Ismail, R., and Ali, R., 2014. Chelation technique for the removal of heavy metals (As, Pb, Cd and Ni) from green mussel, Perna viridis. Indian Journal of Geo-Marine Sciences, 43(3): 372-376. (  0) 0) |
Bacon, J. R., Butler, O. T., Cairns, W. R. L., Cavoura, O., Cook, J. M., Davidson, C. M., et al., 2021. Atomic spectrometry update–A review of advances in environmental analysis. Journal of Analytical Atomic Spectrometry, 36(1): 10-55. DOI:10.1039/d0ja90074e (  0) 0) |
Choi, S. D., Son, H. S., Choi, M., and Park, M. K., 2015. Accumulation features of arsenic species in various fishes collected from coastal cities in Korea. Ocean Science Journal, 50(4): 741-750. DOI:10.1007/s12601-015-0066-5 (  0) 0) |
Ciardullo, S., Aureli, F., Raggi, A., and Cubadda, F., 2010. Arsenic speciation in freshwater fish: Focus on extraction and mass balance. Talanta, 81(1-2): 213-221. DOI:10.1016/j.talanta.2009.11.060 (  0) 0) |
Clowes, L. A., and Francesconi, K. A., 2004. Uptake and elimination of arsenobetaine by the mussel Mytilus edulis is related to salinity. Comparative Biochemistry and Physiology–C Toxicology and Pharmacology, 137(1): 35-42. DOI:10.1016/j.cca.2003.11.003 (  0) 0) |
Dahl, L., Molin, M., Amlund, H., Meltzer, H. M., Julshamn, K., Alexander, J., et al., 2010. Stability of arsenic compounds in seafood samples during processing and storage by freezing. Food Chemistry, 123(3): 720-727. DOI:10.1016/j.foodchem.2010.05.041 (  0) 0) |
Fang, Y., and Zhang, Z., 2020. Arsenic trioxide as a novel antiglioma drug: A review. Cellular and Molecular Biology Letters, 25: 44. DOI:10.1186/s11658-020-00236-7 (  0) 0) |
Farmer, J. G., 1996. Arsenic exposure and health. Applied Geochemistry, 11(3): 493-494. DOI:10.1016/0883-2927(96)81808-7 (  0) 0) |
Grotti, M., Terol, A., and Todolí, J. L., 2014. Speciation analysis by small-bore HPLC coupled to ICP-MS. TrAC-Trends in Analytical Chemistry, 61: 92-106. DOI:10.1016/j.trac.2014.06.009 (  0) 0) |
Hajeb, P., and Jinap, S., 2012. Reduction of mercury from mackerel fillet using combined solution of cysteine, EDTA, and sodium chloride. Journal of Agricultural and Food Chemistry, 60(23): 6069-6076. DOI:10.1021/jf300582j (  0) 0) |
Hiraoka, Y., 1991. Reduction of heavy metal content in Hiroshima Bay oysters (Crassostrea gigas) by purification. Environmental Pollution, 70(3): 209-217. DOI:10.1016/0269-7491(91)90010-T (  0) 0) |
Huerga, A., Lavilla, I., and Bendicho, C., 2005. Speciation of the immediately mobilisable As(III), As(V), MMA and DMA in river sediments by high performance liquid chromatography-hydride generation-atomic fluorescence spectrometry following ultrasonic extraction. Analytica Chimica Acta, 534(1): 121-128. DOI:10.1016/j.aca.2004.11.025 (  0) 0) |
Koch, I., McPherson, K., Smith, P., Easton, L., Doe, K. G., and Reimer, K. J., 2007. Arsenic bioaccessibility and speciation in clams and seaweed from a contaminated marine environment. Marine Pollution Bulletin, 54(5): 586-594. DOI:10.1016/j.marpolbul.2006.12.004 (  0) 0) |
Kösters, J., Diaz-Bone, R. A., Planer-Friedrich, B., Rothweiler, B., and Hirner, A. V., 2003. Identification of organic arsenic, tin, antimony and tellurium compounds in environmental samples by GC-MS. Journal of Molecular Structure, 661-662(1-3): 347-356. DOI:10.1016/j.molstruc.2003.09.005 (  0) 0) |
Li, P., and Gao, X., 2014. Trace elements in major marketed marine bivalves from six northern coastal cities of China: Concentrations and risk assessment for human health. Ecotoxicology and Environmental Safety, 109: 1-9. DOI:10.1016/j.ecoenv.2014.07.023 (  0) 0) |
Lin, Y., Lu, J., and Wu, J., 2021. Heavy metals pollution and health risk assessment in farmed scallops: Low level of Cd in coastal water could lead to high risk of seafood. Ecotoxicology and Environmental Safety, 208: 111768. DOI:10.1016/j.ecoenv.2020.111768 (  0) 0) |
Liu, L., He, B., Yun, Z., Sun, J., and Jiang, G., 2013. Speciation analysis of arsenic compounds by capillary electrophoresis online coupled with inductively coupled plasma mass spectrometry using a novel interface. Journal of Chromatography A, 1304: 227-233. DOI:10.1016/j.chroma.2013.07.034 (  0) 0) |
Liu, Q. K., Yang, C., He, J., Meng, X. H., Cao, L. M., and Liu, B. J., 2021. Depuration cadmium on physiological status and biological response of Chlamys farreri using the combination of ZnSO4, EDTA-Na2 and sodium citrate. Chemosphere, 263: 127802. DOI:10.1016/j.chemosphere.2020.127802 (  0) 0) |
Maher, W. A., Ellwood, M. J., Krikowa, F., Raber, G., and Foster, S., 2015. Measurement of arsenic species in environmental, biological fluids and food samples by HPLC-ICPMS and HPLC-HG-AFS. Journal of Analytical Atomic Spectrometry, 30(10): 2129-2183. DOI:10.1039/c5ja00155b (  0) 0) |
Marcinkowska, M., and Barałkiewicz, D., 2016. Multielemental speciation analysis by advanced hyphenated technique–HPLC/ICP-MS: A review. Talanta, 161: 177-204. DOI:10.1016/j.talanta.2016.08.034 (  0) 0) |
Mawia, A. M., Hui, S., Zhou, L., Li, H., Tabassum, J., Lai, C., et al., 2021. Inorganic arsenic toxicity and alleviation strategies in rice. Journal of Hazardous Materials, 408: 124751. DOI:10.1016/j.jhazmat.2020.124751 (  0) 0) |
Mijošek, T., Filipović Marijić, V., Dragun, Z., Ivanković, D., Krasnići, N., Erk, M., et al., 2019. Comparison of electrochemically determined metallothionein concentrations in wild freshwater salmon fish and gammarids and their relation to total and cytosolic metal levels. Ecological Indicators, 105(6): 188-198. DOI:10.1016/j.ecolind.2019.05.069 (  0) 0) |
Nam, S. H., Oh, H. J., Min, H. S., and Lee, J. H., 2010. A study on the extraction and quantitation of total arsenic and arsenic species in seafood by HPLC-ICP-MS. Microchemical Journal, 95(1): 20-24. DOI:10.1016/j.microc.2009.08.009 (  0) 0) |
Narukawa, T., Suzuki, T., Inagaki, K., and Hioki, A., 2014. Extraction techniques for arsenic species in rice flour and their speciation by HPLC-ICP-MS. Talanta, 130: 213-220. DOI:10.1016/j.talanta.2014.07.001 (  0) 0) |
Papry, R. I., Ishii, K., Mamun, M. A. A., Miah, S., Naito, K., Mashio, A. S., et al., 2019. Arsenic biotransformation potential of six marine diatom species: Effect of temperature and salinity. Scientific Reports, 9(1): 1-16. DOI:10.1038/s41598-019-46551-8 (  0) 0) |
Peng, H. Y., Hu, B., Liu, Q. Q., Yang, Z. L., Lu, X. F., Huang, R. F., et al., 2014. Liquid chromatography combined with atomic and molecular mass spectrometry for speciation of arsenic in chicken liver. Journal of Chromatography A, 1370: 40-49. DOI:10.1016/j.chroma.2014.10.012 (  0) 0) |
Reid, M. S., Hoy, K. S., Schofield, J. R. M., Uppal, J. S., and Le, X. C., 2020. Arsenic speciation analysis: A review with an emphasis on chromatographic separations. TrAC-Trends in Analytical Chemistry: 123. DOI:10.1016/j.trac.2019.115770 (  0) 0) |
Santos, C. M. M., Nunes, M. A. G., Barbosa, I. S., Santos, G. L., Peso-Aguiar, M. C., Korn, M. G. A., et al., 2013. Evaluation of microwave and ultrasound extraction procedures for arsenic speciation in bivalve mollusks by liquid chromatography-inductively coupled plasma-mass spectrometry. Spectrochimica Acta–Part B: Atomic Spectroscopy, 86: 108-114. DOI:10.1016/j.sab.2013.05.029 (  0) 0) |
Sun, M., Liu, G., Lin, H., Zhang, T., and Guo, W., 2018. Effect of salinity on the bioaccumulation and depuration of cadmium in the Pacific cupped oyster, Crassostrea gigas. Environmental Toxicology and Pharmacology, 62(7): 88-97. DOI:10.1016/j.etap.2018.05.018 (  0) 0) |
Wolle, M. M., and Conklin, S. D., 2018. Speciation analysis of arsenic in seafood and seaweed: Part I–Evaluation and optimization of methods. Analytical and Bioanalytical Chemistry, 410(22): 5675-5687. DOI:10.1007/s00216-018-0906-0 (  0) 0) |
Wu, H. F., Liu, X. L., Zhang, X. Y., Ji, C. L., Zhao, J. M., and Yu, J. B., 2013. Proteomic and metabolomic responses of clam Ruditapes philippinarum to arsenic exposure under different salinities. Aquatic Toxicology, 136-137: 91-100. DOI:10.1016/j.aquatox.2013.03.020 (  0) 0) |
Wu, X., Gao, M., Wang, L., Luo, Y. J., Bi, R., Li, L. L., et al., 2014. The arsenic content in marketed seafood and associated health risks for the residents of Shandong, China. Ecotoxicology and Environmental Safety, 102(1): 168-173. DOI:10.1016/j.ecoenv.2014.01.028 (  0) 0) |
Yang, C., Liu, Q. K., Meng, X. H., Cao, L. M., and Liu, B. J., 2020. Depuration of cadmium from Chlamys farreri by ZnSO4, EDTA-Na2 and sodium citrate in short time. Chemosphere, 244: 125429. DOI:10.1016/j.chemosphere.2019.125429 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


